Research Article Open Access
The Effect of CaP Concentration on Corrosion Behavior of Plasma Sprayed Hydroxyapatite Coating on Titanium in Simulated Body Fluid
Gurpreet Singh1*, Hazoor Singh2and Buta Singh Sidhu3
1University College of Engineering, Punjabi University, Patiala, Punjab, India
2Yadavindra College of Engineering, Punjabi University G.K. Campus, Talwandi Sabo, Punjab, India
3Punjab Technical University, Jalandhar, Punjab, India
- Corresponding Author:
- Gurpreet Singh
University College of Engineering
Punjabi University, Patiala, Punjab, India
E-mail: gurpreetsnabha@yahoo.com
Received date May 03, 2013; Accepted date July 04, 2013; Published date July 14, 2013
Citation: Singh G, Singh H, Sidhu BS (2013) The Effect of CaP Concentration on Corrosion Behavior of Plasma Sprayed Hydroxyapatite Coating on Titanium in Simulated Body Fluid . J Biomim Biomater Tissue Eng 18:103. doi: 10.4172/1662-100X.1000103
Copyright: © 2013 Singh G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biomimetics Biomaterials and Tissue Engineering
Abstract
The aim of this work was to evaluate the corrosion behavior of the uncoated and coated titanium (Ti) and study the effect of calcium phosphate (CaP) on corrosion behavior of hydroxyapatite (HA) coatings in simulated body fluid (Ringer’s solution). Three types of coatings: HA, HA + 10 wt% CaP, and HA + 20 wt% CaP on titanium were made using plasma-spraying technique. Structural characterization techniques including X-ray diffraction and scanning electron microscopy/energy dispersive X-ray spectroscopy were used to investigate the crystallinity, microstructure and morphology and of the coatings. Electrochemical potentiodynamic test were performed to determine the corrosion resistance of uncoated and all three coatings in Ringer’s solution. The corrosion resistance of plasma sprayed HA coating on titanium was found maximum.
Keywords
Hydroxyapatite (HA); Calcium phosphate (CaP); Corrosion; Titanium (Ti); Ringer’ solution
Introduction
Implant material used for high load bearing bones such as femoral and tibia bones should possess good bone bonding ability and high fracture toughness [1,2]. But the available bio-ceramics and biocompatible metals neither fulfill the both requirements. The ceramics has low fracture toughness than human cortical bone and no metal has the ability to directly bond with living bone [3]. So coating of bioactive materials such as hydroxyapatite [(Ca10(PO4)6(OH)2), HA] and calcium phosphate [Ca3(PO4)2, CaP] on the metals like titanium and biological steel was proposed as the well-known technique to enhance the bone-bonding ability of metals [4,5]. Furlong and Osborn [6] who were the first to perform the clinical trials of HA-coated implants had reported that HA coatings can successfully enhance clinical success. Delecrin et al. [7] had reported that Calcium phosphate (CaP) coatings promote early bone apposition at the surface of cementless orthopedic prostheses and had given well successful clinical results [8-14].
Titanium (Ti) is commonly used for developing orthopedic and dental implant under load bearing applications. But the high stiffness of titanium in comparison of surrounding bone can leads to the problem of stress shielding and subsequent loosening at the bone– implant interface [15]. In the last many years, continuous research on HA and CaP have not only focused on tissue-coating interface, but also on the problems associated with the coating process and optimization of coating properties for maximum tissue response [16].
There are numerous experimental deposition processes such as thermal spraying [17-22], sputter coating [23-25], pulsed laser ablation [26-29], dynamic mixing [30], dip coating [31-33], sol–gel [34-37], electrophoretic deposition [38-44], biomimetic coating [45,46], and hot isostatic pressing [47]. Plasma spray process is the most commercially, well preferred technique to deposit HA on metallic implants because of high deposition rate and a sufficiently low cost [48-51].
In this work, Atmospheric Plasma Spray technique was employed to spray HA - CaP coatings on Titanium substrate. The as-sprayed coatings were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) techniques. Subsequently corrosion behavior of plasma sprayed HA and HA - CaP coatings on titanium has been investigated by the Tafel extrapolation method in simulated body fluid (Ringer’s solution). The changes in the crystallinity and morphology if any of the exposed samples were analyzed by XRD, SEM and EDX. This study is focused on the effects of adding CaP in HA on the corrosion resistance of HA - CaP coatings.
Experimental Procedure
Materials
Medical grade HA and CaP powders (IFGL Bio Ceramics Limited, Kolkata, India) with particle size distribution of 57-200 μm and 90-300 μm respectively were used as spraying materials. Titanium substrate of size 15 × 15 × 3 mm was plasma sprayed with HA and HA – CaP coatings. The substrate surface was grit blasted with alumina of particle size 50-60 μm at a pressure of 5 bars for 2 minutes to roughen the surface and subsequently air blasted to remove any residual grit before spraying. Because a highly roughened substrate surface exhibited higher bond strength as compared to a smooth substrate surface [52].
Developments of coatings
The mixture of HA and CaP powders were prepared by mechanically stirring the mixture of HA + 10 wt% CaP and HA + 20 wt% CaP powders in a ceramic pot for 30 min. The titanium substrates were coated with following powders compositions: HA, HA + 10 wt% CaP and HA + 20 wt% CaP using Plasma spray (Miller Spray System) at Anod Plasma, Kanpur, India. Hydrogen and argon were used as the fuel and powder carrier gas respectively. The spraying parameters for all the coating are identical and are listed in Table 1.
| Spraying Parameter | Value |
|---|---|
| Arc Current | 500 [Amp] |
| Arc Voltage | 50 [V] |
| Argon Flow Rate | 5 [slpm] |
| Hydrogen Flow Rate | 12 [slpm] |
| Spraying Distance | 75 [mm] |
| Powder Flow Rate | 20-25 [g/min] |
Table 1: Spraying Parameters for HA, HA + 10 wt% CaP and HA + 20wt% CaP Coatings.
Characterization of coatings
The phase structure of the feedstock powder and coatings were analyzed by XPERT-PRO X-ray diffractometer system. In the phase analysis, the radiation source was Cu Kα; the operating generator setting was 45 kV / 40 mA. The feedstock powder and coated samples were scanned over the 2θ range of 10° to 60° and 20° to 60° respectively.
Microstructural investigation was carried out on the surfaces and polished cross-sections of the coatings by SEM (EVO MA 15 ZEISS) coupled with EDX. As-sprayed coatings were cut with a low speed precision saw and mounted in hot resin using a hot mounting press, followed by polishing with emery papers of 220, 320, 400, 600, 800, 1000, and 2000 grades, and finally mirror finished by buffing using an alumina slurry solution on napped cloth. To achieve the desired conductivity for observation in SEM the gold sputter coating were applied to samples using JEOL JFC-1600 auto fine coater. Elemental analysis of the coatings was carried out using an EDX. EDX analysis was used to calculate Ca/P ratio and to display the distribution of elements in the coatings.
Surface roughness
Roughness parameters such as Ra (the arithmetic mean of the departures of the roughness profile from the mean line), Rq (root mean square (RMS) of average roughness), and Rz (average of the highest peaks and the lowest valleys) are measured at five different positions on the surface of the uncoated, HA, HA +10 wt% CaP and HA + 20 wt% CaP coated titanium specimen by using a roughness tester (SJ-201 MITUTOYO), with a filter of Gaussian type for a cut-off wavelength of 0.8 mm. The average value of each parameter at various positions is reported in this work.
Electrochemical corrosion studies
The electrochemical corrosion behavior of the uncoated, HA, HA + 10 wt% CaP and HA + 20 wt% CaP coated titanium specimens were investigated by conducting the potentiodynamic polarization tests. Potentiostat/Galvanostat (Series G-750; Gamry Instruments, Inc. USA), interfaced with a personal computer having specific Gamry electrochemical software ‘DC105’ was used to conduct the test. Ringer’s solution (Nice Chemical Pvt. Ltd. Cochin, India) with chemical composition (in g/L) as 9 NaCl, 0.24 CaCl2, 0.43 KCl, and 0.2 NaHCO3 at pH 7.2 was used as the electrolyte for simulating human body fluid conditions. The titanium specimen and saturated calomel electrode (SCE) were used as working electrode and reference electrode respectively. A graphite rod was used as the counter electrode. The instrument measures and controls the potential difference between a non-current carrying reference electrode and one of the two current carrying electrodes (the working electrode). The parameters for conducting the potentiodynamic scan for calculating the corrosion rate by plotting the Tafel plot are mentioned in Table 2. The initial delay of 24 hour is given for the stabilization of immersed specimen in Ringer’s solution and fresh solution was used for each experiment.
| Parameter | Value |
|---|---|
| Initial Potential | 0.25 [V Vs Open Circuit Potential] |
| Final Potential | - 0.25 [V Vs Open Circuit Potential] |
| Scan Rate | 1 [mV/s] |
| Sample area in Ringer Solution | 1 [cm2] |
Table 2: Parameters for conducting the potentiodynamic scan.
Results and Discussions
XRD analysis
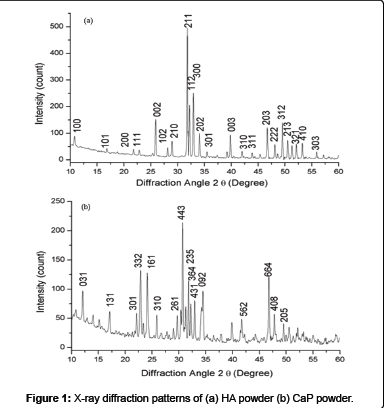
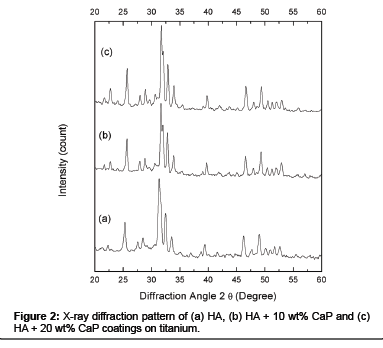
The XRD pattern of HA powder (Figure 1 (a)) and CaP powder (Figure 1 (b)) were composed of crystalline phases and all major peaks belongs to HA and CaP match the JCPDS cards 74-566 and 70-364 respectively. Figure 2 shows XRD patterns of plasma sprayed HA (a), HA + 10 wt% CaP (b), and HA + 20 wt% CaP (c) coating on titanium were crystalline. The rise in peaks of HA + 10 wt% CaP and HA + 20 wt% CaP has been noticed in the range of 200 2θ to 250 2θ because the CaP powder has sharp peaks between this range as compare to HA powder. The rise in peaks is significant in the range of 300 2θ to 350 2θ in HA + 20 wt% CaP coating.
SEM / EDX analysis
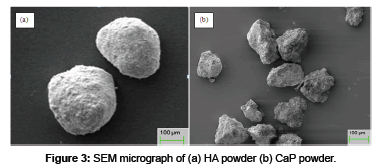
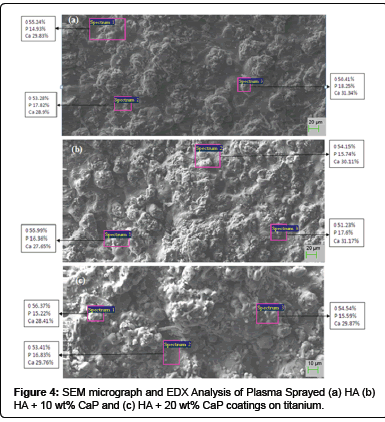
Surface analysis: The morphology of HA and CaP powders (Figure 3) confirm that the HA powder has the spherical shape and CaP powder has the irregular shape with sharp edges. SEM micrograph of plasma spray HA coating (Figure 4 (a)) shows the microstructure consist of fully molten HA particles and appear denser. The EDX analysis shows the presence of Ca, P and O, which are main components of HA powder at three different positions. The average value of Ca/P ratio of the coating is 1.7 which lies in between the guidelines described by the Food and Drug Administarion and in the ISO standards [16,53,54]. The SEM micrograph of HA + 10 wt% CaP coating surfaces (Figure 4 (b)) shows that microstructure consist of pores / voids and unsmooth. As the quantity of irregular shape particles of CaP increases in HA + 20 wt% CaP coating increases the smoothness decreases and pores / voids appear as shown in Figure 4 (c). It is generally believed that if the density of coating surface is higher than the corrosion resistance of the surface increase.
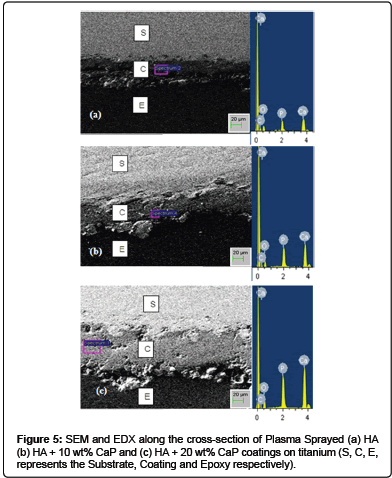
Cross-Sectional analysis: The morphology of the plasma sprayed HA, HA + 10 wt% CaP and HA + 20 wt% CaP coatings on titanium at the cross-section is investigated by SEM and EDX and is shown in Figure 5. The thickness of coatings was measured at four positions and100 ± 20 μm was the average thickness. All the coating shows good bond with the substrate. The EDX analysis showed peaks of calcium and phosphors increases as the content of CaP in HA increased. The increase in proportion of CaP promotes the osteoconduction while HA particles carry the biological apatite precipitation [55]. It has been reported in literature that high soluble coating are more osteoconductive and bioactive in comparison to the stable layers in vivo [56]. The osteointegration of CaP coatings is faster than HA coatings in non – load bearing conditions [7].
Surface roughness
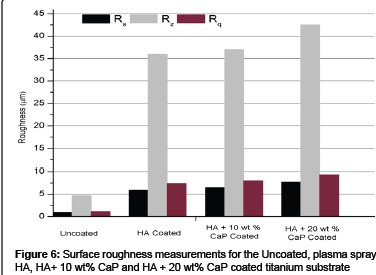
Surface roughness, surface topography, surface energy and chemical composition have been reported as very important factors for implant tissue interaction and to affect the biocompatibility in clinical use [57,58]. High surface roughness will increase the coating and body-fluid interface, and thus increase the dissolution rate and apatite precipitation [16].
The surface roughness parameters (Ra, Rq and Rz) for uncoated, HA, HA + 10 wt% CaP and HA + 20 wt% CaP plasma coated titanium substrates are shown in Figure 6. The average surface roughness (Ra) value for uncoated, HA, HA + 10 wt% CaP and HA + 20 wt% CaP plasma coated titanium substrates are 0.934 ± 0.2 μm, 5.874 ± 0.4 μm, 6.462 ± 0.3 μm and 7.624 ± 0.4 μm respectively. Gross and Babovic [59] reported that plasma sprayed HA coating with a powder particle size of 20-30 μm give a surface roughness of 4-6 μm while incorporating the partially melted particles but the particles size is coarse in this work. The un-melted particles produce rough areas within and on the surface of the coating. Crystalline particles spread over the coating surface when the un-molten particle core is not sufficiently strong to fragment upon impact with the substrate [59]. The smooth areas in the coating are the result of melting of the powder.
Corrosion behavior
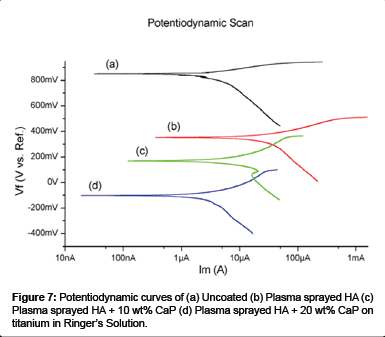
The potentiodynamic scan of the uncoated, plasma sprayed HA, HA + 10 wt% CaP and HA + 20 wt% CaP coating on titanium samples in Ringer’s solution is shown in Figure 7. The corrosion parameters such as anodic tafel slope (ßa), cathodic tafel slope (ßc), corrosion potential (ECorr), and corrosion current density (ICorr) are determined from the potentiodynamic curves by conducting the Tafel extrapolation test.
The results of these corrosion parameters are shown in Table 3. The chance of corrosion in a material depends upon the corrosion current density (ICorr) at a given potential, material will be more corrosion resistant at lower value of ICorr [60-62].
The result of Tafel slope values shows that corrosion current density of uncoated sample in Ringer’s solution is (ICorr = 2.28 μA, ECorr = -152.0 mV) higher than the plasma sprayed HA (ICorr = 1.6 μA, ECorr = -113 mV), HA +10 wt% CaP (ICorr = 1.810 μA, ECorr = -63.0 mV) and HA +20 wt% CaP (ICorr = 2.01 μA, ECorr = -37.90 mV) coated titanium sample. The earlier corrosion studies on HA coatings shows the same kind of results [63-66]. So the analysis of Tafel slope values indicates that the plasma coated HA titanium specimen with lowest ICorr values is the most corrosion resistant specimen among the uncoated, HA + 10 wt% CaP and HA + 20 wt% CaP coated titanium specimens in Ringer’s solution. The results also suggest that the increase percentage of CaP in HA also decreases the corrosion resistance of plasma coated titanium.
| Parameter | Uncoated | HA Coated | HA + 10 wt% CaP Coated | HA + 20 wt% CaP Coated |
|---|---|---|---|---|
| βa [e-3 V/decade] | 64.90 | 104.1 | 115.0 | 136.0 |
| βc [e-3 V/decade] | 286.3 | 743.3 | 114.8 | 385.6 |
| ECorr [mV] | -152.0 | -113.0 | -63.0 | -37.90 |
| ICorr [μA] | 2.28 | 1.6 | 1.810 | 2.01 |
Table 3: Corrosion parameters determined by the Tafel extrapolation test.
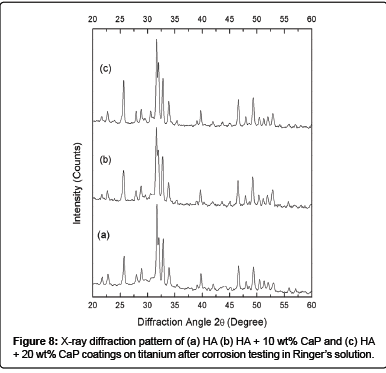
The XRD peaks of HA, HA + 10 wt% CaP and HA + 20 wt% CaP coated titanium (Figure 8) appears more crystalline after electrochemical corrosion testing and the intensity of XRD peaks found to be increased after immersion of samples in Ringer’s solution for 24 hours during corrosion testing. The sharp peaks after immersion indicates the dissolution of the amorphous phases [63]. It is reported that amorphous phases are more soluble then crystalline HA as they encourage the early bone growth [67]. The dissolution was favorable for the early stages of transformation of biological equivalents that act as mediator between osteoclast and osteoblast differentiation [68]. The higher crystalline coating leads to longer implant life, while some implant manufacture prefer a faster dissolving coating to enhance the bone growth [69]. It has been reported in in vivo studies that the phase purity, crystallinity and microstructure of HA coatings affects the biological response of HA coating [70].
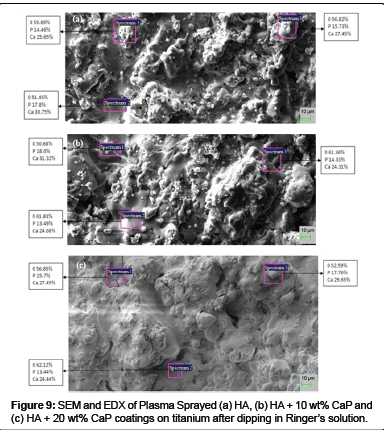
After electrochemical corrosion testing, the compositional changes, if any, on the surfaces of the exposed specimens were further examined by SEM and EDX. The surface morphology of the HA coated samples (Figure 9 (a)) changes to flattened particles and looks smooth and denser after exposure to the corrosion testing in Ringer’s solution.
The morphology of HA - CaP coatings (Figure 9 (b-c)) appears to be more porous and less smooth after 24 hour immersion in Ringer’s solution for corrosion testing. The size of pores and void appear to be increased. The micrograph of HA + 20 wt% CaP coating shows the dissolution of the coating. No cracks were found on both the Ha and HA - CaP coated exposed specimens.
EDX analysis confirms the presence of Ca, P and O elements in all HA and HA - CaP coatings. EDX analyses of exposed specimens shows that Ca and P (at %) decreases by taking the average of elemental composition at three spectrums after 24 hour immersion in ringer solution. The decrease in the values indicates that phosphate accumulate on the surface which suggests that incongruent dissolution of the HA has taken place [63]. No constituent of substrate i.e. titanium found on the surface of any coatings of the exposed samples.
Conclusion
In the present study, plasma spray technique was used to deposit the HA, HA + 10 wt% CaP and HA + 20 wt% CaP on titanium. The following conclusions have been drawn from the study:
1. The plasma sprayed HA + 20 wt% CaP coating is more crystalline than HA and HA + 10 wt% CaP Coating on titanium.
2. The plasma sprayed HA + 20 wt% CaP coating exhibited higher surface roughness (Ra = 7.624 ± 0.4 μm) than HA and HA + 10 wt% CaP Coating on titanium.
3. The electrochemical study showed the corrosion resistance of the titanium is more after the deposition of plasma sprayed HA than uncoated, HA, HA + 10 wt% CaP and HA + 20 wt% CaP coatings on titanium.
Future in vivo studies of plasma sprayed HA, HA + 10 wt% CaP and HA + 20 wt% CaP coated titanium and a complete interpretation of these results can help in assessing their use in clinical applications.
References
- Kato H, Nakamura T, Nishiguchi S, Matsusue Y, Kobayashi M, et al. (2000) Bonding of alkali- and heat-treated tantalum implants to bone. J Biomed Mater Res 53: 28-35.
- Kokubo T, Miyaji F, Kim HM, Nakamura T (1996) Spontaneous formation of bonelike apatite layer on chemically treated titanium metals, J Am Ceram Soc 79: 1127–1129.
- Kim HM, Miyaji F, Kokubo T, Nakamura T (1996) Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J Biomed Mater Res 32: 409-417.
- Kim HM, Miyaji F, Kokubo T, Nakamura T (1997) Bonding strength of bonelike apatite layer to Ti metal substrate. J Biomed Mater Res 38: 121-127.
- Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF (2001) Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 22: 87–96.
- Furlong RJ, Osborn JF (1991) Fixation of hip prostheses by hydroxyapatite ceramic coatings. J Bone Joint Surg Br 73: 741-745.
- Delecrin J, Daculsi G, Passuti N, Duquet B (1994) Specific resorbable calcium phosphate coating to enhance osteoconduction. Cells Mater 4: 51–62.
- D'Antonio JA, Capello WN, Crothers OD, Jaffe WL, Manley MT (1992) Early clinical experience with hydroxyapatite-coated femoral implants. J Bone Joint Surg Am 74: 995-1008.
- Donnelly WJ, Kobayashi A, Freeman MA, Chin TW, Yeo H, et al. (1997) Radiological and survival comparison of four methods of fixation of a proximal femoral stem. J Bone Joint Surg Br 79: 351-360.
- Dorr LD, Wan Z, Song M, Ranawat A (1998) Bilateral total hip arthroplasty comparing hydroxyapatite coating to porous-coated fixation. J Arthroplasty 13: 729-736.
- Geesink RG, Hoefnagels NH (1995) Six-year results of hydroxyapatite-coated total hip replacement. J Bone Joint Surg Br 77:534–547.
- Hardy DC, Frayssinet P, Bonel G, Authom T, Le Naelou SA, et al. (1994) Two-year outcome of hydroxyapatite-coated prostheses. Two femoral prostheses retrieved at autopsy. Acta Orthop Scand 65: 253-257.
- Hardy DC, Frayssinet P, Krallis P, Descamps PY, Fabeck L, et al. (1999) Histopathology of a well-functioning hydroxyapatite-coated femoral prosthesis after 52 months. Acta Orthop Belg 65: 72-82.
- McNally SA, Shepperd JA, Mann CV, Walczak JP (2000) The results at nine to twelve years of the use of a hydroxyapatite-coated femoral stem. J Bone Joint Surg Br 82: 378–82.
- Park JB, Lakes RS (1992) Biomaterials: an introduction, New York: Plenum Press.
- Sun L, Berndt CC, Gross KA, Kucuk A (2001) Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res 58: 570-92.
- Wang H, Eliaz N, Xiang Z, Hsu HP, Spector M, et al. (2006) Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 27: 4192-4203.
- Hijón N, Victoria Cabañas M, Peña J, Vallet-Regí M (2006) Dip coated silicon-substituted hydroxyapatite films. Acta Biomater 2: 567-574.
- Gross KA, Berndt CC (1998) Thermal processing of hydroxyapatite for coating production. J Biomed Mater Res 39: 580-587.
- Gross KA, Berndt CC, Herman H (1998) Amorphous phase formation in plasma-sprayed hydroxyapatite coatings. J Biomed Mater Res 39: 407-414.
- Li H, Khor KA, Cheang P (2002) Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray. Biomaterials 23: 85-91.
- Chen C, Wang D, Bao Q, Zhang L, Lei T (2005) Influence of laser remelting on the microstructure and phases constitution of plasma sprayed hydroxyapatite coatings. Appl Surf Sci 250: 98-103.
- Wolke JG, van der Waerden JP, Schaeken HG, Jansen JA (2003) In vivo dissolution behavior of various RF magnetron-sputtered Ca-P coatings on roughened titanium implants. Biomaterials 24: 2623-2629.
- Yang Y, Kim KH, Ong JL (2005) A review on calcium phosphate coatings produced using a sputtering process--an alternative to plasma spraying. Biomaterials 26: 327-337.
- Thian ES, Huang J, Best SM, Barber ZH, Bonfield W (2005) Magnetron co-sputtered silicon-containing hydroxyapatite thin films--an in vitro study. Biomaterials 26: 2947-2956.
- Zhang MY, Cheng GJ (2011) Pulsed laser coating of hydroxyapatite/titanium nanoparticles on Ti-6Al-4V substrates: multiphysics simulation and experiments. IEEE Trans Nanobioscience 10: 177-186.
- Clèries L, Martínez E, Fernández-Pradas JM, Sardin G, Esteve J, et al. (2000) Mechanical properties of calcium phosphate coatings deposited by laser ablation. Biomaterials 21: 967-971.
- Fernandez-Pradas JM, Clèries L, Martinez E, Sardin G, Esteve J, et al. (2001) Influence of thickness on the properties of hydroxyapatite coatings deposited by KrF laser ablation. Biomaterials 22: 2171-2175.
- Zeng H, Lacefield WR (2000) The study of surface transformation of pulsed laser deposited hydroxyapatite coatings. J Biomed Mater Res 50: 239-247.
- Yoshinari M, Ohtsuka Y, Dérand T (1994) Thin hydroxyapatite coating produced by the ion beam dynamic mixing method. Biomaterials 15: 529-535.
- Choi J, Bogdanski D, Köller M, Esenwein SA, Müller D, et al. (2003) Calcium phosphate coating of nickel-titanium shape-memory alloys. Coating procedure and adherence of leukocytes and platelets. Biomaterials 24: 3689-3696.
- Hijón N, Victoria Cabañas M, Peña J, Vallet-Regí M (2006) Dip coated silicon-substituted hydroxyapatite films. Acta Biomater 2: 567-574.
- Shi D, Jiang G, Bauer J (2002) The effect of structural characteristics on the in vitro bioactivity of hydroxyapatite. J Biomed Mater Res 63: 71-78.
- Jafari S, Taheri MM, Idris J (2011) Advanced Materials Research 341: 48.
- Manso M, Langlet M, Jiménez C, Martínez-Duart JM (2002) Microstructural study of aerosol-gel derived hydroxyapatite coatings. Biomol Eng 19: 63-66.
- Liu DM, Yang Q, Troczynski T (2002) Sol-gel hydroxyapatite coatings on stainless steel substrates. Biomaterials 23: 691-698.
- Zhang S, Wang YS, Zeng XT, Cheng K, Qian DE (2007) Evaluation of interfacial strength and residual stress of sol-gel derived fluoridated hydroxyapatite coatings onTi6Al4V substrates. Eng Fract Mech 74: 1884-1993.
- Agata De Sena L, Calixto De Andrade M, Malta Rossi A, de Almeida Soares G (2002) Hydroxyapatite deposition by electrophoresis on titanium sheets with different surface finishing. J Biomed Mater Res 60: 1-7.
- Vasilescu C, Drob P, Vasilescu E, Demetrescu I, Ionita D (2011) Characterisation and corrosion resistance of the electrodeposited hydroxyapatite and bovine serum albumin/hydroxyapatite films on Ti–6Al–4V–1Zr alloy surface. Corros Sci 53: 992-999.
- Popa VM, Moreno JMC, Popa M, Vasilescu E, Drob P, et al. (2011) Surface and Coatings Technology 205: 4776.
- Aniket, El-Ghannam A (2011) Electrophoretic deposition of bioactive silica-calcium phosphate nanocomposite on Ti-6Al-4V orthopedic implant. J Biomed Mater Res B Appl Biomater 99: 369-379.
- Ma J, Wang C, Peng KW (2003) Electrophoretic deposition of porous hydroxyapatite scaffold. Biomaterials 24: 3505-3510.
- Nie X, Leyland A, Matthews A, Jiang JC, Meletis EI (2001) Effects of solution pH and electrical parameters on hydroxyapatite coatings deposited by a plasma-assisted electrophoresis technique. J Biomed Mater Res 57: 612-618.
- Manso M, Jiménez C, Morant C, Herrero P, Martínez-Duart JM (2000) Electrodeposition of hydroxyapatite coatings in basic conditions. Biomaterials 21: 1755-1761.
- Bigi A, Fini M, Bracci B, Boanini E, Torricelli P, et al. (2008) The response of bone to nanocrystalline hydroxyapatite-coated Ti13Nb11Zr alloy in an animal model. Biomaterials 29: 1730-1736.
- Habibovic P, Barrere F, Van Bliterswijk CA, Groot KD, Layrolle P, et al. (2002) J Am Ceram Soc 517.
- Wie H, Herø H, Solheim T (1998) Hot isostatic pressing-processed hydroxyapatite-coated titanium implants: light microscopic and scanning electron microscopy investigations. Int J Oral Maxillofac Implants 13: 837-844.
- Yang YC, Chang E (2001) Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti-6Al-4V substrate. Biomaterials 22: 1827-1836.
- Gu YW, Loh NH, Khor KA, Tor SB, Cheang P (2002) Spark plasma sintering of hydroxyapatite powders Biomaterials 23: 37-43.
- Ong JL, Chan DC (2000) Hydroxyapatite and their use as coatings in dental implants: a review. Crit Rev Biomed Eng 28: 667-707.
- Herman H (1988) Plasma spray deposition processes. MRS Bull 13: 60-67.
- Nimb L, Gotfredsen K, Steen Jensen J (1993) Mechanical failure of hydroxyapatite-coated titanium and cobalt-chromium-molybdenum alloy implants. An animal study. Acta Orthop Belg 59: 333-338.
- FDA (1992) Calcium phosphate (Ca-P) coating draft guidance for preparation of FDA submissions for orthopedic and dental endosseous implants. Washington, DC: Food and Drug Administration 1-14.
- ISO (1996) Implants for surgery: coating for hydroxyapatite ceramics 1-8.
- Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B (1989) Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res 23: 883-894.
- Dhert WJ, Klein CP, Jansen JA, van der Velde EA, Vriesde RC, et al. (1993) A histological and histomorphometrical investigation of fluorapatite, magnesiumwhitlockite, and hydroxylapatite plasma-sprayed coatings in goats. J Biomed Mater Res 27: 127-138.
- Schwartz Z, Boyan BD (1994) Underlying mechanisms at the bone-biomaterial interface. J Cell Biochem 56: 340-347.
- Lee TM, Tsai RS, Chang E, Yang CY, Yang MR (2002) The cell attachment and morphology of neonatal rat calvarial osteoblasts on the surface of Ti-6Al-4V and plasma-sprayed HA coating: effect of surface roughness and serum contents. J Mater Sci Mater Med 13: 341-350.
- Gross KA, Babovic M (2002) Influence of abrasion on the surface characteristics of thermally sprayed hydroxyapatite coatings. Biomaterials 23: 4731-4737.
- Songur M, Celikkan H, Gokmese F, Simsek SA, Altun NS, et al. (2009) Electrochemical corrosion properties of metal alloys used in orthopaedic implants. J Appl Electrochem 39: 1259-1265.
- Aksakal B, Gavgali M, Dikici B (2010) The effect of coating thickness on corrosion resistance of hydroxyapatite coated Ti6Al4V and 316L SS implants. Mater Eng Perform 19: 894-899.
- Manivasagam G, Dhinasekaran D, Rajamanickam A (2010) Biomedical Implants: Corrosion and its Prevention - A Review. Recent Pat Corros Sci 2: 40-54.
- Sousa SR, Barbosa MA (1996) Effect of hydroxyapatite thickness on metal ion release from Ti6Al4V substrates. Biomaterials 17: 397-404.
- Fathi MH, Salehi M, Saatchi A, Mortazavi V, Moosavi SB (2003) In vitro corrosion behavior of bioceramic, metallic, and bioceramic-metallic coated stainless steel dental implants. Dent Mater 19: 188-198.
- Yen SK, Chiou SH, Wu SJ, Chang CC, Lin SP, et al. (2006) Characterization of electrolytic HA/ZrO2 double layers coatings on Ti-6Al-4V implant alloy. Mater Sci Eng 26: 65-77.
- Cachinho SC, Correia RN (2008) Titanium scaffolds for osteointegration: mechanical, in vitro and corrosion behaviour. J Mater Sci Mater Med 19: 451-457.
- Maxian SH, Zawadsky JP, Dunn MG (1993) Mechanical and histological evaluation of amorphous calcium phosphate and poorly crystallized hydroxyapatite coatings on titanium implants. J Biomed Mater Res 27: 717-728.
- Xue W, Tao S, Liu X, Zheng X, Ding C (2004) In vivo evaluation of plasma sprayed hydroxyapatite coatings having different crystallinity. Biomaterials 25: 415-421.
- Gross KA, Saber-Samandari S (2007) Nano-mechanical properties of hydroxyapatite coatings with a focus on the single solidified droplet. J Aust Ceram Soc 43: 98-101.
- Yang CY, Lin RM, Wang BC, Lee TM, Chang E, et al. (1997) In vitro and in vivo mechanical evaluations of plasma-sprayed hydroxyapatite coatings on titanium implants: the effect of coating characteristics. J Biomed Mater Res 37: 335-345.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22819
- [From(publication date):
July-2013 - Nov 24, 2024] - Breakdown by view type
- HTML page views : 18277
- PDF downloads : 4542