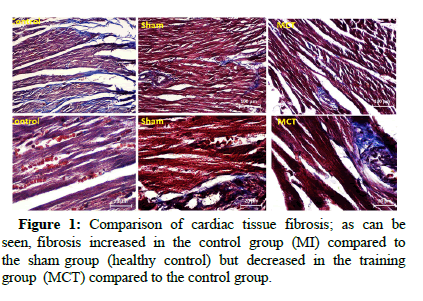
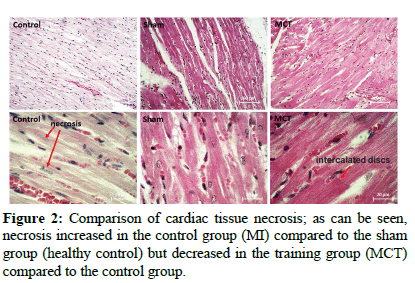
The Effect of Moderate Intensity Training on Necrosis and Fibrosis of Cardiac Tissue and their Mechanisms in Male Rats with Myocardial Infarction
Received: 17-Aug-2022 / Manuscript No. JCPR-22-72119 / Editor assigned: 22-Aug-2022 / PreQC No. JCPR-22-72119 (PQ) / Reviewed: 05-Sep-2022 / QC No. JCPR-22-72119 / Revised: 09-Jan-2023 / Manuscript No. JCPR-22-72119 (R) / Published Date: 24-Jan-2023 DOI: 10.4172/jcpr.1000188
Abstract
Introduction: The aim of this study was to investigate the effect of moderate intensity continuous training on necrosis and fibrosis of cardiac tissue and their mechanisms in male rats with myocardial infarction.
Methods: Thirty wistar rats weighing 180 g-230 g and in the age range of 2-3 months were randomly divided into three groups: Healthy control, control with myocardial infarction and training group with myocardial infarction. The rats in the training group ran on a treadmill with a zero degree slope 5 days a week for 8 weeks. The training started at a speed of 16, which increased to 2 meters per minute after two weeks or 0.2 meters per second per training session. 48 hours after the last exercise session, the rats were sacrificed and their hearts were removed. Necrotic damage, fibrosis rate, HMGB1, TLR4, TNF-α and collagen type I protein levels were measured.
Findings: The results showed that with myocardial infarction, the levels of HMGB1, TLR4, TNF-α, Col I, the rate of fibrosis and necrotic damage increased significantly (P<0.05), but training significantly reduced all these variables (P<0.05). After training, HMGB1, TLR4 and fibrosis rate were still significantly higher than pre-myocardial infarction values (P<0.05) but TNF-α, Col I and necrotic damage were not significantly different from pre myocardial infarction values (P>0.05).
Conclusion: Although myocardial infarction increases inflammation and damage to cardiac tissue, moderate intensity aerobic training may be able to reduce the amount of inflammation and damage to cardiac tissue after a myocardial infarction.
Keywords: Exercise training, Inflammation, Cardiac necrosis, Cardiac fibrosis, Myocardial infarction
Abbreviations
MI (Myocardial Infarction); PTCA (Percutaneous Transluminal Coronary Angioplasty); HMGB1 (High Mobility Group protein Box-1); TLR4 (Toll Like Receptor 4); CXCR4 chemokine receptor (CXC Receptor 4); IL-6 (Interleukin 6); TNF-α, (Tumor Necrosis Factor-alpha); SAM-α (Smooth Muscle actin-alpha); ECM (Extracellular Matrix); TGF-β (Transforming Growth Factor beta); LAD (Left coronary Artery occlusion); ANOVA One way (Analysis of Variance); MICT (Moderate Intensity Continuous Training); HIIT (High Intensity Interval Training)
Introduction
Myocardial Infarction (MI) is one of the leading causes of death worldwide and it is related to the formation of plaques in the inner wall of the artery, which blocks or reduces blood flow to the cardiac and damages the cardiac muscle due to lack of oxygen [1,2]. Currently, several treatment options such as thrombolytic drugs, Percutaneous Transluminal Coronary Angioplasty (PTCA), coronary artery bypass grafting, etc., are available for the acute treatment of MI [3-5].
In addition to medications and surgeries, epidemiological evidence suggests that physical exercise such as climbing stairs, walking and a variety of sports are inversely related to mortality from heart disease [6,7]. Therefore, exercise as a complementary treatment plays an essential role in the treatment of patients with MI. Exercise in MI patients can improve workload, functional capacity, test duration and heart rate response and in addition, it improved the ejection fraction and stroke index by 34.7% and 32%, respectively [8-9]. For several years, it was recommended to avoid exercise and physical activity after MI. However, it is now clear that moderate intensity exercise should be part of a rehabilitation program.
There is growing evidence that exercise reduces myocardial infarction by reducing fibrosis, apoptosis and inflammation [10-12]. However, various studies on animals and humans have shown that concerning the effect of exercise, the results are contradictory [13-16]. These discrepancies may be due to differences in training intensity in different studies. High intensity exercise can put too much strain on the heart of MI patients and low intensity exercise has little physiological effect. Therefore, examining the effect of moderate intensity training can provide good information to researchers. In a randomized controlled clinical trial in humans, training increased quality of life and decreased cardiovascular risk factors; but in another study on rats with myocardial infarction, exercise had no significant effect [17-18].
To better understand the effect of training, we must examine the mechanisms of the effect of training on myocardial infarction. Reduction of oxidative stress and tissue inflammation were identified as key mechanisms that contribute to the beneficial effect of training [19]. Recently, the High Mobility Group Protein Box-1 (HMGB1) has been identified as a cytokine that elicits an inflammatory response to injury [20]. Toll Like Receptor 4 (TLR4) and chemokine receptor CXC Receptor 4 (CXCR4) signaling are activated by HMGB1. In the central nervous system, HMGB1 regulates microglial activation and subsequent production of pro inflammatory cytokines. Previous studies have shown that CXCR4 signaling induces the expression of Interleukin-6 (IL-6) and Tumor Necrosis Factor alpha (TNF-α). Also, TLR4 is a major member of TLRs that plays an important role in various pathological conditions including cardiovascular disease and plays a vital role in myocardial inflammatory conditions such as MI. Regular training has been shown to exert its anti-inflammatory effects through negative regulation of TLR4. When oxidative stress occurs, an increase in TLR4 can increase pro inflammatory factors and the development of inflammation. Increase of TLR4 and pro inflammatory cytokines, including TNF-α and IL-1β, have been reported following acute intense exercise.
MI also causes fibrosis, which plays an important role in cardiac disease and causes deformity of cardiac tissue. Myofibroblasts, which express Smooth Muscle Actin-Alpha (SMA-α), exhibit a secretory and contractile phenotype that is associated with increased extracellular synthesis and deposition of Extracellular Matrix (ECM) proteins, including collagen. Transforming Growth Factor Beta (TGF-β) is the most effective stimulant of myocardial fibrosis, the mechanism of which is stimulation of ECM proteins that one of them is collagen I protein and regular exercise has been shown to inhibit cardiac fibrosis by reducing this protein. However, fewer studies have examined necrosis and fibrosis of the cardiac (both together) and their mechanisms following training. Also, as mentioned, previous studies in this field provide conflicting results, which is probably due to differences in training intensity and therefore in the present study, the moderate intensity of continuous training was examined.
The aim of this study was to investigate the effect of moderate intensity continuous training on necrosis and fibrosis of cardiac tissue and their mechanisms in male rats with myocardial infarction.
Materials and Methods
The present study was performed experimentally. Thirty wistar rats average weighing 275 ± 25 g and in the age range of 2-3 months were purchased from the laboratory animal breeding center of Pasteur Institute in Iran. After transfer to the research environment, the subjects were kept in new conditions for one week. The mice were fed pellets and drinking water was optional. The rats were kept in a polycarbonate cage in an environment with a temperature of 22 ± 2°C, light and dark cycle of 12:12 hours. After one week of adaptation to the laboratory environment, among 30 samples using a simple random sampling design, 20 rats were induced ischemia and were randomly divided into groups.
In the resting phase after infarction induction, rats with FS levels less than 35% using echocardiography (FS ≤ 35) were selected as the subjects of this study. Then, they were randomly divided into three groups: Healthy control (sham), control with myocardial infarction and training group with myocardial infarction. Left coronary artery occlusion (LAD) was performed surgically. Echocardiography was used to assess MI.
Method of induction of ischemia in rats
The rats were first anesthetized by intramuscular injection of a combination of ketamine (150 mg/kg body weight) and xylazine (15 mg/kg body weight), Then their chest hairs were completely shaved and disinfected with 70% alcohol.
After this, the animal is placed lying on its back on a rat surgery bed and the animal's arms and legs are tied. Using an otoscope number three and a green angiocatheter, the animal is intubated under a ventilator (Inter med bear) with a ratio of inhale to exhale one to two and 90 breaths-80 breaths per minute with a volume of 8 ml.
Then, from the left side of the chest, the space between the third and fourth ribs at a rate of 10 mm was done with a razor blade and other horizontal incision surgical instruments so that the heart muscle can be fully visible after removing the chest. With this incision, the anterior descending coronary artery (LAD) was identified as a bright red pulsating spike that flows in the middle of the heart wall from under the left atrium to the apex of the heart.
We closed the LAD artery with 0.6 mm polypropylene suture 1 mm-2 mm below the level of the left atrium and closed it completely by tying two knots at this point. Left ventricular anterior wall infarction was confirmed by sudden discoloration and discoloration of the myocardium and an increase in the ascending pattern of ST on the electrocardiogram during infarction. After LAD occlusion, the chest and muscle layers were sutured using 0.5 proline suture and the animal skin was sutured with 0.3 proline suture, respectively. The operated rat remained under the ventilator until it regained normal consciousness and began to breathe and when the rat regained consciousness, it was detached from the ventilator. In addition, cefazolin and tramadol as antibiotics and analgesics were injected twice a day, one day before surgery and for three days after surgery. It should be noted that a group of rats that were randomly selected as a healthy control group had their chests only opened and then sutured by a surgeon, and no surgical interventions or exercise were performed on them.
Eco cardiography
After surgery and blocking the LAD, the rats were placed on separate shelves for 48 hours. Then, in order to perform echocardiography, they were first anesthetized according to the conditions mentioned in the surgery ward and by an echocardiography specialist in the radiology ward of Shahid Rajaei heart Hospital, using an American made vivid 7 echocardiography device with 10 MHz prop at two days and tenth week after surgery, they underwent echocardiography. During this process, Ejectable Fraction (EF) and left ventricular shortening Fraction Indices (FS) were measured.
After the infarction induction and adaptation period, the rats in the training group ran on a treadmill with a zero degree slope 5 days a week for 8 weeks. The training started with a speed of 16 and according to the formula, the intensity of the training was about 65%-60% VO2 max, which after two weeks of 2 meters per minute or 0.2 meters per second for each training session was increased to their running speed (Table 1).
| Exercise variables | First and second weeks | Third and fourth weeks | Fifth and sixth weeks | Seventh and eighth weeks |
|---|---|---|---|---|
| Exercise duration | 35 minutes | 35 minutes | 35 minutes | 35 minutes |
| Exercise speed (meters per minute) | 16 | 18 | 19-20 | 22 |
| VO2 max | 60%-65% | 60%-65% | 60%-65% | 60%-65% |
Table 1: Training protocol.
48 hours after the last exercise session and after 12 hours of starvation, rats were anesthetized by inhaling chloroform and then sacrificed by beheading. The hearts were carefully separated and immediately immersed in liquid nitrogen and frozen and kept at 75°C for subsequent experiments. The heart tissue of the rats was adjusted by adding buffer containing NaCl, 50 mM Tris-HCl and 12 mM leupeptin using an electric homogenizer and homogenized at 800 rpm. The homogenized samples were then centrifuged at 4°C for 20 minutes at 3000 rpm. RNA extraction was performed using the purchased kit according to the manufacturer's instructions. Levels of HMGB1, TLR4, TNF-α and type I collagen proteins were measured using immunehistochemical staining by invision method. Masson trichrome staining method was used to evaluate the percentage of necrotic damage and hematoxylin and eosin staining method was used to evaluate the rate of fibrosis.
All stages of the research were performed in accordance with the rules of research ethics of working with laboratory animals and were reviewed in the ethics committee of Shahid Rajaee heart hospital in Tehran and approved by obtaining the ethics code IR.SSRI.REC. 1397.374. One way Analysis of Variance (ANOVA) was used to compare the differences between the groups and if it was significant, Tukey post hoc test was used.
Results
Descriptive data, the results of one way ANOVA and Tukey's test are shown in Tables 2 and 3, respectively. The results of necrotic damage and fibrosis are also shown in Figures 1 and 2. The results showed that HMGB1 and TLR4 levels increased significantly with myocardial infarction (P=0.001) but training significantly reduced both of these variables (P=0.001). However, both variables were still significantly higher than before myocardial infarction (P=0.001 and P=0.003, respectively). Also, the levels of TNF-α and Col I increased significantly with myocardial infarction (P=0.001), but training significantly reduced them (P=0.001) so that they were not significantly different from before myocardial infarction (P=0.34 and P=0.45 respectively). The rate of fibrosis with myocardial infarction increased significantly (P=0.001) but training significantly reduced it (P=0.008). However, fibrosis rate was still significantly higher than before myocardial infarction (P=0.006). Necrotic damage with myocardial infarction increased significantly (P=0.001) but exercise significantly reduced the damage (P=0.017) so that it was not significantly different from before myocardial infarction (P=0.072).
| Variables | Groups | Mean ± Std. Deviation | F | P |
|---|---|---|---|---|
| HMGB1 (%) | Healthy control | 31.77 ± 2.18 | 223.9 | 0.001* |
| Control | 67.03 ± 3.40 | |||
| Training | 53.02 ± 1.42 | |||
| TLR4 (%) | Healthy control | 45.57 ± 3.80 | 226.4 | 0.001* |
| Control | 75.33 ± 2.26 | |||
| Training | 54.64 ± 1.88 | |||
| TNF-α (%) | Healthy control | 49.90 ± 3.23 | 125.5 | 0.001* |
| Control | 71.65 ± 3.06 | |||
| Training | 52.10 ± 1.85 | |||
| Col I (%) | Healthy control | 44.63 ± 4.31 | 137 | 0.001* |
| Control | 70.99 ± 1.95 | |||
| Training | 46.68 ± 3.05 | |||
| Fibrosis (%) | Healthy control | 27.86 ± 2.40 | 55.27 | 0.001* |
| Control | 45.17 ± 3.70 | |||
| Training | 34.57 ± 2.19 | |||
| Necrosis (%) | Healthy control | 14.40 ± 3.03 | 20.08 | 0.004* |
| Control | 28.25 ± 2.42 | |||
| Training | 20.70 ± 1.92 | |||
| *Significant at 0.05 | ||||
Table 2: Comparison of variables between three groups (ANOVA).
| Variables | Pair wise comparison | P-value |
|---|---|---|
| HMGB1 | Healthy control-control | 0.001* |
| Healthy control-training | 0.001* | |
| Control-training | 0.001* | |
| TLR4 | Healthy control-control | 0.001* |
| Healthy control-training | 0.003* | |
| Control-training | 0.001* | |
| TNF-α | Healthy control-control | 0.001* |
| Healthy control-training | 0.34 | |
| Control-training | 0.001* | |
| Col I | Healthy control-control | 0.001* |
| Healthy control-training | 0.45 | |
| Control-training | 0.001* | |
| Fibrosis | Healthy control-control | 0.001* |
| Healthy control-training | 0.006* | |
| Control-training | 0.008* | |
| Necrosis | Healthy control-control | 0.001* |
| Healthy control-training | 0.072 | |
| Control-training | 0.017* | |
| *Significant at 0.05 | ||
Table 3: The results of Tukey's test regarding the points of significant difference.
Discussion
According to the findings of the present study, myocardial infarction caused a significant increase in inflammatory factors and fibrosis and necrosis of cardiac tissue, but moderate intensity continuous training significantly reduced these factors and damages (HMGB1, TLR4, TNF-α, Col I, the rate of fibrosis and necrotic damage). Recent research has shown that post MI exercise is associated with reduced mortality and recurrent myocardial infarction, so exercise should be part of a cardiac rehabilitation program. It is said that the start of exercise should be 5 to 7 days after MI. In the present study, after training, HMGB1, TLR4 and fibrosis rates were still significantly higher than pre MI values, but TNF-α, Col I and necrotic damage were almost at pre MI levels and were not significantly different from pre MI values.
In confirmation of the present findings has been shown that a short period of aerobic exercise reduces HMGB1 expression, inhibits microglia activation and decreases pro inflammatory cytokines in the hypothalamus of hypertensive rats. In addition to the classic pro inflammatory cytokines, HMGB1 has recently been identified as a cytokine like peptide that stimulates the expression of pro inflammatory cytokines by activating various cells. Due to its redox status, HMGB1 binds to TLR4 and CXCR4, which ultimately produce tissue inflammation and oxidative stress. Previous studies have shown that in the central nervous system, HMGB1 inhibits decreased microglia activation and tissue inflammation in ischemia and reperfusion models. Giallauria in patients after MI showed that cardiac rehabilitation based on exercise program significantly reduced HMGB1, which was significantly associated with improved oxygen uptake and heart rate recovery. HMGB1 appears to be the link between the autonomic nervous system in the brain and the immune system. In the present study, TLR4 levels also decreased due to training, which was probably due to a decrease in HMGB1. The results of various studies have shown that aerobic training leads to a decrease in TLR4 at the level of protein and gene. Zwagerman also reported a decrease in TLR4 following aerobic training at both protein and gene levels. But the results are contradictory. Studies have shown no significant change in TLR4 after aerobic training and even studies have shown an increase in TLR4 after aerobic training at both protein and gene levels. These differences in the results of different studies can be due to differences in training intensity. However, most studies have reported that aerobic training reduces TLR4. It has also been shown that 10 months of aerobic training has a greater effect on reducing inflammatory factors compared to strength and flexibility training. But the immunological beneficial effects of aerobic training occur when this training is performed with moderate intensity. In contrast, Zheng observed an increase in TLR2 and inflammatory cytokines, including TNF-α, following regular, moderate intensity badminton training. Researchers have attributed these results to possible improvements in the body's resistance to invasion by pathogens in response to regular exercise; this indicates that an increase in these receptors does not necessarily indicate a negative impact on health, although more research is needed. However, strenuous exercise can increase inflammation and people who run long distances have been reported to have an increased risk of atherosclerosis and coronary heart disease. It has also been observed that 24 hours of continuous physical activity increases circulating levels of inflammatory cytokines. In fact, strenuous exercise increases plasma levels of cortisol and catecholamines, which in turn reduce immune function. In intense exercise, high levels of muscle oxidative stress can increase ROS and inflammation, but moderate intensity regular exercise can reduce inflammation.
One of the reasons for the anti-inflammatory effect of training can be attributed to weight loss. Inflammation due to obesity alters the phenotype of macrophages and T cells in adipose tissue, leading to changes in the production of pro inflammatory and anti-inflammatory cytokines. It has been suggested that free fatty acids can lead to inflammation in adipose tissue by activating macrophages, TLR2 and TLR4, which peak with activation of NF-κB and increased expression of pro inflammatory cytokines such as TNF-α. In the present study, training significantly reduced TNF-α in rat with MI, possibly due to a decrease in HMGB1 and a consequent decrease in TLR4. On the other hand, Phillips reported a decrease in TNF-α without decreasing TLR4 expression following resistance training, but in another study, a decrease in TLR4 protein content after 10 days of Moderate Intensity Continuous Training (MICT) and High Intensity Interval Training (HIIT) observed. In contrast, nickel showed an increase in these receptors at the gene and protein levels in marathon runners. What is clear is that in future researches, different training intensities should be further compared. Low grade chronic inflammatory characteristics are involved in the pathogenesis of various diseases, which TNF-α is one of the inflammatory parameters reported in this field. In confirmation of the present findings, it has been suggested that moderate intensity aerobic exercise can lead to a decrease in TLR4, which in turn leads to a decrease in inflammatory cytokines such as TNF-α and an increase in anti-inflammatory cytokines such as IL-10. For these training effects, training intensity plays a very decisive role, so that the desired effects occur at moderate intensity. The moderate intensity of training in the present study led to a significant reduction in cardiac tissue necrosis in MI. However, what the effects of exercise are depends on other physiological characteristics of individuals that should be considered in future researches.
The present results also showed that both collagen I and cardiac fibrosis were significantly reduced due to training. Cardiac fibrosis is a complex process involving several intervening pathways, but TGF-β and collagens play an important role in cardiac fibrosis. Previous studies have shown that TGF-β causes scar tissue to form during scar formation. It also causes fibroblasts to become myofibroblasts. Myofibroblasts are present in the abnormal myocardium and are characterized by the presence of α-SMA rich microfilament contractile devices. Persistence of myofibroblasts leads to excessive scarring. Most studies have shown that the expression of collagen I and collagen III in cardiac fibroblasts is significantly increased. On the other hand, it has been reported that the collagen profile changes after training in cardiac. For example, Thomas observed that 10 weeks of treadmill exercise resulted in an up regulation of collagen concentration (collagen percentage) in the left ventricular septum of rats. However, the results are also contradictory, so that Burgess did not observe any change in left ventricular collagen after 10 weeks of treadmill training. Woodiwiss also reported no significant effect of 16 weeks of running on myocardial collagen in rats. Jin also showed that mRNA levels of type I and III rat collagen did not change significantly after 13 weeks of treadmill training. But Kwak reported an increase in rat collagen after 12 weeks of training. We observed a decrease in collagen I and subsequent decrease in fibrosis after training, but it seems that the role of exercise training on cardiac collagen needs to be further investigated.
Conclusion
Although myocardial infarction increases inflammation and damage to cardiac tissue, moderate intensity aerobic training may be able to reduce the amount of inflammation and damage to cardiac tissue after a myocardial infarction. Inflammatory markers and damage to cardiac tissue evaluated in the present study were all reduced by exercise and some of them did not show a significant difference compared to pre MI values. It is possible that if the training period was longer, all the variables would have reached the level of pre MI values that should be considered in the future.
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
References
- Mcaloon CJ, Boylan LM, Hamborg T (2016) The changing face of cardiovascular disease 2000-2012: An analysis of the world health organisation global health estimates data. Int J Cardiol 224: 256-264.
[Crossref] [Google Scholar] [PubMed]
- Lu L, Liu M, Sun R, Zheng Y, Zhang P (2015) Myocardial infarction: Symptoms and treatents. Cell Biochem Biophys 72: 865-867.
[Crossref] [Google Scholar] [PubMed]
- Sorensen JT, Maeng M (2015) Regional systems of care for primary percutaneous coronary intervention in ST elevation myocardial infarction. Coron Artery Dis 26: 713-722.
[Crossref] [Google Scholar] [PubMed]
- Song PS, Kim MJ, Jeon KH, Lim S, Park JS, et al. (2015) Efficacy of postprocedural anticoagulation after primary percutaneous coronary intervention for ST segment elevation myocardial infarction: A post-hoc analysis of the randomized innovation trial. Medicine (Baltimore) 98: e15277.
[Crossref] [Google Scholar] [PubMed]
- Lhermusier T, Ohayon P, Boudou N, Bouisset F, Campelo-Parada F, et al. (2019) Re-endothelialisation after synergy stent and absorb bioresorbable vascular scaffold implantation in acute myocardial infarction: Cover AMI study Trials 20: 1-9.
[Crossref] [Google Scholar] [PubMed]
- Paffenbarger RS Jr, Hyde RT, Wing AL, Hsieh CC (1986) Physical activity, all cause mortality and longevity of college alumni. N Engl J Med 314: 605-613.
[Crossref] [Google Scholar] [PubMed]
- Xing Y, Yang SD, Wang MM, Feng YS, Dong F et al. (2020) The beneficial role of exercise training for myocardial infarction treatment in elderly. Front Physiol 11: 270.
[Crossref] [Google Scholar] [PubMed]
- Andjic M, Spiroski D, Ilic Stojanovic O, Vidakovic T, Lazovic M, et al. (2016) Effect of short term exercise training in patients following acute myocardial infarction treated with primary percutaneous coronary intervention. Eur J Phys Rehabil Med 52: 364-369.
[Google Scholar] [PubMed]
- Chursina TV, Molchanov AV (2006) Bicycle exercise in the free load regimen and hemodynamics in inpatients with ischemic heart disease. Vopr Kurortol Fizioter Lech Fiz Kult 5: 5-8.
[Google Scholar] [PubMed]
- Puhl SL, Muller A, Wagner M, Devaux Y, Böhm M, et al. (2015) Exercise attenuates inflammation and limits scar thinning after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 309: 345-359.
[Crossref] [Google Scholar] [PubMed]
- Xu X, Wan W, Powers AS, Ji LL, Lao S, et al. (2008) Effects of exercise training on cardiac function and myocardial remodeling in post myocardial infarction rats. J Mol Cell Cardiol 44: 114-122.
[Crossref] [Google Scholar] [PubMed]
- Maessen MF, Eijsvogels TM, Stevens G, van Dijk AP, Hopman MT (2017) Benefits of lifelong exercise training on left ventricular function after myocardial infarction. Eur J Prev Cardiol 24: 1856-1866.
[Crossref] [Google Scholar] [PubMed]
- Libonati JR (2003) Exercise and diastolic function after myocardial infarction. Med Sci Sports Exerc 35: 1471-1476.
[Crossref] [Google Scholar] [PubMed]
- Otsuka Y, Takaki H, Okano Y, Satoh T, Aihara N, et al. (2003) Exercise training without ventricular remodeling in patients with moderate to severe left ventricular dysfunction early after acute myocardial infarction. Int J Cardiol 87: 237-244.
[Crossref] [Google Scholar] [PubMed]
- Kubo N, Ohmura N, Nakada I, Asu T, Katsuki TA, et al. (2004) Exercise at ventilatory threshold aggravates left ventricular remodeling in patients with extensive anterior acute myocardial infarction. Am Heart J 147: 113-120.
[Crossref] [Google Scholar] [PubMed]
- Liao Z, Li D, Chen Y, Huang R, Zhu K, et al. (2019) Early moderate exercise benefits myocardial infarction healing via improvement of inflammation and ventricular remodelling in rats. J Cell Mol Med 23: 8328-8342.
[Crossref] [Google Scholar] [PubMed]
- Peixoto TC, Begot I, Bolzan DW, Machado L, Reis MS, et al. (2015) Early exercise based rehabilitation improves health related quality of life and functional capacity after acute myocardial infarction: A randomized controlled trial. Can J Cardiol 31: 308-313.
[Crossref] [Google Scholar] [PubMed]
- De Waard MC, Van Der Velden J, Bito V, Biesmans L, Boontje NM, et al. (2007) Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res 100: 1079-1088.
[Crossref] [Google Scholar] [PubMed]
- Masson GS, Costa TS, Yshii L, Fernandes DC, Soares PP, et al. (2014) Time dependent effects of training on cardiovascular control in spontaneously hypertensive rats: Role for brain oxidative stress and inflammation and baroreflex sensitivity. PLoS One 9: e94927–e94937.
[Crossref] [Google Scholar] [PubMed]
- Lotze MT, Tracey KJ (2005) High Mobility Group Box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol 5: 331-342.
[Crossref] [Google Scholar] [PubMed]
Citation: Soori R, Mosayebi Z, Pournemati P, Akbarnejad A (2023) The Effect of Moderate Intensity Training on Necrosis and Fibrosis of Cardiac Tissue and their Mechanisms in Male Rats with Myocardial Infarction. J Card Pulm Rehabi 7:188. DOI: 10.4172/jcpr.1000188
Copyright: © 2023 Soori R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1336
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1084
- PDF downloads: 252