Research Article Open Access
The Effect of 28 Days of Beta-alanine Supplementation on Exercise Capacity and Insulin Sensitivity in Individuals with Type 2 Diabetes Mellitus: A Randomised, Double-blind and Placebo-controlled Pilot Trial
Nealon RS*, Sukala WR, Coutts RA and Zhou S
Southern Cross University, Lismore, NSW, Australia
- *Corresponding Author:
- Rhenan Nealon
Southern Cross University
Lismore,NSW, Australia
Tel: 6126659 3777
E-mail: Rhenan.nealon@uon.edu.au
Received Date: January 20, 2016; Accepted Date: September 28, 2016; Published Date: October 05, 2016
Citation: Nealon RS, Sukala WR, Coutts RA, Zhou S (2016) The Effect of 28 Days of Beta-alanine Supplementation on Exercise Capacity and Insulin Sensitivity in Individuals with Type 2 Diabetes Mellitus: A Randomised, Double-blind and Placebo-controlled Pilot Trial. Sports Nutr Ther 2: 111. doi: 10.4172/2473-6449.1000111
Copyright: © 2016 Nealon RS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Nutrition Science Research
Abstract
Objective: To determine the effects of 28 days of BA supplementation on exercise capacity as measured by time to volitional failure during an incremental treadmill test in individuals with T2DM. Methods: Participants undertook a modified Naughton treadmill test to assess exercise capacity and a fasting blood test before and after the supplementation period. Participants consumed four grams of BA (n=7) per day or the equivalent amount of maltodextrin (n=5) for 28 days and maintained their lifestyle habits during the supplementation period. Results: Twenty-eight days of BA supplementation significantly increased the time to volitional failure during the treadmill exercise test by m=135.2, SD=(± 81.3) seconds (21%), p=0.001, as detected by general linear model analysis with repeated measures. There was significant interaction of pre to post by group in fasting blood glucose, p=0.046. A strong negative correlation was found between the changes in the time to volitional failure during exercise testing and the changes in fasting blood glucose in the beta-alanine treatment group, r=− 0.92, p<0.01. Conclusion: This study has provided new evidence that BA supplementation can increase exercise capacity in individuals with T2DM.
Keywords
Beta-alanine; Maltodextrin; Type 2 Diabetes mellitus; Placebo; Randomised; Carnosine; GLUT4
Background
Type 2 diabetes mellitus (T2DM) accounts for nearly 90% of all diabetes cases [1]. It is characterized by insulin resistance and is frequently a combined result of nutrition, genetics and the environment. Approximately 347 million people worldwide were estimated to have diabetes in 2012 and this number growing rapidly [1]. It is considered a risk factor for cardiovascular disease (CVD) and is known to instigate metabolic impairments that can lead to other comorbid conditions such as peripheral neuropathy. The total cost of diabetes in Australia was approximately $10.3 billion in 2008 and is estimated well above $60 billion per year worldwide [1,2]. Diabetes Australia (2008) expects this cost to increase every year as a further 280 Australians develop diabetes every day.
It is recommended that individuals with T2DM partake in regular exercise to reduce body fat stores, particularly intra myocellular triglyceride (IMTG) [3]. An improvement in exercise capacity by one metabolic equivalent of activities (MET) is associated with an 18% reduction in mortality risk in individuals with T2DM [4]. Furthermore, regular exercise contributes to reduced CVD risk factors, and improved glycemic control [5]. However, exercise therapy provided by clinical exercise physiologists (EP) may be a slow process and requires adequate time and planning in order to reach the desired physiological outcomes.
Reduced exercise capacity in the initial stages of exercise therapy may cause individuals to discontinue exercise treatment due to impatience, loss of motivation and poor self-esteem [6]. A lack of supplementary methods that increase exercise capacity in the initial stages of EP treatment could contribute to the lack of adherence to structured exercise programs in T2DM.
Carnosine is a cytoplasmic dipeptide found in human skeletal muscle and the central nervous system and was first isolated in 1900 [7,8]. It is known to enhance buffering capacity in muscle in exercise of increasing intensities and a reduction in this di-peptide may impair exercise capacity [9]. Significant reductions in intramuscular carnosine have been found in individuals with T2DM [10]. Correspondingly, it has been discovered that individuals with T2DM are more likely to have a reduced capacity for exercise than healthy individuals [4,11]. Impaired muscle contraction secondary to low pH and reduced buffering capacity may preclude individuals from attaining sufficient exercise intensities and duration necessary to oxidise IMTG, which may enhance insulin signalling and, consequently, insulin sensitivity.
Beta-alanine (BA) is a dietary supplement which has been experimentally shown to augment muscle carnosine and exercise capacity in healthy individuals, but no previous trials have evaluated its effectiveness in individuals with T2DM [12].
Research aim
To determine whether consuming four grams of BA per day for 28 days would increase exercise capacity in individuals with T2DM measured by time to volitional failure during the modified Naughton treadmill exercise test.
Hypothesis
Consuming four grams of BA per day for 28 days would significantly increase time to volitional failure in individuals with T2DM during the modified Naughton treadmill exercise test.
Methods
Ethical considerations
An application using the National Ethics Application Form (NEAF) was lodged, reviewed and approved by the Southern Cross University Human Research Ethics Committee (approval number ECN-13-086).
Research design
This was a 28-day double-blind, randomised and placebo-controlled trial with two parallel treatment groups (BA supplementation = experimental group, placebo = control group). Before and after the supplementation intervention period, participants completed an incremental treadmill exercise test and a fasting blood test to determine plasma and glucose content, and estimate insulin sensitivity and resistance using HOMA2 software.
Participants
Statistical power and target sample size was calculated using G*Power3 software [13]. After noting the results of a previous randomized controlled trial with a similar research design [14], the following data was entered into G*Power3; effect size (ES)=0.5, alpha level (a)=0.05, power (1-ß)=0.8, r=0.5, 2 groups and 0 correlation between groups. A priori estimation indicated that 12 participants, with six in each treatment group, would be the minimum number for detection of the intervention effect using ANOVA with repeated measures for within and between group interactions.
The inclusion criteria for participation used for this trial were:
- = 18 years;
- Diagnosed with T2DM;
- Diabetes is managed with diet or oral hypoglycemic medications (and not receiving exogenous insulin injections);
- No changes in diabetes medications in the previous two months;
- Stable cardiac history for previous six months;
- Signed consent from GP or specialist.
Experimental procedure
To minimize extraneous variables, subjects were instructed to maintain their usual lifestyle habits. Weekly phone interviews were conducted to monitor changes in medication, physical activity, and other self-reported lifestyle factors.
On day 0 of the trial, participants met with the principal investigator for a familiarisation and screening session at Southern Cross University, Lismore, NSW, Australia.
On day one and 33 or 34 of the trial, participants attended Sullivan Nicolaides Pathology laboratory to provide a blood sample, which was assayed for plasma glucose and insulin content. Fasting plasma glucose and insulin scores were entered into the homeostasis model assessment version 2 (HOMA2) software to estimate insulin sensitivity and insulin resistance. The participants were then randomised using software located at http://www.randomizer.org [15] to receive either BA or placebo treatment for 28-days by an independent person who was not participating in this research project. The researchers and participants were not aware of the treatment allocation.
After blood collection, participants were provided 24 to 48 hours to recover before returning to the Southern Cross University Exercise Physiology Laboratory on day three of the study for the baseline incremental treadmill exercise test. A follow up treadmill exercise test was completed on day 31 or 32 of the trial, no later than 48 hours after consuming the final two capsules in the supplementation protocol.
Supplementation
This trial used four grams of pure BA (Body Science International) and placebo supplementation per day for 28 days (total consumption of 112 grams). Slow release BA was not available, therefore four grams of BA or placebo was split in to three separate doses per day to minimise the paraesthesia associated with BA consumption above 800 mg per dose [9,16] Maltodextrin was chosen due to its close appearance to BA and to remain consistent with BA double-blind randomised and placebo-controlled trials [17-20]. The dose used in this trial was not considered high enough to elicit a heighted insulin response or elevate fasting blood sugar.
Each capsule contained 667 mg of BA or maltodextrin powder. The four-gram dose was met by a daily supplementation regimen of two capsules taken three times daily (breakfast, lunch and dinner) to total a consumption of 112.06 gram in 168 capsules. Twenty-five capsules chosen at random were weighed for quality control to ensure the mean weight was within 2% of the desired 667 mg per capsule. The mean weight of two separate batches of BA capsules was within 0.7% and 1.3% of the desired 667 mg per capsule.
Data analysis
Data were analyzed for normality using skewness, kurtosis and frequency histograms before the general linear model (GLM) analysis with repeated measures was performed to compare participants’ response to BA and placebo treatments. A 95% confidence interval (CI) was employed to specify whether the odds ratios were within a range in which the true population was assumed to lie [21]. Significance was accepted at p<0.05 for the determination of a significant change as a result of the intervention.
T-tests were used to determine differences in demographics between groups at baseline and also for differences between levels of physical activity (IPAQ questionnaire results. Correlations between any significant changes that were discovered were analyzed using Pearson product-moment correlation coefficient, and a correlation larger than ± 0.5, was deemed to be a strong correlation [22].
Results
Participant characteristics
Twelve participants were eligible for participation and were randomized to receive BA (n=7), or placebo (n=5). However, due to abnormal responses on the modified II-lead echocardiograph (ECG), one participant from each group was excluded from the treadmill exercise test but was still included in all other pre and post data analyses (Tables 1 and 2).
| Characteristic | All participants | Beta-Alanine Group | Placebo Group | Significance (p =) |
|---|---|---|---|---|
| Number of participants n) | (12 total, 10 exercise test outcomes) | (7 total, 6 exercise test outcomes) | (5 total, 4 exercise test outcomes) | |
| Age (years) | 64 (6.4) | 62 (4.6) | 66(6.4) | 0.23 |
| Gender (M/F) | 9M /3F | 5M /2F | 4M /1F | |
| Height (m) | 1.729 (0.088) | 1.709 (0.071) | 1.756 (0.110) | 0.38 |
| Weight (kg) | 98.96 (24.63) | 90.99 (11.95) | 110.12 (34.96) | 0.20 |
| BMI | 32.8 (5.81) | 30.92 (2.53) | 35.19 (8.50) | 0.25 |
| Fasting Blood Glucose (mmol.L1) | 8.24 (2.82) | 8.16 (2.34) | 8.36 (3.68) | 0.91 |
| Fasting Insulin (mU.L-1) | 10.67 (5.07) | 10.71 (6.32) | 10.60 (3.29) | 0.97 |
| Beta-cell function (%) | 68.0 (50.40) | 64.70 (48.50) | 72.67 (58.30) | 0.80 |
| Insulin Sensitivity (%) | 63.2 (24.80) | 67.5 (31.20) | 57.2 (12.50) | 0.51 |
| Insulin Resistance | 1.79 (0.69) | 1.79 (0.89) | 1.80 (0.36) | 0.97 |
| Time to volitional failure (seconds) | 715.8 (192.8) | 745.5 (220.0) | 671.3 (162.4) | 0.58 |
| METs | 5.57 (1.65) | 5.27 (1.91) | 5.02 (1.30) | 0.23 |
| Maximum RPE | 7.1 (1.5) | 7.2 (1.0) | 7.0 (2.2) | 0.87 |
| Max Heart Rate | 132.7 (21.1) | 134.0 (17.8) | 131.8 (24.6) | 0.88 |
Table 1: Baseline participant characteristics. Results are presented in APA format as means with standard deviations in brackets.
| Characteristic | All participants | Beta-Alanine Group | Placebo Group | Significance(p =) |
| n | (12 total, 10 exercise test outcomes) | (7 total, 6 exercise test outcomes) | (5 total, 4 exercise test outcomes) | |
| Height (m) | 1.729 (0.088) | 1.709 (0.071) | 1.756 (0.110) | 0.38 |
| Weight (kg) | 99.09 (26.05) | 90.97 (11.96) | 112.93 (40.28) | 0.20 |
| BMI | 33.21 (6.25) | 31.02 (2.63) | 36.8 (9.57) | 0.16 |
| Fasting Blood Glucose (mmol.L-1) | 7.94 (2.78) | 7.14 (1.63) | 9.06 (3.81) | 0.26 |
| Fasting Insulin (mU.L-1) | 10.75 (4.65) | 9.14 (2.19) | 13.00 (6.44) | 0.17 |
| Beta-cell function (%) | 69.48 (45.20) | 70.57 (43.47) | 67.96 (52.72) | 0.93 |
| Insulin Sensitivity %) | 62.08 (22.07) | 70.03 (17.86) | 50.94 (24.41) | 0.15 |
| Insulin Resistance | 1.84 (0.79) | 1.50 (0.37) | 2.32 (1.00) | 0.07 |
| Time to volitional failure (seconds) | 798.4 (194.8) | 880.7 (185.7) | 675.0 (150.0) | 0.10 |
| METs | 5.57 (1.54) | 6.06 (1.75) | 4.83 (0.86) | 0.82 |
| Maximum RPE | 6.9 (1.4) | 7.0 (1.1) | 6.75 (1.9) | 0.80 |
| Max Heart Rate | 132.65 (21.1) | 136.1 (21.1) | 132.7 (18.8) | 0.80 |
Table 2: Post-supplementation participant characteristics. The data are presented as means with standard deviations in brackets.
Primary outcome measures
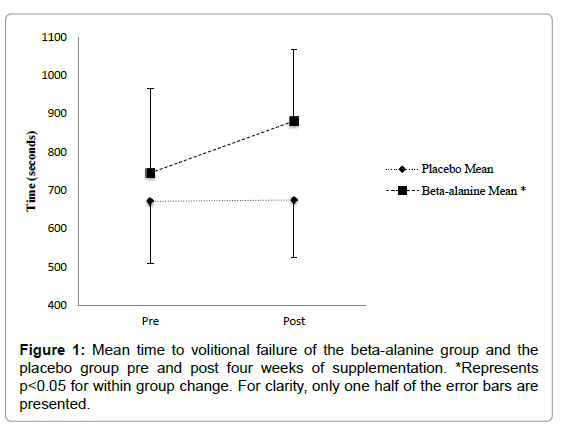
Time to volitional failure: The general linear model with repeated measures analysis showed a significant effect of supplementation on time to volitional failure, F (1, 10)=10.54, p=0.012. There was also a significant interaction of pre to post by group, F (1, 10)=5.16, p=0.015. Participants in the BA group showed a significant increase in time to volitional failure M=135.2 (± 81.3) seconds (21%), p=0.001, 95% CI (72.8 - 197.6), while there was no significant change in the mean time to volitional failure in the placebo group, m=3.8 (± 26.4) seconds, p=0.91, 95% CI (-72.8, 80.8) (Figure 1).
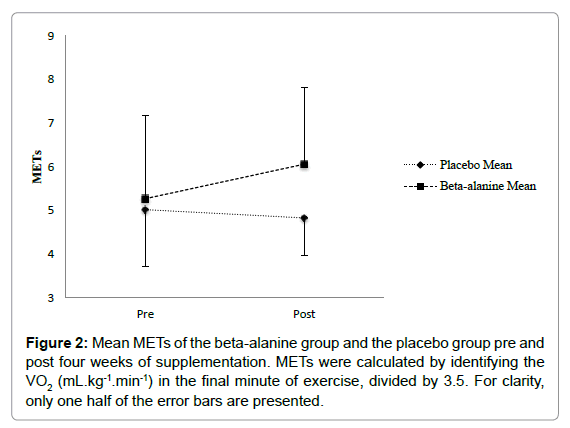
Metabolic equivalent of task (MET): The general linear model with repeated measures analysis showed no significant effect from pre to post supplementation in METs, F (1, 10)=0.36, p=0.565. There was also no significant interaction of pre to post by group F (1, 10)=0.96, p=0.365. Furthermore, there were no significant changes from pre to post supplementation in METs in the placebo or BA group, p=0.81, 95% CI (- 1.97, 1.59), and p=0.24, 95% CI (- 0.67, 2.24), respectively (Figure 2).
Secondary outcome measures
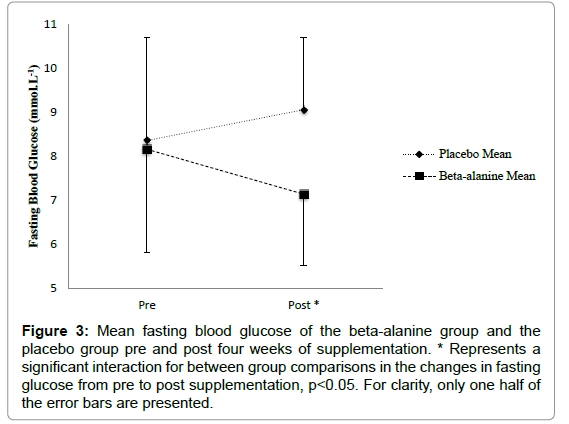
Fasting blood glucose: The general linear model with repeated measures analysis showed no significant effect of supplementation on fasting blood glucose F (1, 10)=0.17, p=0.686. There was no significant change from pre to post supplementation in fasting blood glucose in the BA group, p=0.06, 95% CI (- 0.58, 1.98) and no significant change in the placebo group, p=0.25, 95% CI (- 2.1, 0.71). However, there was a significant interaction of pre to post by group F (1, 10)=5.16, p=0.046. The BA group reduced non-significantly by m=1.01 (± 1.48) mmol.L-1, p=0.06, from pre to post supplementation while the placebo group experienced a mean non-significant increase in fasting blood glucose of 0.70 (± 0.94) mmol.L-1, p=0.09 from pre to post supplementation. There were no other changes in secondary outcome measures (Figure 3).
Figure 3: Mean fasting blood glucose of the beta-alanine group and the placebo group pre and post four weeks of supplementation. * Represents a significant interaction for between group comparisons in the changes in fasting glucose from pre to post supplementation, p<0.05. For clarity, only one half of the error bars are presented.
Correlations in time to volitional failure and fasting blood glucose
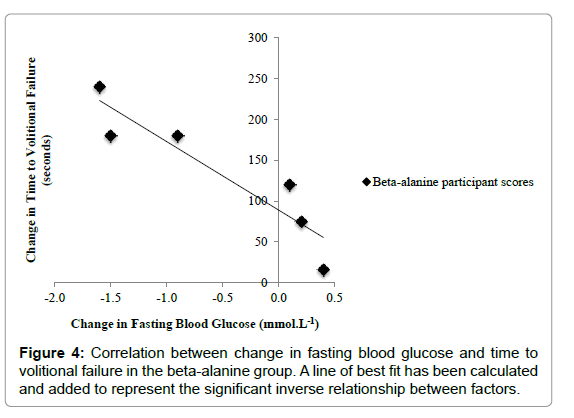
There was a strong negative correlation between the change from pre to post supplementation in the time to volitional failure and fasting blood glucose in all participants, r (8) = - 0.84, p<0.01. Further analysis revealed a strong negative relationship between change from pre to post supplementation in the time to volitional failure and fasting blood glucose in the BA group, r (4) = - 0.92, p<0.01. There was no significant correlation between change from pre to post supplementation in the time to volitional failure and fasting blood glucose in the placebo treatment group, r (2)= - 0.08, p=0.81 (Figure 4).
Discussion
Supplementation with BA for 28 days significantly improved exercise capacity in individuals with T2DM, as measured by time to volitional failure during the modified Naughton treadmill exercise test. There was also a significantly greater reduction in fasting blood glucose after BA supplementation compared to placebo. There were no significant within group changes in fasting blood glucose, insulin or HOMA2 values. However, improvements in exercise capacity were strongly and negatively correlated with changes in fasting blood glucose.
To the researchers’ knowledge, only two previous BA supplementation trials used incremental treadmill testing and required that participants made no other physical activity, exercise or lifestyle changes. Jordan et al. [14,19] found that BA supplementation significantly delayed the onset of blood lactate during incremental exercise testing. Despite using the same supplementation time period as the present trial (28-days) the authors did not report changes in time to exhaustion, total exercise time or other measures consistent with the results of the present trial, which prevents the ability to directly compare findings of [14] with our results. Del Favero et al. [19] reported a significant increase in time to exhaustion during treadmill exercise testing in healthy individuals aged 60-80 years. The improvement in time to volitional failure experienced by the participants with T2DM in our trial was 8.8% larger than the 12.2% improvement in time to exhaustion found by del Favero et al. [19]. Participants in the trial by del Favero et al. [19] consumed 3.2 grams of BA per day for 12 weeks (total BA consumption = 268.8 grams), which is 2.4 times greater than the total amount of BA consumed by participants in the present trial (112 grams). Del Favero et al. [19] found intramuscular carnosine stores in the gastrocnemius of the BA treated participants were significantly increased by 85.4%, measured by proton magnetic resonance imagery. Reviews have concluded that BA supplementation produces a doseresponse relationship with carnosine augmentation and improvements in exercise testing measures that rely on intramuscular buffering capacity [9,23]. Although participants in the present trial consumed 156.8 grams less than del Favero et al. [19] participants, the discrepancy between results may be explained by the possible differences in baseline intramuscular carnosine stores. As previously discussed, one prior trial found significantly reduced intramuscular carnosine stores in individuals with T2DM [10]. Although we did not directly measure intramuscular carnosine, it may be possible that the participants in the present study had lower baseline stores of intramuscular carnosine than individuals in other BA supplementation randomised and controlled trials. This may have stimulated a greater increase in intramuscular carnosine and a subsequently greater increase in exercise capacity than the trial by del Favero et al. [19].
Reaching volitional failure involves continual exercise to an intensity likely to result in the accumulation of myocellular hydrogen ions, which contributes to a reduction in blood pH and impairs muscle contraction capacity [24]. This is likely because myocellular hydrogen ions can block the release of calcium into the sarcoplasmic reticulum, thus disrupting the ability of calcium to diffuse into troponin and
stimulate muscle contraction [25,26]. The accumulation of hydrogen ions can be counteracted by buffers including carnosine, which may permit the attenuation of pH decline below optimal myocellular contractual range [27]. Recent reviews agree that BA supplementation is an effective method of increasing intramuscular carnosine [23,28,29]. The significant increase in time to volitional failure in the experimental treatment group is therefore likely to be due to an increase in intramuscular carnosine content, secondary to BA supplementation. Although we did not find a significant change in METs, the significant increase in time to volitional failure found in the BA treatment group indicates an enhanced capacity for exercise. Combined with the knowledge that structured exercise training improves peak METs and glycemic control in individuals with T2DM [5] and that it has been advocated that an increase in exercise capacity of one MET is associated with an 18% reduction in mortality risk in individuals T2DM, regardless of the baseline exercise capacity [4]. An enhanced capacity to exercise at increasing intensities before the initiation of an exercise program may ease the transition into a structured exercise program, which may improve METs and glycemic control in individuals with T2DM.
No previous trials have investigated the effects of BA supplementation on fasting blood glucose. However, there are two possible mechanisms to explain this finding. These include a possible improvement in intracellular glucose transport in the 48-hour period post exercise testing, and a possible increase in insulin secretion in response to BA supplementation. The significantly increased time to failure in the BA treatment group may have resulted in greater capacity for glucose metabolism in the time leading up the to the post supplementation fasting blood test, which was 24 to 48 hours after exercise testing in all participants. This is consistent with a review by Borghouts and Keizer (2000) who stated that glucose uptake and insulin sensitivity is elevated for at least 16 hours post exercise. It may be possible that the significant increase in time to volitional failure in the BA treatment group may have resulted in a greater depletion of muscle and liver glycogen stores and subsequent glucose removal from blood stream at a larger extent than in the placebo group. However, the difference in the mean time to volitional failure at post intervention exercise testing was not significantly different between groups, p=0.10, and we did not find any changes in insulin sensitivity or insulin resistance estimated by HOMA2 software in either group. Therefore it is unlikely that exercise testing influenced glucose uptake to a greater extent in the BA group compared to placebo.
Alternatively, the significantly greater reduction in fasting glucose in the BA than placebo group may be due to an increase in insulin secretion resulting from amino acid supplementation [30,31]. Metabolism of alanine has been found to enhance insulin secretion from isolated islet and clonal beta-cells of human pancreas [30]. This may have facilitated a larger amount of cellular glucose uptake than placebo treatment. However, there were no significant differences in fasting plasma insulin content in either group from pre to post supplementation. Alternatively, mammalian target of rapamycin (mTOR), a serine/threonine protein kinase that regulates protein synthesis and cell growth, proliferation and survival, is said to be activated by amino acid supplementation [32,33]. Protein synthesis is a process that requires energy and is said to contribute to a large share of adenosine triphosphate (ATP) expenditure at rest [32]. It is possible that mTOR may have been more efficient at rest in the BA group and a larger amount of plasma glucose was transported to intracellular cytoplasm to produce ATP, which is required for protein synthesis. However, no previous trials have specifically investigated the effects of BA supplementation of insulin secretion, or mTOR activity. These speculations require further research.
There was a significant and negative relationship between changes in fasting glucose and time to volitional failure in the BA group, and the total cohort [22]. Considering that there was no significant correlation found in placebo treatment, the strong correlation found in the total cohort is likely due to the contribution from BA treatment. Correlation does not indicate causation and there are mechanisms to consider when interpreting this relationship.
A single acute bout of cycle exercise at 60-70% VO2max has been documented to increase GLUT4 translocation to the intramyocellular plasma membrane in the vastus lateralis of individuals with T2DM, measured by open muscle biopsy, by 74 (± 20) %, p<0.05, beyond resting values [34]. The post intervention treadmill exercise test in the present trial may have increased intracellular glucose transport in the 24 to 48 hours before the post intervention fasting blood test, and may have reduced plasma glucose to a larger extent than if the exercise test was performed after fasting blood tests. However this would negate the significant difference in the changes in fasting blood glucose from pre to post intervention between groups as both groups undertook the same incremental treadmill exercise test at the same intensity, with no significant differences in time to volitional failure at the post supplementation testing time point.
Another explanation for the strong inverse relationship between the changes in fasting glucose and time to volitional failure is a possible increase in intracellular adenosine tri-phosphate (ATP) production to provide energy for muscular contraction. Circulating plasma glucose stimulates insulin secretion, which causes a signalling cascade and results in glucose transport to intracellular cytoplasm. During moderate intensity exercise that continues for more than 60 seconds, before an accumulation of lactic acid and hydrogen ions contribute to fatigue, the source of energy production is largely determined by the availability of substrate [24,35]. The significantly greater reduction in fasting blood glucose, coupled with the significantly greater increase in time to volitional failure during exercise testing experienced in BA than placebo group, could suggest that BA treated participants had larger intracellular glycogen content during exercise testing. This could have allowed for greater ATP production through glycolysis and delayed fatigue to a greater degree than placebo treatment.
Limitations
Outside of budget and time constraints involved in completing this trial, the main limitation of this study is the relatively low number of participants, irrespective of the target sample size being achieved. This may have increased the likelihood of type 1 and 2 errors [36]. Controlling for lifestyle and habitual physical activity levels were restricted to subjective self-reported measures administered in person and through telephone conversations. Self-reported measures that rely on memory and interpretation of questions can increase participant bias and errors [37]. Supplementation adherence was also restricted to self-reporting, which may have led to participants providing researchers with false information in order to continue with participation. The short supplementation period of 28 days may not have been long enough to elicit the desired response on blood outcome measures. Typically, HbA1c reveals glycated hemoglobin over the lifetime of the red blood cells (2-3 months) [38]. Therefore 28 days may not have been long enough to elicit a long-term response on measures of fasting blood glucose, fasting insulin, HOMA2 insulin sensitivity and HOMA2 insulin resistance.
Recommendations
Future trials should investigate the mechanism that explains the significantly greater decrease in fasting glucose after 28 days of BA supplementation than placebo treatment with or without an exercise test. This is the first trial to date to investigate the effects of BA supplementation on fasting glucose, so there are no previous trials to compare with. Furthermore, the strong inverse relationship between changes in exercise capacity and fasting glucose in the BA treatment group has been speculatively explained in this trial, but cannot be directly explained due to the lack of previous trials investigating correlations in changes as opposed to measuring the correlations in the pre-existing exercise capacity and fasting glucose. The correlation in the present trial was discovered from changes in a longitudinal manner, whereas previous research has investigated exercise capacity and fasting glucose in a cross-sectional manner. Future trials should investigate whether improvements in exercise capacity secondary to BA supplementation, and other methods that do not involve an exercise intervention, are associated with a reduction in fasting glucose or an improvement in HbA1c.
The increase in time to volitional failure during the modified Naughton treadmill exercise test has been attributed in the present trial to a possible increase in intramuscular carnosine stores. However, intramuscular carnosine was not directly measured in this trial. Future trials investigating the effects of BA supplementation in individuals with T2DM should measure intramuscular carnosine content by muscle biopsy or proton magnetic resonance imagery to determine whether individuals with T2DM have reduced intramuscular stores, whether BA supplementation significantly increases exercise capacity in individuals with T2DM, and also whether there are significant positive correlations between intramuscular carnosine stores, exercise capacity and glycemic control in individuals with T2DM.
As HbA1c is commonly used to monitor diabetic management, future trials that are carried out over periods of 2 months (60 days) or more should measure HbA1c to determine whether there were any long-term changes in glycemic control. Other factors to consider for future research include: larger sample sizes, increased daily dose of BA, use of slow release BA and the inclusion of an exercise intervention.
Conclusion
This study has provided new evidence that BA supplementation can increase exercise capacity in individuals with T2DM. Moreover, there was a significantly greater reduction in fasting blood glucose in BA the treatment group than placebo treatment after 28 days of supplementation. The changes in the time to volitional failure were strongly and inversely correlated with the changes in fasting blood glucose. However, the role BA supplementation played in these changes requires further research.
Acknowledgements
The researchers would like to acknowledge BodyScience International, who provided financial support for blood analysis, printing of posters and thesis binding. BodyScience International also provided Beta-alanine product and empty label canisters at no cost. Furthermore, the contribution by Sullivan Nicolaides Pathology, who collected blood samples at no cost and provided a discounted price for blood analysis, was greatly appreciated.
A living stipend was provided by the research Centre for Tourism, Leisure and Work of Southern Cross University to the principal researcher Rhenan Nealon, to assist with living expenses while undertaking research.
To the author’s knowledge, no conflicts of interest exist from this research.
Author Contributions
Rhenan Nealon was the lead investigator and author for this research project as it was completed to satisfy an honours degree in Health and Human Sciences. Dr William Sukala was lead supervisor and contributed largely to the editing of thesis as well as to data collection and research methodology. Professor Shi Zhou and Dr Rosanne Coutts also contributed to the research methodology, data collection and to the editing of the manuscript.
References
- http://www.who.int/mediacentre/factsheets/fs312/en/index.html
- (2008) Diabetes Australia, The growing cost of obesity in: three years on: Australia.
- Kelley D, Goodpaster B, Storlien L(2002) Muscle triglyceride and insulin resistance. Annu Rev Nutr 22: 325-346.
- Nylen E, Kokkinos P, Myers J, Faselis C (2010) Prognostic effect of exercise capacity on mortality in older adults with diabetes mellitus. J Am Geriatr Soc 58: 1850-1854.
- Maiorana A, Gerard OD, Carmel G, Roger T, Daniel G (2002) Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract 56: 115-123.
- Qiu S, SunZ, Cai X, Liu L, Yang B (2012) Improving patients' adherence to physical activity in diabetes mellitus: a review. Diabetes Metab J 36: 1-5.
- Gulewitsch W, Amiradzhibi S (1900) Uber der carnosin, eineneueorganische base des fleischextrakten. Berichte der Deutschen Chemischen Gesellschaft 33:1902-1903.
- Harris R, Wise JA, Price KA, Kim HJ, Kim CK, et al. (2012) Determinants of musclecarnosine content. Amino Acids 43: 5-12.
- Caruso J, Charles J, Unruh K, Giebel R, Learmonth L, et al.(2012) Ergogenic effects of ß-Alanine and carnosine: proposed future research to quantify their efficacy. Nutrients 4: 585-601.
- Gualano B, Everaert I, Stegen S, Artioli GG, Taes Y,et al. (2011) Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids 43: 21-24.
- Ugur-Altun B, Altun A, Tatli E, Tugrul A (2005) Factors related to exercise capacity in asymptomatic middle-aged type 2 diabetic patients. Diabetes Res Clin Pract 67: 130-136.
- Hobson R, Saunders G, Ball G, Harris RC, Sale C, et al. (2012) Effects of ß-alanine supplementation on exercise performance: a meta-analysis. Amino Acids 43: 25-37.
- Faul F, Edgar E, Albert GL, Axel B (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175-191.
- Jordan T, Lukaszuk J, Misic M, Umoren J (2010) Effect of beta-alanine supplementation on the onset of blood lactate accumulation (OBLA) during treadmill running: Pre/post 2 treatment experimental design. J Int Soc Sports Nutr 7: 20.
- http://www.randomizer.org
- Décombaz J, Beaumont M, Vuichoud J, Bouisset F, Stellingwerff T, et al. (2012) Effect of slow-release ß-alanine tablets on absorption kinetics and paresthesia. Amino Acids 43: 67-76.
- Hill C, Harris RC, Kim HJ, Harris BD, Sale C, et al. (2007) Influence of ß-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32:225-233.
- Saunders B, Sale C, Harris RC, Sunderland C (2011) Effect of beta-alanine supplementation on repeated sprint performance during the Loughborough Intermittent Shuttle Test. Amino Acids 43: 39-47.
- Favero Sd, Roschel H, Solis MY, Hayashi AP, Artioli GG, et al. (2012) Beta-alanine (CarnosynTM) supplementation in elderly subjects (60-80 years): effects on muscle carnosine content and physical capacity. Amino Acids 43: 49-56.
- Saunders B, Sunderland C, Harris RC, Sale C (2012) ß-alanine supplementation improves YoYo intermittent recovery test performance. J Int Soc Sports Nutr 9: 39.
- Allen P, Bennett K (2010) SPSS Statistics Version 22: A Practical Guide (18th edn.). Cengage Learning, Australia.
- Cohen J (1988) Statistical power analysis for the behavioural sciences (2ndedn.). Hillside NJ: Erlbaum.
- Stellingwerff T, Decombaz J, Harris RC, Boeschet C (2012) Optimizing human in vivo dosing and delivery of b-alanine supplements for muscle carnosine synthesis. Amino Acids 43: 57-65.
- Green H (2010) Mechanisms of muscle fatigue in intense exercise. J Sports Sci 15: 247-256.
- Edwards R (1981) Human muscle fatigue: physiological mechanism. P Medical, Wiley, London.
- Green H (2004) Membrane excitability, weakness, and fatigue. Can J Appl Physiol 29: 291-307.
- Smith ECB (1938) The buffering of muscle in rigor; protein, phosphate and carnosine. J Physiol 92: 336-342.
- Culbertson J, Kreider RB, Greenwood M, Cookeet M (2010) Effects of beta-alanine on muscle carnosineand exercise performance: a review of the current literature. Nutrients 2: 75-98.
- Derave W, Everaert I, Beeckman S, Baguet A (2010) Muscle carnosine metabolism and b-alanine supplementation in relation to exercise and training. Sports Medicine 40: 247-263.
- Anuradha C (2009) Amino acid support in the prevention of diabetes and diabetic complications. Curr Protein Pept Sci 10: 8-17.
- Ansurudeen I, Sunkari VG, Grünler J, Peters V, Schmitt CP, et al. (2012) Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids 43: 127-134.
- Deldicque L, Theisen D, Francaux M (2005) Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur J Appl Physiol 94: 1-10.
- Fingar D, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151-3171.
- Kennedy, John W, Hirshman, Michael F, Gervino, et al.(1999) Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 48: 1192-1197.
- Powers S, Howley E (2009) Exercise physiology: theory and application to fitness and performance (9th edn.). McGraw Hill, US.
- Zhanga S, Caob J, Ahna C (2010) Calculating sample size in trials using historical controls. Clinical Trials 7: 343-353.
- Concatio J, Shah N, Horwitz R (2000) Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 342: 1887-1892.
- O'Sullivan CJ, Hynes N, Mahendran B, Andrews EJ, Avalos G, et al. (2006) Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg 32: 188-197.
Relevant Topics
- Aminoacid Suppliments
- Bodybuilding Nutrition
- Clinical Sports Nutrition
- Creatine Sports Nutrition
- Diet
- Fitness Nutrition
- Food and Nutrition
- Gym Suppliments
- Herbal Suppliments
- Micronutrients
- Natural Suppliments
- Nutrition Sport Fitness
- Nutritional Health
- Protein Diet
- Protein Suppliments
- Sports Nutrition Suppliments
- Vitamin Supplement
Recommended Journals
Article Tools
Article Usage
- Total views: 16214
- [From(publication date):
December-2016 - Dec 03, 2024] - Breakdown by view type
- HTML page views : 15408
- PDF downloads : 806