Research Article Open Access
The Effect of In-Vivo Collagen Cross-Linking Procedure on the Material of Intracorneal Ring Segments
Haris Sideroudi1*, Georgios Labiris1, Amaia Soto-Beobide2, Irfan Perente3, Georgios Voyiatzis2, Athanassios Chrissanthopoulos2, Hanefi Cakir3 and Vassilios Kozobolis11Eye Institute of Thrace, Democritus University, Alexandroupolis, Greece
2Foundation for Research and Technology – Hellas / Institute of Chemical Engineering Sciences (FORTH/ICE-HT), Patras, Greece
3Turkiye Hospital, Istanbul, Turkey
- Corresponding Author:
- Haris Sideroudi
Eye Institute of Thrace
1st building of Medical School
Democritus University of Thrace
Dragana-Alexandroupolis P.O. 68100, Greece
Tel: +302551039891
Fax: +302551039891
E-mail: harissid@alex.duth.gr
Received date:: September 29, 2015; Accepted date:: November 20, 2015; Published date:: November 27, 2015
Citation: Sideroudi H, Labiris G, Soto-Beobide A, Perente I, Voyiatzis G, et al. (2015) The Effect of In-Vivo Collagen Cross-Linking Procedure on the Material of Intracorneal Ring Segments. J Biotechnol Biomater 5:209. doi:10.4172/2155-952X.1000209
Copyright: © 2015 Sideroudi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The material of Intracorneal Rings Segments (ICRS) has seemed to be polymerized after the absorption of Riboflavin and UV illumination in laboratory settings. This study aimed to assess the potential impact of in-vivo corneal crosslinking (CXL) on the chemical composition of ICRS. Within this contex, three ICRS samples (S1, S2 and S3) were extracted from patients' cornea, which formerly had undergone CXL treatment. Alterations on the chemical structure of PMMA rings were studied using Ultraviolet-Visible (UV-Vis) spectroscopy and Fourier Transform-Infrared (FT-IR) spectroscopy. An extracted ICRS from a patient who didn’t underwent CXL treatment, was also used as reference (S0). UV-Vis spectroscopy didn't identify any change in the specimens S1, S2 and S3. Nevertheless, FT-IR spectroscopic analysis showed alterations in the spectra of ICRS material of samples S1, S2 and S3, mainly at the 2800 to 3200 cm-1 spectral region [modification in band intensities of CH2 (2850 cm-1 and 2925 cm-1) and CH3 (2950cm-1)]. In conclusion, our results suggest crosslinking reaction in ICRS material after in-vivo CXL treatment. This should be taken into consideration prior to any CXL treatment of post ICRS implanted cornea.
Keywords
Corneal crosslinking; FT-IR spectroscopy; INTACS; Keratoconus; UV-Vis spectroscopy
Introduction
Intracorneal rings (ICRS) are semicircular segments of poly (methyl methacrylate) -PMMA-, that are implanted in the corneal periphery medium either by conventional or laser assisted methods. These implants were initially developed to correct myopia by modifying the corneal curvature. Colin et al. were the first to report on the efficacy of these implants in keratoconus [1]. Corneal ectasias (e.g., keratoconus, pellucid marginal degeneration, laser-induced iatrogenic ectasia) are associated with progressive irregular astigmatism and corneal stroma degeneration and these vision-correcting methods attempt to regularize the front surface of the cornea while maintaining the existing biomechanical status of the underlying stroma.
In ectatic corneas, ICRS have traditionally been used in order to minimize aberrations. However ICRS alone could not halt corneal ectasia. Therefore, a series of studies proposed that ICRS implantation should be combined with corneal crosslinking treatment, using 365nm UV-A irradiation with riboflavin (CXL). It is known that CXL treatment attempts to stabilize and strengthen the ectatic cornea by creating new covalent bonds between the stromal collagen fibrils [2,3]. Both modalities exert a distinct beneficial impact on the ectatic cornea that aims to: (1) improve the corneal topography and the visual ability and (2) stabilize the ectatic cornea over time.
Former investigator assessed the impact of UV illumination on PMMA material has been thoroughly investigated [4-7]. Specifically, photodegradation and side chain cleavage is induced by UV irradiation below 250 nm and leads to chemical reactions such photopolymerization, crosslinking or curing [8,9]. Infrared (IR) spectroscopy is among the prevalent modality for assessing the structure, the purity and chemical modification of materials.
However the impact of standard CXL treatment on ICRS material has not been explored. Within this context, primary objective of this work was the assessment of the potential impact of the CXL treatment on the chemical composition of ICRS.
Methods
Settings
The study protocol adheres to the tenets of the Helsinki Declaration and written informed consent was obtained from all participants. All patients were diagnosed and treated at the Turkiye Hospital in Istanbul, Turkey.
Surgical technique
Four patients had femtosecond laser–assisted (IntraLase Femtosecond Laser 60 Megahertz (IntraLase, Irvine, California, USA/ AMO's IntraLase™ FS Technology) placement of ICRS (Keraring model SI-6 Kerarig Mediphacos - Ophthalmic) that consisted of two sterile, non-pyrogenic, crescent shaped, 160-degree PMMA segments (Samples S0 and S11, S12, S13). The thickness of the segments was 250 μm. The axis of incision was the steepest axis of manifest refraction. The depth of the ring channels was set at 300 μm by intraoperative pachymetry as long as 75% of the thinnest pachymetry reading was above 350 μm. The Keraring Calculation Guidelines 2009 (Mediphacos - Ophthalmic) was used for selection of ICRS.
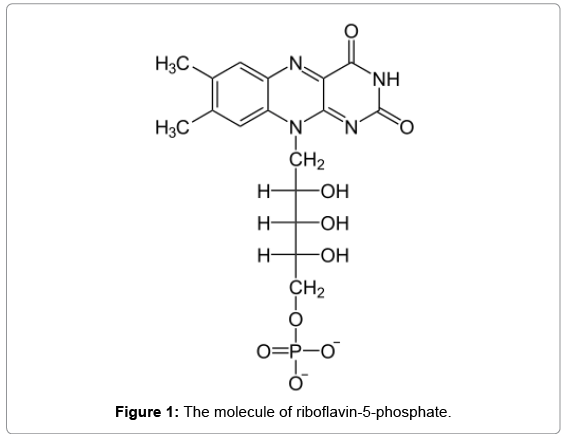
Immediately after ICRS implantation, corneal CXL was performed under sterile conditions by the (Peschke Meditade GmbH UV-X crosslinking system), in three of four patients (samples S11, S12, S13). The corneal epithelium was removed before treatment. Riboflavin (Figure 1) in dextran drops (0.1%) was then administered topically for 30 minutes. After the administration of riboflavin, the corneal surface was rinsed thoroughly with a sterile solution. The cornea was then exposed to 365 nm UV-A light with the CXL system for 30 minutes at an irradiance level of 3 mW/cm2. A soft contact lens bandage was placed to minimize pain.
Approximately 8 months after the operation ICRS were removed due to refractive mis- correction (4 cases). For removal an inverted Sinskey hook was used to engage the segment hole, from beneath, and it was pulled out of the tunnel, approximately 8 months.
The extracted sample S0 was not treated by CXL and was used as reference. After INTAC’s extraction, they were rinsed with sterile balance salt solution (BSS) and become dried in ambient condition in darkroom for about an hour. Afterwards each specimen was placed in black bag, was transferred and spectroscopic measurements (FTIR and UV-Vis) was performed 3 days after removal.
Instrumentation for UV-Vis electronic absorption measurements
UV-Vis absorption spectra were obtained with a modified UV/ VIS/NIR spectrophotometer (Perkin–Elmer, Lambda 900, California, USA). A Perkin–Elmer Transfer optics device (B220-5302) allows the sample beam to be directed and focused on the sample via a monofiber. A second monofiber collects the light, which passes through the sample, allowing remote measurements. The two ‘‘quartz’’ fiber optic cables 1.5 m long each were especially manufactured by HELL-MA GmBH and Co KG Germany and were suitable for use in the wavelength range 220-1100 nm.
For all experiments the spectral range was from 600 nm to 250 nm in steps of 0.2 nm.
FT-IR spectroscopy
All, spectra were acquired using the Attenuated Total Reflection (ATR) MIRacle (PIKE technologies, Madison, Wisconsin) accessorize in an IR EQUINOX55 spectrometer (Bruker, Germany). ATR Infrared Spectroscopy is a rapid technique and much easier to use than typical FT-IR spectroscopy by preparing small pellets with KBr. The great advantage is that ATR FT-IR is a nondestructive method and samples are measured without any pre-treatment. All spectra were taken at a spectral resolution of 2 cm-1 between wavenumbers range 500-3500 cm-1, using an ATR diamond cell at room temperature. Samples and background were scanned with 200 scans. The spectra of ICRS samples, were calibrated based on the peak at 1722 cm-1 assigned to C=O vibration. Measurements were repeated at least three times for each sample and the average spectrum was calculated.
Results
UV-Vis spectorscopy
In figure 2, the UV-Vis absorption spectrum of the riboflavin solution is shown in the spectral range of 250–600 nm; absorption bands with peak maxima at 445, 365 and 265 nm correspond to the characteristics bands of riboflavin in aqueous solution [10]. In the same figure, the UV-Vis spectrophotometric scans of samples S0 and the average spectrum of samples S11, S12 and S13 are shown, as well. In the spectral window from 300 to 600 nm, no absorption contribution occurred, neither to sample S0 [11] nor to treated samples (S11, S12 and S13).
FT-IR spectroscopy
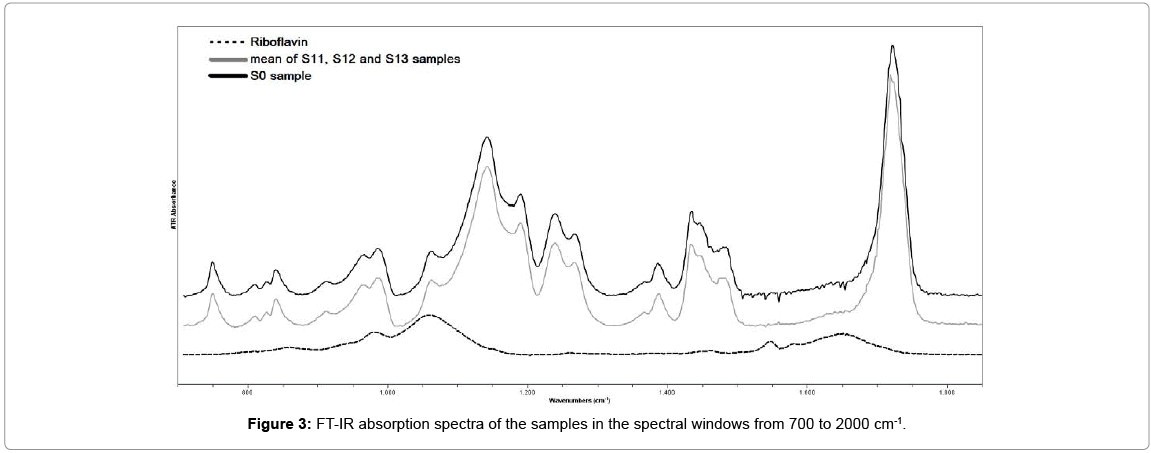
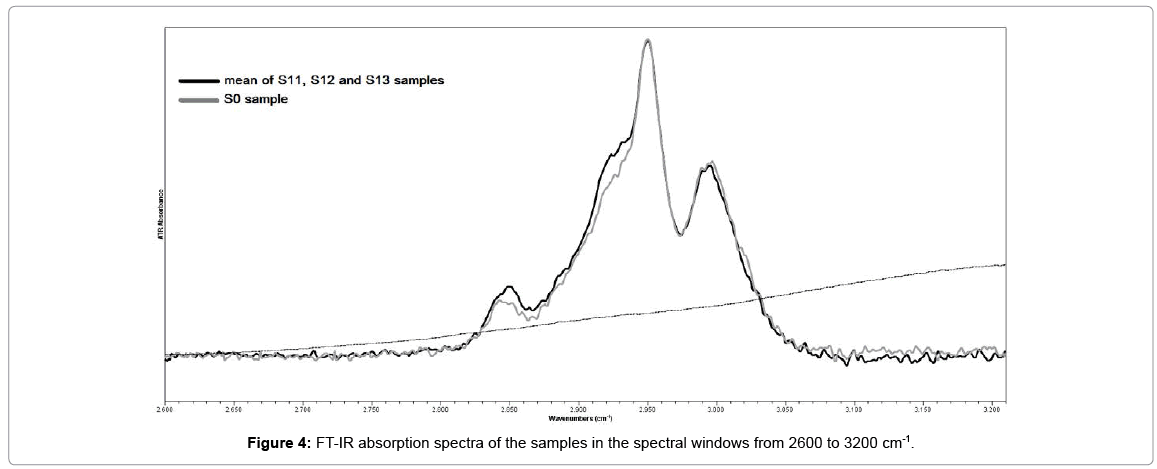
Figures 3 and 4 demonstrate the FT-IR spectrum of the sample S0, in two different spectral windows. The assignments of the principal absorption bands are presented in Table 1. The absorptions around 2850, 2925 and 2950 cm-1 characterized the (C-H) stretching vibrations of (CH2) and (CH3) respectively. The stretching vibration of C=O bonds associated with the ester groups peaked around 1722cm-1. The two doublet bands at 1270 & 1239cm-1 and 1190 & 1142cm-1 were due to the (C-O) stretching vibrations of ester groups [12,13].
| Measured Wavenumbers (cm-1) | Significant Contribution |
|---|---|
| 750 | C=O out of plane |
| 807 | C=O in plane bending |
| 826 | C-O-C symmtertic stretching |
| 838 | CH2 rocking |
| 911 | CH3 rocking |
| 964 | C-C stretching |
| 984 | C-C stretching |
| 1062 | CH3 twisting |
| 1140 | CH3 twisting |
| 1185 | CH3 wagging |
| 1237 | C-O stretching |
| 1263 | C-O stretching |
| 1387 | CH2 deformation |
| 1433 | CH3 deformation |
| 1445 | CH3 deformation |
| 1478 | CH3 deformation |
| 1719 | C=O stretching |
| 2850 | C-H symmetric streching in CH2 |
| 2925 | C-H antisymetricstreching in CH2 |
| 2950 | C-H antiymetricstreching in CH3 |
| 2994 | C-H antisymetricstreching in CH3 |
| INTACS: Intracorneal Ring Segments | |
Table 1: FT-IR band positions of INTACS material and their assignments.
The FT-IR absorption spectrum of riboflavin solution is also presented in Figures 3 and 4 for comparison. The assignments of the strongest absorption bands was based on spectroscopic data of previous studies and presented in Table 2. The 857 cm-1, 980 cm-1 and 1060 cm-1 bands were associated with the PO4 group of riboflavin-5-phosphate [14,15]. The 1259, 1374, 1403, 1454, 1544 and 1581 cm-1 bands were associated with vibrations of the isoaloxazine rings of riboflavin molecule [16,17]. The intense peak at 1650 cm-1 and the sharp peak with a maximum around 3300 cm-1 was due to the water molecule [18].
| Measured Wavenumbers (cm-1) | Significant Contribution |
|---|---|
| 857 | P-O-P bending |
| 980 | vs (PO3-2) |
| 1060 | vas (PO3-2) |
| 1259 | v(C4aΝ3), v(C4C4a) |
| 1374 | v(C5aC6), ν(Ν10,C10a) |
| 1403 | ν(C2Ν3), ν(C2Ν1) |
| 1454 | ν(C7C8) |
| 1541 | ν(Ν1C10a), ν(C4aΝ5) |
| 1581 | ν(Ν1C10a), ν(Ν10C10a) |
| 1548 | v(Ν1C10a), |
| 1580 | v(Ν5C4a), v(Ν1C10a) |
| 1650 | ν(C2=O) |
Table 2: FT-IR band positions of riboflavin solution and their assignments.
Figure 3 show that there is no difference between the spectra of sample S0 and the mean spectrum of sample S1 in this lower spectral window.
The most significant changes in the mean spectrum of samples S1 compared to the spectrum of sample S0 occurred at the 2800 to 3200 cm-1 spectral region (Figure 4). Particularly a change was noted in the ratio of band intensities of CH2 and CH3. The increase in the band intensities assigned to symmetric (2850 cm-1) and antisymmetric (2925cm-1) stretching of methylene group (CH2) at carbonyl towards the band intensity of antisymmetric stretching (2950 cm-1) of methyl group (CH3), suggested that crosslinking reaction occurs in the material [19,20].
Discussion
The key indication for CXL is to delay the progression of corneal ectasia [21]. On the other hand ICRS implantation is a minimally invasive surgical technique that is used to treat ectatic corneas [22,23]. Although CXL arrests or delay the progression of the ectatic process without significantly changing the cornea shape, ICRS implantation significantly flattens and regularizes it without affecting its biomechanical properties.
Previous studies suggested that the combination of the two techniques possibly delivers a combined beneficial impact. Pretreatment with ICRS implantation would significantly reshape the cornea by flattening and regularizing it, and subsequent CXL would stabilize the newly shaped cornea. Alternatively, the CXL could be performed prior to implantation of ICRS.
Chan et al. showed that the addition of CXL to the ICRS procedure resulted in better outcomes than the ICRS insertion alone [24]. Further to a simple additive effect of the procedure, the authors proposed an additional local effect in the ICRS with CXL group due to enhancement of the ICRS. Moreover, Wollensan et al. have shown that the collagen changes induced in the cornea, increased the overall biomechanical rigidity by 4.5 times, and the placement of an ICRS segment possibly altered the pattern and distribution of collagen changes [3]. This in turn might have led to increased rigidity locally or across the ICRS segment, producing further flattening.
Furthermore Coskunseven et al. suggested that ICRS implantation followed by the CXL procedure produced better results in keratoconus than CXL followed by ICRS implantation [25]. They proposed that a stiffer cornea is induced by the CXL, decreases the flattening effect of ICRS implantation. In addition, authors agreed with Chan et al. that the additional stiffening of the cornea, especially around the channel of the ICRS, could cause an additive effect when sequential treatment is selected [24].
Kilic et al. reported that combined ICRS and CXL treatment with intracorneal riboflavin injection was effective in keratoconic eyes and they proposed that limited and localized riboflavin penetration around the ICRS might increase the effect of the segments [26].
Recently our team explored the impact of CXL treatment on the chemical structure of ICRS in an effort to explain the additional local effect that is detected in cases when ICRS precedes CXL treatment [27]. The experiments followed the standard CXL protocol of corneal crosslinking treatment in a laboratory setting. We found that the CXL procedure leads to the crosslinking effect of the ICRS core. The translational potential of those results was the strengthening of the polymer and the further stiffening of the area near the implant. Nevertheless the basic limitation of the aforementioned study was that PMMA photopolymerization was confirmed in a laboratory setting.
In present study we detected that crosslinking effect occurred in the ICRS core material, after in-vivo CXL treatment (30 min) with UV-A irradiation (365 nm, 18 mW) and riboflavin solution. Indeed, after the treatment and the extraction of ICRS the ratio of symmetric (2980 cm-1) and antisymmetric (2925 cm-1) valence vibrations of methylene groups (CH2), changed toward an increase in the intensity of the high frequency peak (2950 cm–1) which is assigned to anti-symmetric vibrations of methyl groups (CH3) at carbonyl. This indicates an elongation of the chain of intermolecular crosslinks and an inhomogeneity of reaction products. Photopolymerization occurs both in the corneal stroma and ICRS material.
ICRS core is made of Poly (methyl methacrylate), more often called PMMA that is a commonly used low cost thermoplastic polymer with numerous applications in clinical practice. Crosslinking is a process where the long chains of polymers are linked together increasing the molecular mass of the polymer. In order to obtain cross-linked polymers, it is necessary to generate free radicals in the system that will induce a free radical chain polymerization of monomers and oligomers. Photo initiators have an essential role in this process, since they are excited under UV irradiation leading to the formation of the active radicals that start the polymerization mechanism. In all cases, the chemical structure of the polymer is altered through the crosslinking process.
Properties of polymer products that can be improved by crosslinking include: Mechanical properties, such as tensile strength; Scratch resistance; Performance at higher temperatures, often with an increase in the melting temperature; Resistance to chemicals because of lowered solubility in organic solvent; Gas permeation reduction and shape memory retention.
Aforementioned statement possibly explains why there is additional stiffening and corneal flattening especially across the ICRS after CXL procedure. UV-A irradiation and riboflavin triggered the crosslinking of the PMMA material of ICRS. This in turn stiffened the ICRS core.
Since changes in FTIR spectroscopy may or may not indicate any change the mechanical properties; Biomechanical properties of a CXL treated ICRS itself; need to be investigated in order to explain any differentiated ICRS-mediated effect after CXL procedure.
In conclusion, the outcome of this study confirms the observations of the polymerization of the ICRS material after in-vivo CXL treatment.
This effect should be taken into consideration:
a) Wen selecting combine treatments in keratectasia
b) When assessing the therapeutic outcome
c) When observing the overall biomechanical status of the cornea
Moreover we consider that our results bare a significant translational potential, since advanced ICRS products with modified properties may be developed after CXL preparation.
Acknowledgements
The manuscript has been presented at the 10th International Congress of Corneal Cross-Linking, December 5+6, 2014, Zurich, Switzerland.
References
- Colin J, Cochener B, Savary G, Malet F (2000) Correcting keratoconus with intra-corneal rings. J Cataract Refract Surg 26: 1117-1122.
- Spoerl E, Huhle M, Seiler T (1998) Induction of cross-links in corneal tissue. Exp Eye Res 66: 97-103.
- Wollensak G, Spoerl E, Seiler T (2003) Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg 29: 1780-1785.
- Caykara T, Guven O (1999) UV degradation of poly(methyl methacrylate) and its vinyltriethoxysilane containing copolymers. PolymDegradStabil 65:225-229.
- Nagai N, Matsunobe T, Imai T (2005) Infrared analysis of depth profiles in UV-photochemical degradation of polymers. PolymDegradStabil 28:224-233.
- Wochnowski C, Metev S, Sepold G (2000) UV–laser-assisted modification of the optical properties of polymethylmethacrylate. Appl Surf Sci 154:706-711.
- Wochnowski C, Shams Eldin MA, Metev S (2005) UV-laser-assisted degradation of polymethyl (methacrylate). PolymDegradStabil 89:252-264.
- Estler RC, Nogar NS (1986) Mass spectroscopic identification of wavelength dependent UV laser photoablation fragments from polymethylmethacrylate. ApplPhysLett 49:1175-1177.
- Hiraoka H (1977) Radiation chemistry of poly(methacrylates). IBM J Res Dev 21:121-130.
- OSTER G, BELLIN JS, HOLMSTROM B (1962) Photochemistry of riboflavin. Experientia 18: 249-253.
- Zidan H, Abu-Elnader M (2005) Structural and optical properties of pure PMMA and metal chloride-doped PMMA films. Phys Rev B: Condens Matter 355:308-317.
- Dirlikov St, Koening L (1980) Carbon-Hydrogen stretching and bending vibrations in the Raman spectra of Poly(methylmethacrylate). J Raman Spectrosc 9:150-154.
- Lipschitz I (1982) The vibrational spectrum of poly(methyl methacrylate): a Review. PolymPlastTechnolEng 19:53-106.
- Barth A, Montele W (1998) ATP-Induced Phosphorylation of the Sarcoplasmic Reticulum Ca2+ ATPase: Molecular Interpretation of Infrared Difference Spectra. Biophys J 75: 538-544.
- Vonach R, Lend B, Kellner R (1997) Modulation of the pH in the Determination of Phosphate with Flow Injection and Fourier Transform Infrared Detection. Analyst 122:525-530.
- Rieff B, Mathias G, Bauer S, Tavan P (2011) Density functional theory combined with molecular mechanics: the infrared spectra of flavin in solution. PhotochemPhotobiol 87: 511-523.
- Spexard M, Immeln D, Thöing C, Kottke T (2011) Infrared spectrum and absorption coefficient of the cofactor flavinin water. VibSpectrosc 57:282-287.
- Maréchal Y (2011) The molecular structure of liquid water delivered by absorption spectroscopy in the whole IR region completed with thermodynamics data. J MolStruct 1004:146-155.
- Killdeeva NR, Perminov PA, Vladimirov LV, Novikov VV, Mikhailov SN (2009) About Mechanism of Chitosan Crosslinking with Glutaraldehyde. Russ J BioorgChem 35: 360-369.
- Namouchi F, Smaoui H, Guermazi H, Fourati N, Zerrouki C, et al. (2009) Study of charge relaxations after thermal aging in poly (methyl methacrylate). PhysProcedia 2:961-970.
- Wollensak G, Spoerl E, Seiler T (2003) Riboflavin/ultraviolet-A induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 135:620-627.
- Coskunseven E, Kymionis GD, Tsiklis NS, Atun S, Arslan E, et al. (2008) One-year results of intrastromal corneal ring segment implantation (KeraRing) using femtosecond laser in patients with keratoconus. Am J Ophthalmol 145: 775-779.
- Kymionis GD, Siganos CS, Tsiklis NS, Anastasakis A, Yoo SH, et al. (2007) Long-term follow-up of Intacs in keratoconus. Am J Ophthalmol 143: 236-244.
- Chan C, Sharma F, Wachler B (2007) Effect of inferior-segment Intacs with and without C3-R on keratoconus. J Cataract Refract Surg 33: 75-80.
- Coskunseven E, Jankov MR 2nd, Hafezi F, Atun S, Arslan E, et al. (2009) Effect of treatment sequence in combined intrastromal corneal rings and corneal collagen crosslinking for keratoconus. J Cataract Refract Surg 35: 2084-2091.
- Kilic A, Kamburoglu G, Akinci A (2012) Riboflavin injection into the corneal channel for combined collagen crosslinking and intrastromal corneal ring segment implantation. J Cataract Refract Surg 38: 878-883.
- Sideroudi H, Labiris G, Soto-Beobide A, Voyiatzis G, Chrissanthopoulos A, et al. (2015) The effect of Collagen Crosslinking procedure on the material of Intracorneal Ring Segments. Curr Eye Res 40:592-615.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 11614
- [From(publication date):
December-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10734
- PDF downloads : 880