The Effect of Novel Combination Therapy with Azelastine Hydrochloride and Fluticasone Propionate in Allergic Rhinitis
Received: 22-Jul-2016 / Accepted Date: 24-Aug-2016 / Published Date: 31-Aug-2016 DOI: 10.4172/2161-119X.1000261
Abstract
Background: Allergic rhinitis is a prevalent condition in the community and impairs quality of life. Treatment often requires combination of different topical treatment. We wanted to assess the effect of combination steroid and antihistamine nasal spray in patients who have failed primary therapy. Secondary aims were to analyse the multidomain impact of rhinitis, financial costs incurred and impact on quality of life. Method: We analysed fifty-three patients who were referred to the specialist hospital having failed primary care treatment for nasal and sinus disease. The MSNOT-20 is a disease specific validated questionnaire to identify rhinitis and its response to treatment. Patients were re-assessed following one month of treatment use. Skin prick testing and nasal inspiratory peak flow were also used. Results: All subjects had improvement in their symptoms following treatment (31.6 (10.69) vs. 11 (4.18)-mean and standard deviation before and after treatment respectively, p<0.05). There was improvement in each subgroup, statistically significant in all except the emotional subgroup. Common allergens identified in sufferers were grass, house dust mite and tree pollen. The majority of patients were in the working age bracket and 90% had to take time off work due to their symptoms. Conclusion: There is significant impact on quality of life and education/work-based complications of rhinitis. Combination intranasal steroid with intranasal antihistamine is effective at improving symptomatology of allergic rhinitis, this was also demonstrated through patient comments. MSNOT-20 is once again proven as a useful tool in detecting rhinitis, its impact and disease response to treatment.
Background
Seasonal allergic rhinitis, or hay fever as it is also known, is a prevalent condition effecting 1 in 4 persons in the UK [1,2], in fact it is becoming increasingly common especially in developed countries [3-5]. Rhinitis itself means inflammation of the nasal mucous membrane and often precedes sinusitis (inflammation of the lining of the paranasal sinuses), it is rare for sinusitis to occur without coexisting rhinitis and as such the most appropriate term for both is rhinosinusitis [6,7].
Allergic rhinitis has been found to be one of the commonest causes of presentation to primary care [8], causing nasal congestion, rhinorrhea, itching and/or sneezing. Allergic rhinoconjucntivitis is the associated watery eyes, itching, burning/irritability, redness and injection of the conjunctiva which has been documented in 71% of European patients who concurrently had nasal symptoms [9].
Skin prick testing (SPT) has been shown to be superior to patientreported allergen identification or allergens as identified on allergy history take [10]. One meta-analysis reported that on SPT the top three allergens identified in 15 developed countries (covering Europe including the UK, USA and Australia) were house dust mite, grass pollen and cat (median prevalence across all centres 21.7%, 16.9% and 8.8%, respectively) [11].
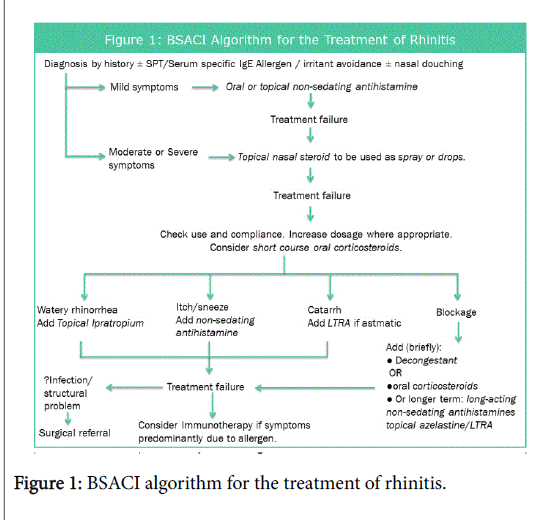
Allergic rhinitis has been shown to be a risk factor for developing asthma, often preceding it, in fact it has been shown that optimal management of allergic rhinitis may improve coexisting asthma control [12-14]. Rhinitis has also been shown to affect quality of life as well as impacting on education and productivity. The British Society of Allergy and Clinical Immunology (BSACI) have developed a pathway for the treatment of Rhinitis, adapted in Figure 1 [15,16].
One meta-analysis looked into the role of intranasal corticosteroids in patients with allergic rhinitis (n=2,267) and showed that it provides significantly greater relief of nasal congestion than oral antihistamines [17]. It is, however, combination therapy which has proven to not only improve symptomatology but also be found to be more convenient and effective when used by patients [18-20].
The MSNOT-20 is a validated, disease specific questionnaire (Appendix 1) which can identify rhinitis and rhinosinusitis, its associated impact on quality of life and disease response to treatment in the adult population, its modified version has proven similar qualities in the paediatric/young person’s population of 11-16 year old [1].
The primary aim of this study is to assess the effect of combination nasal spray (azelastine hydrochloride and fluticasone propionate) on disease in patients who have failed primary therapy in the GP setting. Secondary aims include evaluation of the cost of disease to patients and impact on quality of life, relationship between the different subgroups defined by MSNOT-20 questionnaire and comparison between allergic and non-allergic rhinitis.
Methods
Inclusion criteria- patients being referred from their GP to the ENT and Allergy department at Royal National Throat, Nose and Ear hospital, London due to nasal and sinus symptoms. These patients showed no response to optimal primary care treatment along the Figure 1 algorithm or practice-specific guidelines. Participants were enrolled in a four-month period, February-May 2016.
Exclusion criteria- patients with nasal polyposis
The tool used was the MSNOT-20 questionnaire which has been proven to be diagnostic for rhinitis [1]. It consists of three sections; section one comprises of demographic details, section two is the disease specific section and section three is the quality of life section. Its disease specific section is split into subgroups each of which is composed of the following questions from this section:
Nasal: questions 1, 2, 3, 19
Paranasal: 5, 6, 7, 8, 9, 10-can be further split into the below for further analysis
Sinus: 5, 6, 10
Ear: 7, 8, 9
Sleep: 11, 12, 13,14
Social: 15, 16, 17
Emotional: 18, 20
On first presentation to clinic patients had a full clinical assessment, completed the MSNOT-20 questionnaire and, if relevant, had skin prick test and nasal inspiratory peak flow. The management plan including pharmaceutical option was discussed; if eligible and clinically appropriate (using treatment guidelines and clinical experience) treatment was advised with the aforementioned combination nasal spray and, following informed consent, initiated as per manufacturers guidelines; 1 spray in each nostril twice a day in those over 12 years. Where needed patient information leaflet (Appendix 2) and clinical demonstration of the correct technique when using a nasal spray was discussed.
As this particular brand combination was initially not available at the index hospital a GP prescription was provided. After four weeks, patients were asked to repeat section two of the MSNOT-20 questionnaire again, results were sent back to the researchers.
Data was collated, statistical analysis was carried out and the results were represented through bar charts, graphs and tables.
Results
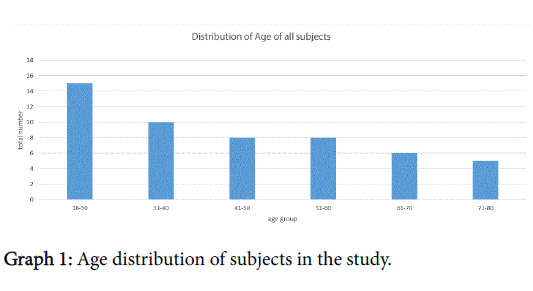
There were 53 eligible subjects in this study, 30 women 3 of whom had been pregnant within the last 12 months. The age range was 18-78 years with 83% of patients between 18-65 years of age; just under thirty percent of these were between the ages of 18-30, breakdown in Graph 1.
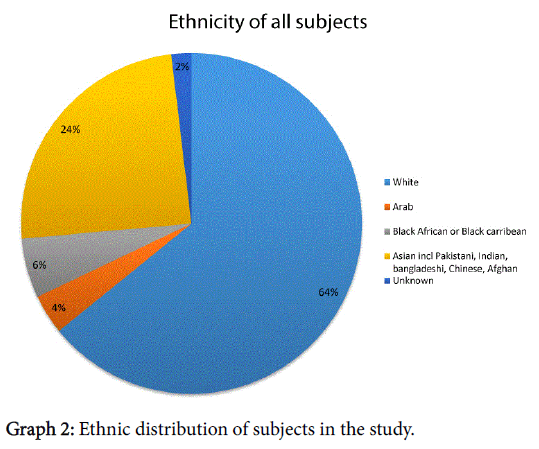
The majority of subjects described themselves as Caucasian/white, the ethnicity was distributed as shown in Graph 2.
Forty-nine percent of subjects were employed with occupations including office workers, banking staff, media industry and more. Thirteen percent were retired, eight percent were students, nine were housewives whilst the remaining were either unemployed or did not disclose their occupation.
Only 44 of the 53 had analysable responses to the question on accommodation. The majority lived in a house or bungalow, 33 of the 44, whilst the remaining 11 lived in a flat or maisonette.
Of the cohort eleven percent were current smokers, ranging from 1 to 20 cigarettes per day. The remaining had no smoking history and only one person did not answer the question.
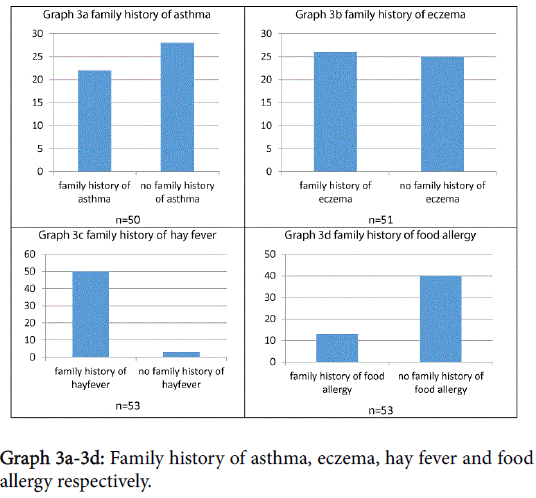
Subjects were asked about family history of potentially atopic disease, as shown in Graphs 3a-3d. Statistical analysis showed that, in this sample, there was no significant correlation between symptom severity and family history of asthma/eczema/food allergy/hay fever, p>0.1.
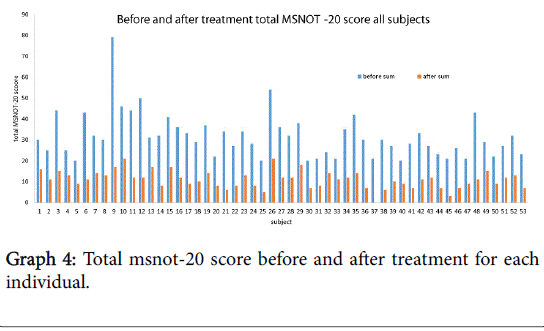
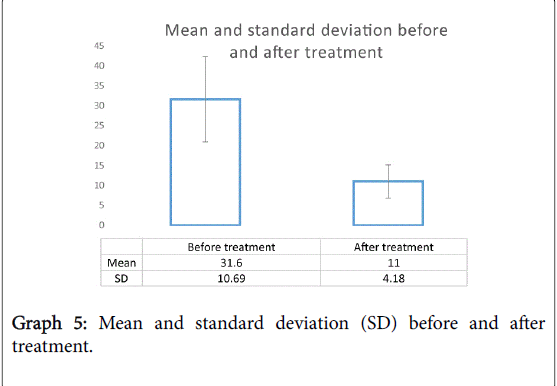
The total MSNOT-20 score is the sum of the symptom severity rating from each of the 20 disease specific questions in section 2 of the questionnaire. There was a statistically significant (p<0.05) decrease in the total MSNOT-20 score following treatment, Graph 4 shows the improvement in total MSNOT-20 score after treatment for each subject whilst Graph 5 represents the overall mean and standard deviations for before and after treatment groups.
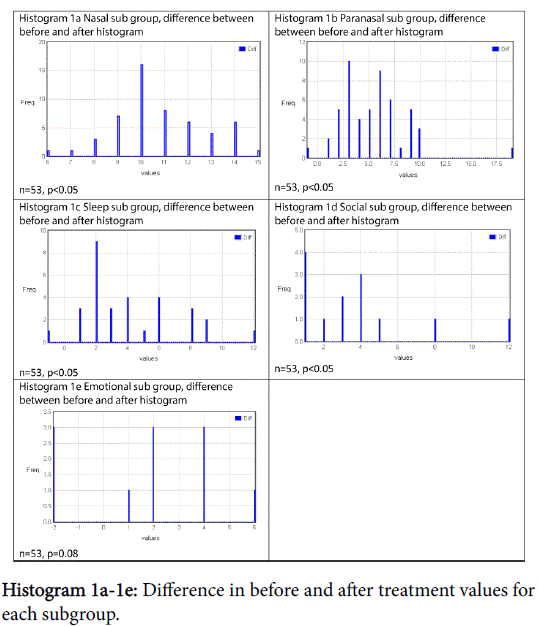
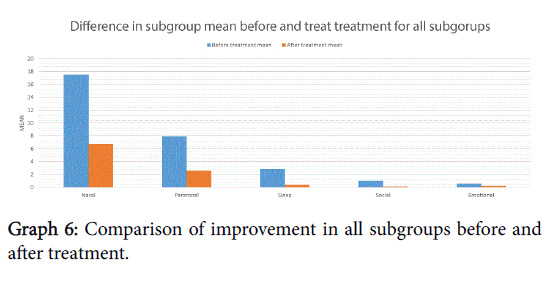
There was a statistically significant improvement following treatment in all subgroups except emotional, shown in Graph 6 and Table 1. Histograms 1a-1e show the proportion of the difference within the subgroup following treatment.
Correlation of the different subgroups using Spearman rank showed that the total MSNOT-20 score correlated most strongly with the nasal subgroup (R=0.5117, n=53, p<0.05). Subsequently the nasal group had strong correlation with paranasal (R=0.3868, p<0.05), sleep (R=0.3126, p<0.05) and social scores (R=0.3247, p<0.05). Emotional subgroup was seen to correlate strongest with the paranasal subgroup (R=0.4702, p<0.05).
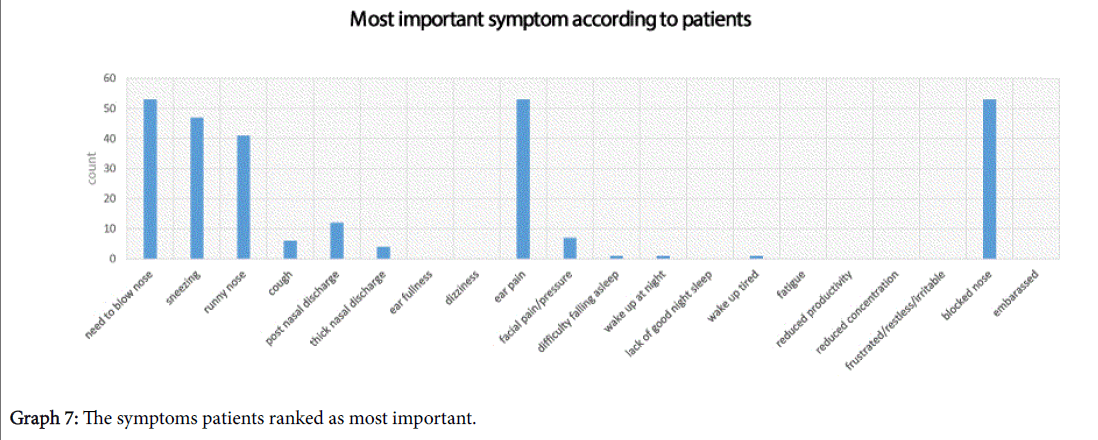
Subjects were asked to highlight the most important symptoms for them, they were allowed to choose a maximum of 5 and their responses have been shown in Graph 7. The top 3 symptoms which patients ranked the highest were; need to blow the nose, ear pain and blocked nose.
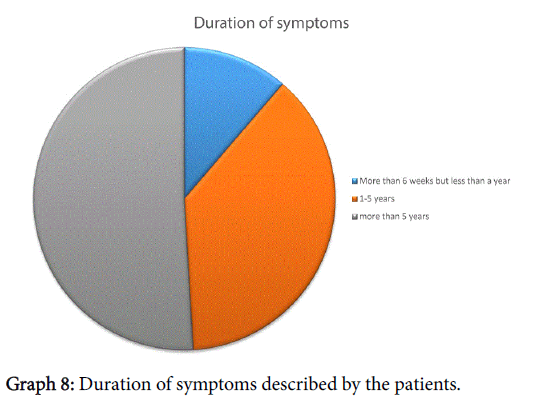
When asked about duration of symptoms, everyone had had symptoms for more than 6 weeks, further breakdown shown in Graph 8. All patients had received treatment by the GP, just under half (24/53) had to consultant their GP 3 or more times (maximum number of consultation were 6). Thirty of the fifty-three patients enrolled also sought help from their Chemist whilst 4/53 had also tried alternative therapies. Forty percent had seen an Ear, Nose and Throat specialist in the past.
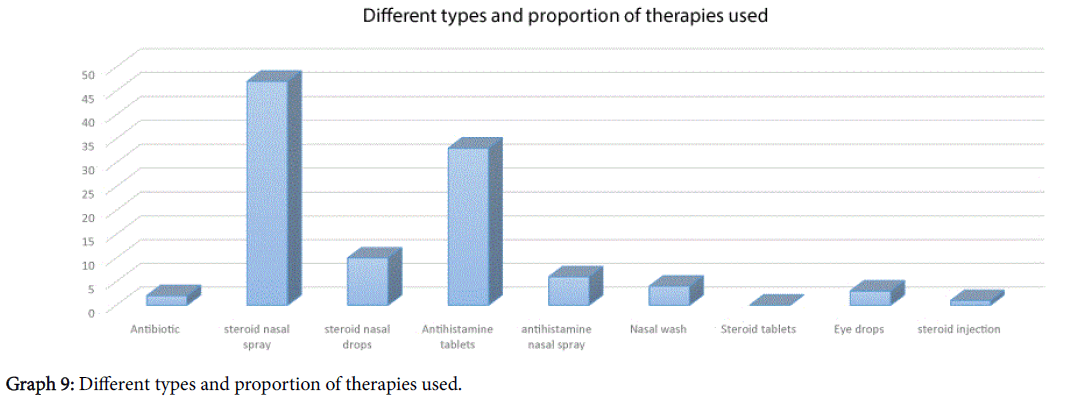
The types of treatment that patients had received have been outlined in Graph 9, however one third of patients (18/53) said that the treatment did not “work in any way” for their symptomatology.
Fifty-one percent of people used their treatment on most days. Twenty-six percent used their treatment once or twice a day whilst 17% used it once or twice a week. The remaining 6% only used their treatment once or twice a year. The financial burden on patients was significant. Sixty percent of patients spent between £5-£20 every month on treatment with 9% saying they had to spend more than £20 in a month, the highest monthly cost of treatment quoted by this cohort was £50.
Of all those enrolled onto the study, 17% had previously had an operation on their nose. Though their total MSNOT-20 score was, on average, higher than their counterparts who had not previously had a nose operation, this was not statistically significant (p=0.2).
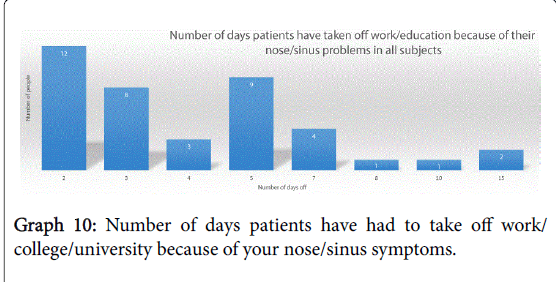
Their symptoms meant that 90% of subjects (46 of the 51 analysable responses) had to take time off work or education because of their nose/sinus symptoms, with the numbers of days taken off ranging from between 2-15, breakdown shown in Graph 10.
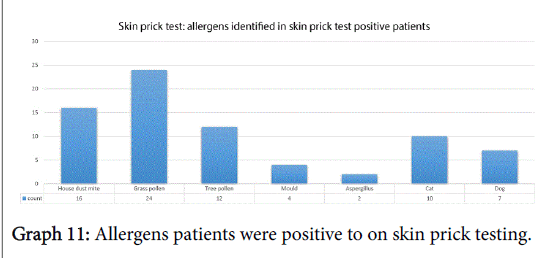
Fifty-nine percent of subjects (31/53) had a positive skin prick test (SPT), 9 were negative on SPT and the remaining 15 subjects were not eligible for testing according to their initial referral, as per Trust protocol. The allergens patients were sensitive to are shown in Graph 11.
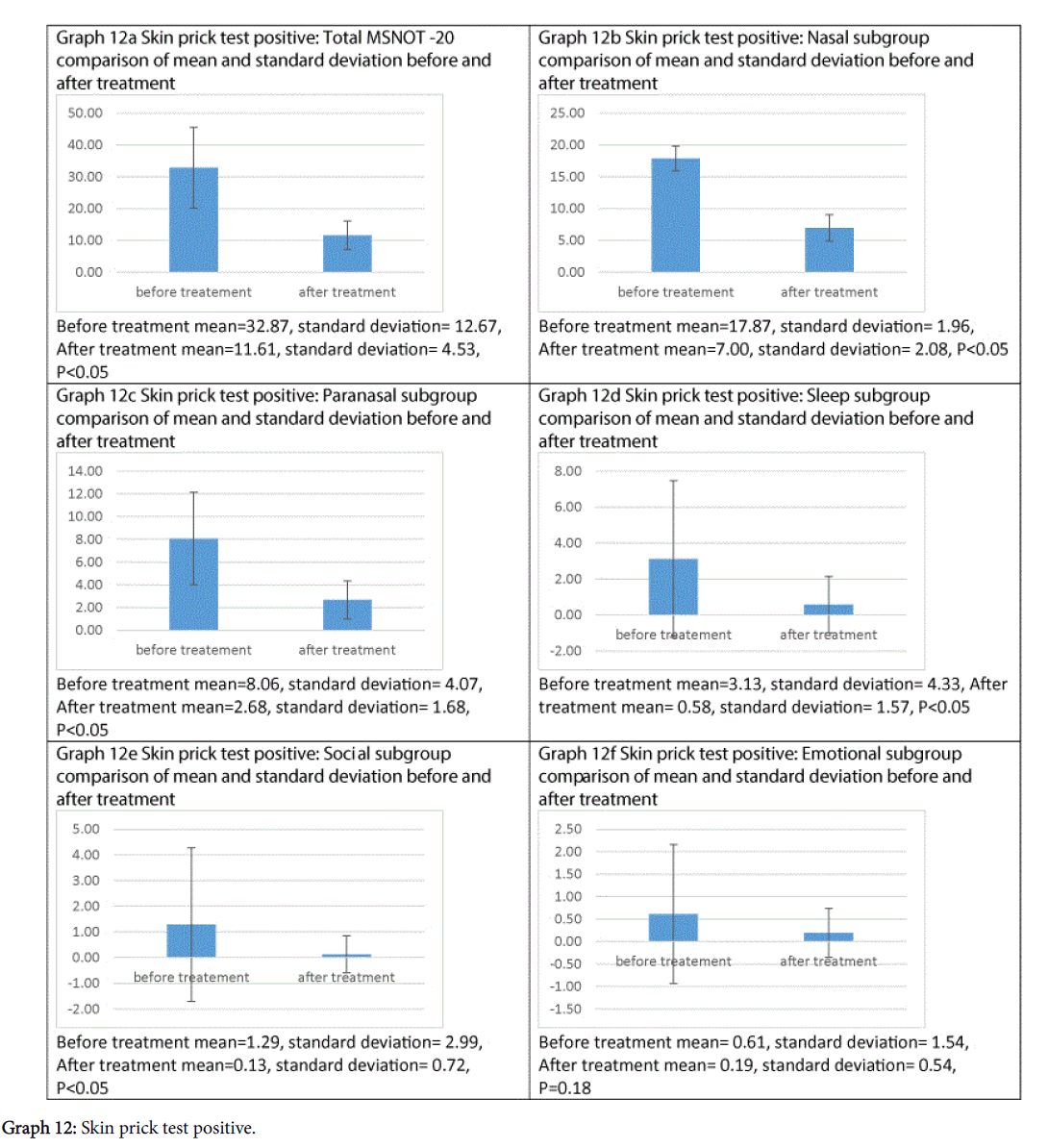
Treatment has been shown to be effective at improving symptomatology in each subgroup and the total MSNOT-20 score of all patients who had allergic rhinitis, as shown in Graphs 12a-12f, all of which were statistically significant except emotional subgroup.
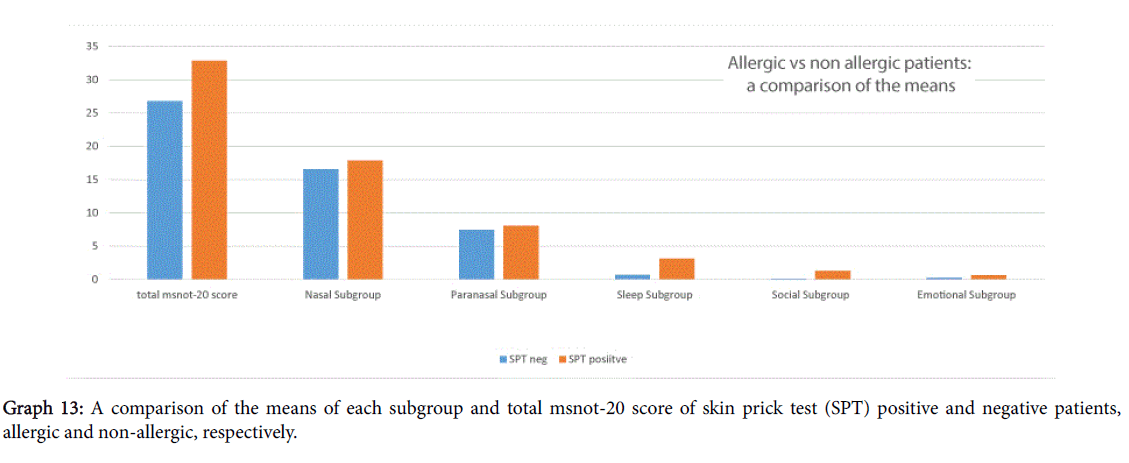
A comparison of scores between skin prick test positive and negative, i.e., allergic and non-allergic patients, shown in Graph 13, showed that allergic patients had worse total MSNOT-20 score and also more severe symptomatology in all domains, however this was not deemed statistically significant, p>0.2.
The nasal inspiratory peak flow was analysable in 28 people, of which 61% had an abnormal reading. Unfortunately, there were not enough valid samples to analyse the peak flow values of patients.
Patients were asked to comment, should they wish, following onemonth use of the spray. The following excerpts are quoted here, with consent:
One patient describing his condition in his own words prior to treatment: “very debilitating and persistent problem”
The following are the positive comments of using the therapy:
“very beneficial”, the patient subsequently wrote how he has stopped using other non-steroidal symptomatic treatments
“having a positive effect on my allergy symptoms. It feels noticeably clear when (I) breath and the swelling seems to have subsided”
“quite effective and helped me a lot going through the spring hay fever season…it has also helped (in) improving my breathing condition… I would like to keep on taking ‘Dymista’ ”
“was far more effective than [market competitor trade names quoted]”
“my nasal passages felt clearer and unblocked and the post nasal drip seems diminished”
A couple of patients mentioned the side effect they noted:
“the taste it leaves in my mouth…perhaps a more natural taste would give more confidence to me and others taking your medicine”
“the worst part was the taste”
Discussion
This treatment can significantly improve symptomatology in those suffering with allergic rhinitis, improving both the total MSNOT-20 score and that of each of the five subgroups, however this is not statistically significant in the emotional subgroup, which maybe as a consequence of the reduced prevalence of these symptoms before treatment in our cohort.
The correlations between the total disease severity score and different subgroups were analysed, please note that the closer the correlation score is to 1, the stronger relationship [21]. Our study showed that the total MSNOT-20 score correlates strongest with the nasal subgroup implying how effective and targeted treatment at the nose can have a huge influence on the disease severity as a whole. In itself the nasal subgroup correlates with the paranasal (its strongest relationship) followed by sleep then social. This proves that vast impact that nasal symptoms have on different domains of a person’s health. We found that emotional score was strongest associated with the paranasal subgroup highlighting this lesser recognised impact of dysfunction of the air sinus’s and ear symptoms.
We identified the top three allergens as grass, house dust mite and tree pollen which is similar to those found in a large international meta-analysis whose top three were house dust mite, grass and cat [11]. This shows that a significant number of our patients, hence indicative of the referrals to hospital received, suffer from allergic rhinitis and the need for effective treatment in our patients. In fact, we saw how allergic rhinitis patients had a worse severity score in each domain and in their total score also though this wasn’t deemed statistically significant.
Our study supported the evidence that allergic rhinitis is a significant cause of presentation to the GP [8]. In our cohort all patients had consulted their GP about their condition with some having up to 6 consultations about their nasal and sinus symptoms. This condition is also a burden on specialist care services with 40% of our cohort having previously seen an Ear, Nose and Throat specialist prior to enrolment in this study.
It is interesting to note that 1 in 10 of our patients were current smokers. Smoking has been shown to be associated with high prevalence of chronic rhinitis at a dose dependant effect [22]. Even though we were not able to identify its correlation with symptom severity presently, this highlights the need to promote greater patient education and support in smoking cessation initiatives.
This study shows how sufferers are reduced to having to take time off work due to the nasal and/or sinus symptoms and as the majority of our cohort is within the working age bracket, this has significant consequences for both the individual and economy as a whole. This is not the only financial consequence, indeed 60% of patients had to spend £5-£20 a month on treatment with almost one in ten paying £20 or more per month, combine this with the fact that all the patients had had their symptoms for more than 6 weeks, hence unlikely acute infection, with the maximum duration of time quoted as >5 years, this proves the huge burden rhinitis has on its sufferers. It is also important to note that a third of patients said that the treatment they took did not work for them. Subsequent treatment with combination nasal spray was then proven to improve their symptoms and has been found in previous studies to be more convenient and effective when used by patients [18-20].
The top three symptoms that patients rated as the most troublesome were need to blow the nose, ear pain and blocked nose which shows the significance nasal symptoms have in the subjective disease experience, previous studies [15] have also proven how nasal disease impacts on quality of life including social limitation, time off school and reduced work productivity.
This study once again proved the useful tool of the MSNOT-20 in research into Rhinitis in identifying disease, quantifying its impact in different domains of health and health related quality of life and measuring response to treatment in all these domains.
Conclusion
This study has shown that combination of intranasal steroid with intranasal antihistamine in one novel spray improves symptoms of those suffering from allergic rhinitis in patients who have failed conventional primary care therapy. Patients felt this improvement and commented positively to this effect.
This study has also shown that rhinitis impairs quality of life, negatively impacting on education and/or work leading to patients taking up to 15 sick days as well as incurring significant financial cost.
The MSNOT-20 has been proven to be a quick, effective and reliable tool for exploring rhinitis, its effect on quality of life and having a multi-domain assessment of response to treatment.
Conflict of Interest
A.S.S received a financial grant from Meda Pharmaceuticals. Meda Pharmaceuticals had no role in study design, collection, analysis, interpretation of data, writing the report or decision to submit for publication.
Acknowledgement
We would like to thank Dr Peter Nightingale, Statistician, University of Birmingham, for his help in the statistical analysis.
References
- Sami A (2010) Epidemiology of rhinitis in secondary school children using MSYPQ and comparison with modified SNOT-20 used in adult community based survey. European Academy of Allergy and Clinical Immunology 25.
- Kariyawasam HH, Scadding GK (2010) Seasonal allergic rhinitis: fluticasone propionate and fluticasone furoate therapy evaluated. J Asthma Allergy 3: 19-28.
- Emanuel M (1995) Hay fever, a post industrial revolution epidemic: A history of its growth during the 19th century. Clinical & Experimental Allergy 18:295-304.
- Strachan D (1995) Epidemiology of hay fever: Towards a community diagnosis. Clinical &Experimental Allergy 25:296-303.
- von Mutius E, Weiland SK, Fritzsch C, Duhme H, Keil U (1998) Increasing prevalence of hay fever and atopy among children in Leipzig, East Germany. Lancet 351: 862-866.
- Fokkens W, Lund V, Mullol J (2007) Â European Position Paper on Rhinosinusitis and Nasal Polyps group (2007) European position paper on rhinosinusitis and nasal polyps. RhinolSuppl : 1-136.
- Lanza DC, Kennedy DW (1997) Adult rhinosinusitis defined. Otolaryngol Head Neck Surg 117: S1-7.
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. (2008) Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 63 Suppl 86: 8-160.
- Canonica G, Bousquet J, Mullol J, Scadding G, Virchow J (2007) A survey of the burden of allergic rhinitis in europe. Allergy 62:17-25.
- Smith HE, Hogger C, Lallemant C, Crook D, Frew AJ (2009) Â Is structured allergy history sufficient when assessing patients with asthma and rhinitis in general practice? J Allergy ClinImmunol 123:646-650.
- Bousquet P, Chinn S, Janson C, Kogevinas M, Burney P, et al. (2007) Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the european community respiratory health survey I. Allergy 62:301-309.
- Ryan D, van Weel C, Bousquet J, Toskala E, Ahlstedt S, et al. (2008) Primary care: the cornerstone of diagnosis of allergic rhinitis. Allergy 63: 981-989.
- Greiner AN1, Hellings PW, Rotiroti G, Scadding GK (2011) Allergic rhinitis. Lancet 378: 2112-2122.
- Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, et al. (2010) Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy ClinImmunol 126: 466-476.
- Sami AS, Scadding GK (2014) Rhinosinusitis in secondary school children-part 2: main project analysis of MSNOT-20 Young Persons Questionnaire (MSYPQ). Rhinology 52: 225-230.
- Sami AS, Scadding G (2013) Management of allergic rhinitis in schools. British Journal of School Nursing 8:119-123.
- Nathan RA (2008) The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. ClinTher 30: 573-586.
- Carr W, Bernstein J, Lieberman P, Meltzer E, Bachert C, et al. (2012) A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy ClinImmunol 129: 1282-1289.
- Sami AS, Ahmed N (2014) Dymista (c) nasal spray with multifocal analysis of its impact on the rhinitis disease experience. Otolaryngology: Open Access 4: 1-5.
- Meltzer EO, LaForce C, Ratner P, Price D, Ginsberg D, et al. (2012) Â MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate) in the treatment of seasonal allergic rhinitis: A randomized, double-blind, placebo-controlled trial of efficacy and safety 33:324-332.
- Eriksson J, Ekerljung L, Sundblad BM, Lötvall J, Torén K, et al. (2013) Cigarette smoking is associated with high prevalence of chronic rhinitis and low prevalence of allergic rhinitis in men. Allergy 68: 347-354.
Citation: Sami AM, Ahmed N, Ahmed S (2016) The Effect of Novel Combination Therapy with Azelastine Hydrochloride and Fluticasone Propionate in Allergic Rhinitis. Otolaryngol (Sunnyvale) 6:261. DOI: 10.4172/2161-119X.1000261
Copyright: © 2016 Sami AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13435
- [From(publication date): 9-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 12525
- PDF downloads: 910