The Diagnostic Value of HPV E6/E7 mRNA Test in Young Women with Cervical Squamous Intraepithelial Lesion
Received: 28-Jan-2022 / Manuscript No. dpo-22-52733 / Editor assigned: 31-Jan-2022 / PreQC No. dpo-22-52733(PQ) / Reviewed: 14-Feb-2022 / QC No. dpo-22-52733 / Revised: 17-Feb-2022 / Manuscript No. dpo-22-52733(A) / Accepted Date: 17-Feb-2022 / Published Date: 24-Feb-2022 DOI: 10.4172/2476-2024.S10.1000002
Abstract
Objective: At present, the HPV DNA test is used to triage young female patients with abnormal cytology. Still, it is not suitable to precisely identify the population with persistent HPV infection. The purpose of this study was to evaluate the diagnostic value of HPV E6/E7 mRNA test in young women with abnormal cytology by comparing HPV DNA test.
Methods: A total of 258 young women aged 20 to 29 years, with squamous cell abnormalities on the cervical cytology, were enrolled in this study between January 2015 and December 2019. All patients were subject to HPV DNA test, HPV E6/E7 mRNA test, colposcopy biopsy, and histopathological examination. A comparative analysis of the diagnostic performance of the HPV DNA test and HPV E6/E7 mRNA test was conducted according to the histological diagnosis (CIN II and CIN II+ were defined as high-grade squamous intraepithelial lesion+ (HSIL+)).
Results: The results showed that HPV E6/E7 mRNA test had a higher specificity of 47.3% (40.0%-55.1%) for HSIL+ compared to HPV DNA test that had specificity of 16.0% (11.0%-22.6%) in young women(P<0.01). The HPV E6/E7 mRNA test presented high rates of specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV), which were 92.1%(86.0%-96.0%), 62.1%(42.4%-78.7%), 92.1%(85.9%-95.8%), respectively, compared to that of HPV DNA, which were 15.8%(10.4%-23.2%),14.6%(9.40%-21.9%), and 71.0%(51.8%-85.1%), respectively (P<0.01) in young women with mildly abnormal cytology (ASC -US and LSIL). Yet, with severe abnormal cytology (ASC-H and HSIL), HR-HPV test was similar to HPV E6/E7 mRNA test in sensitivity(x2=0.98, P=0.322), specificity (x2=0.938, P=0.333), PPV(x2=0.074, P=0.786) and NPV (x2=0.00, P=1.000).
Conclusion: Compared to the HPV DNA test, the HPV E6/E7 mRNA test has better clinical value in screening cervical cancer and predicting the risk of HSIL+ in young women, especially those with mild abnormal cytology.
Keywords: E6/E7 mRNA testing; HPV DNA test; Cervical cancer;Cytology; Immune system; Mental disorders
Abbreviations
HPV: Human Papilloma Virus; NILM: Negative for Intraepithelial Lesions or Malignancy; ASC-US: Atypical Squamous Cell of Undetermined Significance; ASC-H: Atypical Squamous Cells cannot exclude high-grade squamous intraepithelial lesion; LSIL: Lowgrade Squamous Intraepithelial Lesion; HSIL: High-grade Squamous Intraepithelial Lesion; PPV: Positive Predictive Value; NPV: Negative Predictive Value
Introduction
According to an epidemiological survey, most young women (age< 30 years) tend to experience transient HPV infection. However, due to young women’s strong autoimmunity to eliminate the HPV virus, they often have no obvious clinical symptoms. Although cytological screening is still the main method for cervical screening in the clinic setting, it has no uniform standard, and it is vulnerable to subjective factors. Therefore, in some cases, its repeatability and accuracy are poor. HPV DNA test has high sensitivity and high NPV, which can make up for the short comings of cytology; however, its specificity and PPV are low [1-4].Therefore, it is particularly important to explore method with high specificity and good PPV in order to provide accurate and reliable detection for young women with persistent HPV infection.
Current studies have confirmed that the definite cause of cervical cancer is a persistent infection of HR-HPV. The replication of HR-HPV is directly related to the differentiation of cervical epithelial cells. In the process of HPV infection, the viral genome is integrated into the human genome, in which oncogenes E6 and E7 bind to and regulate cell gene products, alter cell growth cycle and DNA repair, resulting in genomic instability and leading to cell immortalization. The oncogenes E6 and E7 are expressed in cells with later stages of differentiation on the surface and intermediate layers. Therefore, the integration of E6 and E7 genes on the host chromosome caused by persistent infection is necessary for the malignant transformation of certain epicells. The detection of HPV E6/E7 gene can be used to identify the risk of persistent HPV infection and cervical lesions in women [5,6]. HPV E6/ E7 mRNA test has been proposed as a biomarker for HPV oncogene expression. Previous studies have shown that the HPV E6/E7 mRNA test has a very good clinical sensitivity and higher specificity compared to the HPV DNA test [7,8].Therefore, HPV E6/E7 gene detection has been researched as a critical clinical auxiliary diagnostic technology by European Genital Infection and Tumor Research Institutions in recent years [9].
A prospective study revealed that E6/E7 mRNA–positive young women (25-34 years old) had a high incidence of CIN Ⅱ lesions,which suggested that HPV E6/E7 mRNA is valuable for cervical cancer screening in young women [10]. The aim of this study was to examine the application of HPV E6/E7 mRNA test in the diagnosis of cervical lesions in young women under 30 years old with different cytological grades.
Materials and Methods
Study population
This diagnostic accuracy study conforms to the ethical standards and has been approved by the Medical Ethics Association of Quzhou Hospital Affiliated to Wenzhou Medical University. The study population consisted of women who attended an outpatient gynecological screening at the gynecology department of Quzhou Hospital Affiliated to Wenzhou Medical University between January 2015 and December 2019. Women meeting these conditions were enrolled in our study:(1) TCT results higher than ASCUS (ASCUS+);(2) 20-29 years old;(3) sexual life history >3 years; (4) patients agreed to receive the treatment and signed the informed consents. Exclusion criteria: patients with tumors, blood system, immune system, and mental disorders; patients with severe heart, liver, kidney, and other organ dysfunction; those who cannot cooperate with the treatment or follow-up on time.
All women underwent the following procedures: HR-HPV DNA assay, HPV E6/E7 mRNA test, colposcopy biopsy, and histopathological examination.
TCT test
We removed the mucus and secretions from the cervical surface, used a disposable cervical brush to penetrate the cervical canal, rotated 5 to 10 times in the same direction, and put the cervical brush, from which the cells were brushed off into a bottle containing cell preservation solution for rinsing. The TCT results were classified according to the 2001 Bethesda System (TBS): as Negative for Intraepithelial Lesions or Malignancy(NILM), Atypical Squamous Cell Of Undetermined Significance (ASC-US), Atypical Squamous Cells cannot exclude High-grade squamous intraepithelial lesion(ASC-H), the Low-Grade Squamous Intraepithelial Lesion (LSIL), the High- Grade Squamous Intraepithelial Lesion (HSIL), Squamous Cell Carcinoma (SCC), atypical glandular cells with undefined significance, and Adenocarcinoma (AC). All TCT were independently viewed and diagnosed by two senior cytology doctors. In this study, results higher than ASCUS (ASCUS+) were regarded as positive.
HR-HPV DNA test
Materials and reagents for Cervista HPV detection technology are from Hologic Company, USA. The sample collection method was the same as for the TCT test. The results for HPV DNA assay were divided into A5/A6 group (including HPV51, 56 and 66); A7 group (including HPV 18, 39, 45, 59 and 68) and A9 group (including HPV 16, 31, 33, 35, 52 and 58) according to genetic relationship. DNA extraction and cervical detection were carried out according to the instructions of the kit.
HPV E6/E7 mRNA test
The sample collection method was the same as for TCT and HR- HPV DNA detection. We used the QuantiVirus HPV E6/E7 mRNA assay (Kodia, Xinxiang, China) to detect the 14 high-risk types of E6/ E7 mRNA, namely, HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58,59, 66, and 68, according to the manufacturer’s instructions. The assay used a nucleic acid hybridization procedure based on branched-chain DNA hybridization technology. E6/E7 mRNA was quantitatively detected by five key steps: sample splitting decomposition, making and testing buffer, board distribution, signal amplification, and reading board. In each sample, the result of E6/E7 mRNA was marked as a light unit. Special data calculation software can convert the light unit to copy number under the fact of light emission directly related to the amount of HPV mRNA. If the copy number was equal or greater than 1.0, the result of E6/E7 mRNA was positive. If it was less than 1.0, the result was negative [11].
Colposcopy and histological diagnosis
After wiping off cervical secretions, acetic acid white and iodine test were performed, respectively. Three, six, nine, and twelve points were routinely taken from patients with negative acetic acid white and iodine test, while those with abnormal acetic acid white and positive iodine test had a multi-point biopsy in abnormal areas. The tissue specimens, which were fixed by formalin and embedded by paraffin, were stained with H and E for histopathological examination. Histological diagnostic criteria refer to the classification system of female genital tumors revised by WHO in 2014[12]. All specimens were independently examined and diagnosed by two senior pathologists. In this study, we set pathological results higher than HSIL ((including CIN Ⅱ, CINⅡ+) as pathological positive, and the results lower than LSIL (including CINⅡ, CINⅡ−) were considered negative.
Statistical analysis
SPSS 19.0 statistical software was used for statistical analysis. The chi-square test was used to compare the counting data between groups. All reported P-values were two-sided, and P-value of <0.05 was considered statistically significant.
Results
A total of 258 women with a median age of 25 ± 1.3 years who met the inclusion criteria were consecutively included in this study. Among 258 cases, there were 95 (36.8%), 73 (28.3%), 21 (8.1%), and 69 (26.7%) ASCUS, LSIL, ASC-H, and HSIL cases, respectively. Of the 220 (85.3%) women with positive HR-HPV results, 64 (29.1%) belonged to the A5/ A6 group, 57 (25.9%) to the A7 group, and 99 (45.0%) to the A9 group. The numbers of HPV E6/E7 mRNA positive and pathological positive were 159 (61.6%) and 89 (34.5%), respectively.
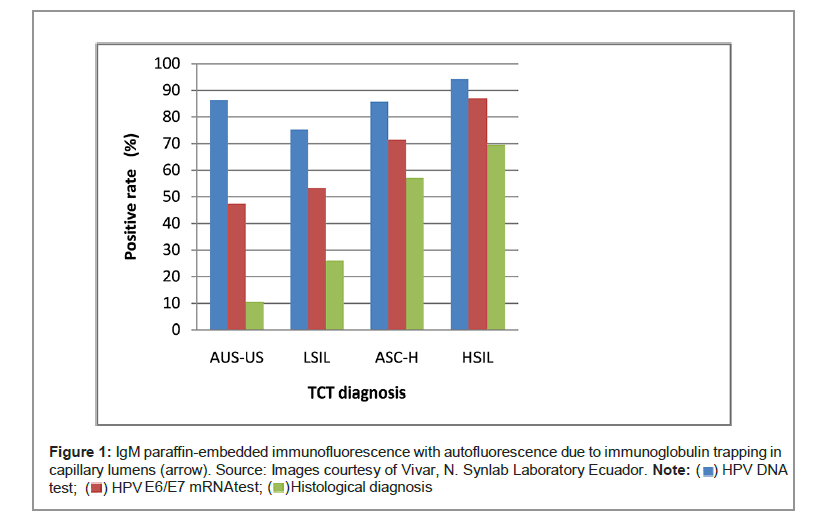
The positive rate of HPV DNA, HPV E6/E7 mRNA, and histological diagnosis in different TCT grade: The positive rates of HPV DNA test in different TCT grade were 86.3% (82/95) for ASC-US, 75.3% (55/73) for LSIL, 85.7% (18/21) for ASC-H, and 94.2% (65/69) for HSIL. HPV E6/ E7 mRNA test revealed 47.4% (45/95) positive rate for ASC-US, 53.4% (39/73) positive rate for LSIL, 71.4% (15/21) positive rate for ASC-H, and 87.0% (60/69) positive rate for HSIL. For histological diagnosis, 10.5% (10/95) positive rates for ASC-US, 26.0% positive rates (19/73) for LSIL, 57.1% positive rates (12/21) for ASC-H, and 69.6% positive rates for (48/69) HSIL were found. The positive rate of HPV E6/E7 mRNA test and histological diagnosis increased along with an increase in TCT grade (Figure 1).
Screening results of HR-HPV and E6/E7 mRNA in young women: First, we analyzed the performances of the HR-HPV and HPV E6/ E7 mRNA in all young women (summarized in Table 1). HPV E6/E7 mRNA test is similar to HR-HPV test with sensitivity (78.1% vs. 87.6%, x2=2.57, P=0.160), PPV (44.0% vs. 35.5 %, x2=2.85, P=0.109) and NPV (80.8% vs. 71.1%, x2=1.53, P=0.251) and there was no significant difference between the two methods. However, the specificity of HPV E6/E7 mRNA was significantly higher than that of HPV DNA (47.3% vs. 16.0%, x2=38.41, P=0.000).
| N | HR-HPV DNA Positive n (%) | HPV E6/E7 mRNA Positive n (%) | |
|---|---|---|---|
| Histological diagnosis positive | 89 | 78(87.6%) | 70(78.7%) |
| Histological diagnosis negative | 169 | 142(84.0%) | 89(52.7%) |
| Sensitivity | 87.6% | 78.7% | |
| (95% CI) | 78.6%-93.4% | 68.4%-86.3% | |
| Specificity | 16.0% | 47.3%* | |
| (95% CI) | 11.0%-22.6% | 40.0%-55.1% | |
| PPV | 35.5% | 44.0% | |
| (95% CI) | 29.2%-42.2% | 36.2%-52.1% | |
| NPV | 71.1% | 80.8% | |
| (95% CI) | 53.9%-84.0% | 71.4%-87.8% |
Table 1: Comparison between two methods in relation to histopathological diagnosis in young women histological diagnosis higher than HSIL ((including CIN II, CIN II+) as histological diagnosis positive and the results lower than LSIL (including CIN I, CIN−) were considered negative. ‡PPV=Positive Predictive Value, NPV=Negative Predictive Value. §*Compared with the specificity of HPV DNA, P<0.01.
Screening results of HR-HPV DNA and E6/E7 mRNA in young women with mildly abnormal cytology: We further analyzed the performances of the HR-HPV DNA and HPV E6/E7 mRNA in young women with mildly abnormal cytology (ASC-US and LSIL) (Table 2). Compared with HR-HPV DNA, the HPV E6/E7 mRNA method presented high rates of specificity (x2=162.69, P=0.000), PPV(x2=30.55, P=0.000), and NPV (x2=10.89, P=0.003), namely 92.1%, 62.1%, 92.1% in HPV E6/E7 mRNA and 15.8%, 14.6%, 71.0% in HR-HPV DNA, respectively. HPV E6/E7 mRNA was similar to HR-HPV DNA with sensitivity (62.1% vs. 69.0%, x2=0.31, P=0.783).
| N | HR-HPV DNA Positive n(%) | HPV E6/E7 mRNA Positive n(%) | |
|---|---|---|---|
| Histological diagnosis Positive | 29 | 20(69.0%) | 18(62.1%) |
| Histological diagnosis Negative | 13 | 117(84.2%) | 11(7.9%) |
| Sensitivity | 9 | 69.0% | 62.1% |
| (95%CI) | 49.0%-84.0% | 42.4%-78.7% | |
| Specificity | 15.8% | 92.1%* | |
| (95%CI) | 10.4%-23.2% | 86.0%-96.0% | |
| PPV | 14.6% | 62.1%** | |
| (95%CI) | 9.4%-21.9% | 42.4%-78.7% | |
| NPV | 71.0% | 92.1%*** | |
| (95%CI) | 51.8%-85.1% | 85.9%-95.8% |
Table 2: Comparison between two methods in young women with mildly abnormal cytology histological diagnosishigher than HSIL ((including CIN II, CIN II+) as histological diagnosis positive and the results lower than LSIL (including CIN I, CIN−) were considered negative. ‡PPV= Positive Predictive Value, NPV=Negative Predictive Value. §* Compared with the specificity of HR-HPV, P<0.01. **Compared with the PPV of HR-HPV, P<0.01. *** Compared with the NPV of HR-HPV, P<0.01.
Screening results of HR-HPV DNA and E6/E7 mRNA in young women with severe abnormal cytology: We analyzed the performances of the HR-HPV DNA and HPV E6/E7 mRNA in young women with severe abnormal cytology (ASC-H and HSIL) (Table 3). HPV E6/ E7 mRNA test is similar to HR-HPV DNA assay with sensitivity (88.3%vs.95.0%, x2=0.98, P=0.322), specificity (26.7%vs.13.3%, x2=0.938, P=0.333), PPV (70.7%vs.68.7%, x2=0.074, P=0.786) and NPV (53.3%vs.57.1%, x2=0.00, P=1.000) and there was no significant difference between the two methods.
| N | HR-HPV DNA Positive n (%) | HPV E6/E7 mRNA Positive n (%) | |
|---|---|---|---|
| Histological diagnosis Positive | 60 | 57(95.0%) | 53(88.3%) |
| Histological diagnosis Negative | 30 | 26(86.7%) | 22(73.3%) |
| Sensitivity | 95.0% | 88.3% | |
| (95% CI) | 85.2%-98.7% | 76.8%-97.8% | |
| Specificity | 13.3% | 26.7% | |
| (95% CI) | 4.4%-31.6% | 13.0%-46.2% | |
| PPV | 68.7% | 70.7% | |
| (95% CI) | 57.4%-78.2% | 58.9%-80.3% | |
| NPV | 57.1% | 53.3% | |
| (95% CI) | 20.2%-88.2% | 27.4%-77.7% |
Table 3: Comparison between two methods in young women with severe abnormal cytology histological diagnosis higher than HSIL ((including CIN II, CIN II+) as histological diagnosis positive and the results lower than LSIL (including CIN I, CIN−) were considered negative. ‡PPV= Positive Predictive Value, NPV=Negative Predictive Value.
Discussion
HPV E6/E7 mRNA is a specific substance. E6 and E7 genes in the early coding region of HPV are viral oncogenes, which are not normally expressed. However, if viral DNA is integrated into the host cell DNA, E6 and E7 genes will be highly expressed and uncontrolled, thus causing cervical cancer. The transcription products E6/E7 mRNA of E6 and E7 genes can reflect the activity of oncogenes. Therefore, the detection of E6/E7 mRNA can avoid repeated HPV detection and judge whether HR-HPV is persistent infection, thus reducing the confusion of doctors and patients with transient infection. Yet, the HPV DNA test can only indicate the carrying status of the virus.
HPV E6/E7 mRNA test is widely used in diagnosing cervical diseases and has obvious advantages compared with HPV DNA assay. HPV E6 / E7 mRNA test can detect E6 / E7 mRNA of 14 kinds of high- risk HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68), which is of high value for the diagnosis and postoperative follow-up of high- level CIN, and can reduce unnecessary treatment [13-15]. HPV E6/E7 expression, which was only present in CIN Ⅰ-Ⅲ diagnosed patients, is a robust indicator of cytological atypical that is better correlated with progressive lesions than HPV DNA assay [16]. A prospective study revealed that women positive on the HPV E6/E7 mRNA test have a greater risk of cervical lesions’ malignant progression and deserve greater attention and earlier check-ups [17].
There are many studies on the performance of HPV E6/E7 mRNA test in diagnosing cervical diseases. Some reports have suggested that HPV E6/E7 mRNA test is more sensitive and specific than the HPV NDA test [18-20]. It has also been suggested that the HPV E6/E7 mRNA test is only more specific than the HPV DNA test, having no difference in sensitivity [21]. Nonetheless, some studies suggest that HPV E6 / E7 mRNA test is more sensitive for the diagnosis of high-level CIN [22]. The research results of each scholar are different due to the different research backgrounds, sample, and research methods.
In this study, the positive rate of HPV DNA assay was 85.3%, which was much higher than that of the HPV E6/E7 mRNA test (61.6%).This relatively high percentage of HPV DNA infection in cases with positive cervical cytology is consistent with some previously published studies [18,19,23]. There was an association between E6/E7 mRNA expression and histology (OR=106.12) [24]. Data analysis from our study showed an association between the results of HPV E6/E7 mRNA, histological diagnosis, and the TCT grade. The positive rate of HPV E6/E7 mRNA and histological diagnosis increased with the upgrade of TCT. However, there was no significant correlation between HPV DNA positive rate and TCT grade.
The performances of the HPV DNA assay and HPV E6/E7 mRNA test were analyzed in young women. HPV DNA was similar to HPV E6/ E7 mRNA in sensitivity, PPV, and NPV, while there was no significant difference between the two methods. Still, the specificity of HPV E6/ E7 mRNA (47.3%) was significantly higher than that of HPV DNA (16.0%) (P<0.01). In the study of Munkhdelgeret al that included 188 women with the squamous intraepithelial cervical lesion, HPV E6/E7 mRNA testing was more specific than HPV DNA assay (85.00% versus 40.83%) [25]. In their study, Drage Dabeski et al. screened a group of 128 sexually active women aged 20 to 59 years with squamous cell abnormalities on the cervical cytology, reporting a greater specificity (88.89%) and greater positive predictive value (93.59%) of HPV E6/ E7 mRNA test [23]. The specificity of the HPV E6/E7 mRNA test in our study was significantly lower than in the above studies, probably because of the differences in age and number of cases.
Benevolo et al. suggest that HPV E6/E7 mRNA can serve as a better triage test than HPV DNA to reduce colposcopy referral in both ASC- US and LSIL because of being more specific than HPV DNA assay (82% in ASC-US, 76% in LSIL, versus 29% in ASC-US,13% in LSIL)[26].
Zhuetal pointed out that HPV E6/E7 mRNA testing could be used as a valuable test in such a setting with higher specificity for the detection of high-grade cervical neoplasia compared to HPV DNA detection without losing sensitivity in women with ASCUS [21]. Based on the above research, we further analyzed the performances of the HPV DNA assay and HPV E6/E7 mRNA test in young women with mildly abnormal cytology (ASU-US and LSIL). The HPV E6/E7 mRNA test presented high rates of specificity, PPV, and NPV, which were 92.1%, 62.1%, 92.1%, respectively, compared to that of HPV DNA, which were 15.8%, 14.6%, 71.0%,respectively (P<0.01).Yet, with severe abnormal cytology(ASC-H and HSIL), HR-HPV DNA assay is similar to HPV E6/ E7 mRNA testing in sensitivity (x2=0.98, P=0.322),specificity (x2=0.938, P=0.333), PPV(x2=0.074, P=0.786) and NPV (x2=0.00, P=1.000); and there was no significant difference between the two methods. Our results are similar to the above results. Still, in addition to specificity, the PPV and NPV were higher than that of HPV DNA, which further highlighted the advantage of HPV E6/E7 mRNA in young women with mildly abnormal cytology.
The present study has a few limitations. None of the women in this study had a diagnosis of SCC by TCT, and there were a limited number of women with severe abnormal cytology as well as HPV DNA positive. Therefore, future studies with a larger sample size are warranted.
Conclusion
The results of our study suggested that the HPV E6/E7 mRNA test was more specific than HPV DNA assay in young women and that the HPV E6/E7 mRNA test presented high rates of specificity, PPV, and NPV compared to HPV DNA assay, especially in those with mild abnormal cytology. Based on the above-reported data, we believe that HPV E6 /E7 mRNA testing has significant clinical value in screening cervical cancer and predicting the risk of HSIL+ in young women, especially those with mild abnormal cytology.
Author’s contributions
XX collected cases material; JW analyzed and interpreted the patient data and was a major contributor in writing the manuscript. All authors have read and approved the manuscript
Acknowledgements
Not applicable
Conflict of interest statement
The authors declare that they have no competing interests.
Funding sources
There was no financial support
Statement of Ethics
This study conforms to the ethical standards and has been approved by the Medical Ethics Association of Quzhou Hospital Affiliated to Wenzhou Medical University and written informed consent was obtained prior to the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Berkowitz RP (2013) 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 122: 393.
[Crossref] [Google Scholar] [Pubmed]
- Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M et al. (2012) Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 30: F88-99.
[Crossref] [Google Scholar] [Pubmed]
- Zou R, Xie W, Wang H, Wang J, Xiao L, et al. (2016) Establishment and Application of a Method for High-Risk Human Papillomavirus Genotyping in Cervical Cancer Tissue. Clin Lab 62:1075-1085.
[Crossref] [Google Scholar] [Pubmed]
- Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, et al. (2008)Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine.26:K29-K41.
[Crossref] [Google Scholar] [Pubmed]
- Cattani P, Siddu A, D'Onghia S, Marchetti S, Santangelo R, Vellone VG, et al. (2009) RNA (E6 and E7) assays versus DNA (E6 and E7) assays for risk evaluation for women infected with human papillomavirus. J Clin Microbiol. 47:2136-2141.
[Crossref] [Google Scholar] [Pubmed]
- Carter JR, Ding Z, Rose BR (2011) HPV infection and cervical disease: a review. Aust N Z J Obstet Gynaecol 512:103-108.
[Crossref] [Google Scholar] [Pubmed]
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, et al. (2009) Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol. 45:S55-S61.
[Crossref] [Google Scholar] [Pubmed]
- Szarewski A, Ambroisine L, Cadman L, Austin J, Ho L, et al. (2008) Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol Biomarkers Prev 17:3033-3042.
[Crossref] [Google Scholar] [Pubmed]
- Cuschieri K, Wentzensen N (2008) Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev.17:2536-2545.
[Crossref] [Google Scholar] [Pubmed]
- Granados R, Tellez-Safina H, Solis I, Mateos F, Rodriguez-Barbero JM, et al. (2017) Cervical cancer screening cotesting with cytology and MRNA HPV E6/E7 yields high rates of CIN2+ lesions in young women. Diagn Cytopathol 45:1065-1072.
[Crossref] [Google Scholar] [Pubmed]
- Liu TY, Xie R, Luo L, Reilly KH, He C, et al. (2014) Diagnostic validity of human papillomavirus E6/E7 mRNA test in cervical cytological samples. J Virol Methods. 196:120-125.
[Crossref] [Google Scholar] [Pubmed]
- Kurman RJ, Carcangiu ML, Herrington CS (2014) World Health Organisation Classification of Tumours of the Female Reproductive Organs: International Agency for Research on Cancer.
- Origoni M, Cristoforoni P, Carminati G, Stefani C, Costa S, et al. (2015) E6/E7 mRNA testing for human papilloma virus-induced high-grade cervical intraepithelial disease (CIN2/CIN3): a promising perspective. Ecancermedicalscience 9:533.
[Crossref] [Google Scholar] [Pubmed]
- Ho CM, Pan KY, Chen YY, Huang CY, Chen YL, et al. (2015) Clinical performance of multiplex high-risk e6 mrna expression in comparison with hpv dna subtypes for the identification of women at risk of cervical cancer. J Med Virol 87:1404-1412.
[Crossref] [Google Scholar] [Pubmed]
- Duvlis S, Popovska-Jankovic K, Arsova ZS, Memeti S, Popeska Z, et al. (2015) HPV E6/E7 mRNA versus HPV DNA biomarker in cervical cancer screening of a group of Macedonian women. J Med Virol. 87:1578-1586.
[Crossref] [Google Scholar] [Pubmed]
- Tüney İ, Altay A, Ergünay K, Önder S, Usubütün A, et al. (2017) HPV types and E6/E7 mRNA expression in cervical samples from Turkish women with abnormal cytology in Ankara, Turkey. Turk J Med Sci. 47:194-200.
[Crossref] [Google Scholar] [Pubmed]
- Bruno MT, Ferrara M, Fava V, Barrasso G, Panella MM (2018) A prospective study of women with ASCUS or LSIL pap smears at baseline and HPV E6/E7 mRNA positive: a 3-year follow-up. Epidemiol Infect. 146:612-618.
[Crossref] [Google Scholar] [Pubmed]
- Pan D, Zhang CQ, Liang QL, Hong XC (2019) An efficient method that combines the ThinPrep cytologic test with E6/E7 mRNA testing for cervical cancer screening. Cancer Manag Res 11:4773-4780.
[Crossref] [Google Scholar] [Pubmed]
- Wang HY, Lee D, Park S, Kim G, Kim S, et al. (2015) Diagnostic Performance of HPV E6/E7 mRNA and HPV DNA Assays for the Detection and Screening of Oncogenic Human Papillomavirus Infection among Woman with Cervical Lesions in China. Asian Pac J Cancer Prev. 16:7633-7640.
[Crossref] [Google Scholar] [Pubmed]
- Pruski D, Millert-Kalinska S, Lewek A, Kedzia W (2019) Sensitivity and specificity of HR HPV E6/E7 mRNA test in detecting cervical squamous intraepithelial lesion and cervical cancer. Ginekol Pol.90:66-71.
[Crossref] [Google Scholar] [Pubmed]
- Zhu Y, Ren C, Yang L, Zhang X, Liu L (2019) Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS. BMC Cancer. 19:271.
[Crossref] [Google Scholar] [Pubmed]
- Pierry D, Weiss G, Lack B, Chen V, Fusco J (2012) Intracellular human papillomavirus E6, E7 mRNA quantification predicts CIN 2+ in cervical biopsies better than Papanicolaou screening for women regardless of age. Arch Pathol Lab Med. 136:956-960.
[Crossref] [Google Scholar] [Pubmed]
- Dabeski D, Duvlis S, Basheska N, Antovska V, Stojovski M, et al. (2019) Comparison Between HPV DNA Testing and HPV E6/E7 MRNA Testing in Women with Squamous Cell Abnormalities of the Uterine Cervix. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 40:51-58.
[Crossref] [Google Scholar] [Pubmed]
- Bruno MT, Ferrara M, Fava V, Rapisarda A, Coco A et al. (2018) HPV genotype determination and E6/E7 mRNA detection for management of HPV positive women. Virol J 15:52.
[Crossref] [Google Scholar] [Pubmed]
- Munkhdelger J, Choi Y, Lee D, Kim S, Kim G, et al. (2014) Comparison of the performance of the NucliSENS EasyQ HPV E6/E7 mRNA assay and HPV DNA chip for testing squamous cell lesions of the uterine cervix. Diagn Microbiol Infect Dis. 79:422-427.
[Crossref] [Google Scholar] [Pubmed]
- Benevolo M, Vocaturo A, Caraceni D, French D, Rosini S, et al. (2011) Sensitivity, specificity, and clinical value of human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for cervical cytology and HPV DNA test. J Clin Microbiol 49:2643-2650.
[Crossref] [Google Scholar] [Pubmed]
Citation: Wang J, Xu X, (2022) The Diagnostic Value of HPV E6/E7 mRNA Test in Young Women with Cervical Squamous Intraepithelial Lesion. Diagnos Pathol Open S1: 002. DOI: 10.4172/2476-2024.S10.1000002
Copyright: © 2022 Wang J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 2083
- [From(publication date): 0-2022 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1587
- PDF downloads: 496