The Contributory Mechanism of Alteration in Prorenin-Renin Homeostasis in the Pathogenesis of Diabetic Nephropathy
Received: 15-Jul-2022 / Manuscript No. cmb-22-69269 / Editor assigned: 16-Jul-2022 / PreQC No. cmb-22-69269(PQ) / Reviewed: 29-Jul-2022 / QC No. cmb- 22-69269 / Revised: 01-Sep-2022 / Manuscript No. cmb-22-69269(R) / Accepted Date: 08-Sep-2022 / Published Date: 08-Sep-2022 DOI: 10.4172/1165-158X.1000242
Abstract
Background: Diabetic nephropathy is part of the microvascular complication of diabetes mellitus along side neuropathy and retinopathy. Many mechanism has been presented as the pathophysiology of diabetic nephropathy but this could actually be attributed to the renin angiotensin system. The alteration in prorenin and renin homeostasis has been reported in patients with diabetes mellitus, it’s noticed to reduction in conversion of prorenin to renin there by leading to accumulation of prorenin binding to (pro)renin.
Aim: This article is targeted at explaining the contributory roles of the alteration in the prorenin and renin homeostasis in the pathogenesis of diabetic nephropathy and to explain why some the drugs that act along renin angiotensin pathways especially angiotensin receptor blockers can be very helpful in the management of diabetic nephropathy.
Methods: A careful literature search was made on some scientific databases such as PubMed, EMBase, Google Scholar, Research Gate and others using a very sensitive search strategy on researches that are related to effects of renin-angiotensin pathway on diabetic nephropathy focusing mainly on the use Handle Receptor Protein (HRP) and Angiotensin Receptor Blocker (ARB) in the management of diabetic nephropathy.
Results: The review of different articles shows HRP especially when use alongside Angiotensinogen Converting Enzyme Inhibitor (ACEI) increases plasma renin activities, reduces progression and development of glomeruli sclerosis, tubular injury, podocyte injury and proteinuria. Also, ARBs such as losartan and irbesartan was found to reduce relative risk for primary composite endpoint death of nephrons, serum creatinine level, and progression to endstage renal disease.
Conclusion: Binding of prorenin which accumulate in diabetic mellitus to (pro)renin receptor causes non-proteolytic activation at higher concentration and also release of profibrotic molecules which are injurious to the nephrons . This can be inhibited by the use of Handle Region Peptide (HRP) which is a competitive antagonist of prorenin or use of angiotensin receptor blocker which aid binding of angiotensin II to AT2 receptor leading to vasodilatory, antiinflammatory anti-proliferative, antihypertrophic and antifibrotic effects which antagonize those produced by prorenin.
Keywords
Diabetic Nephropathy; Prorenin; Renin; Handle Region Peptide (HRP); Angiotensin Receptor Blocker (ARB)
Introduction
The microvascular complications of diabetes mellitus include retinopathy, neuropathy and nephropathy; some of this complications has been linked to biochemical alteration. Diabetic Nephropathy is the major cause of end-stage renal disease and also leading cause of diabetes mellitus-related morbidity and mortality worldwide.
Diabetic nephropathy is characterized by hypertrophy of glomeruli, glomerular hyper-filtration, microalbuminuria, progressive glomerulosclerosis and tubulointestitial fibrosis leading reduction of GFR [1].
Renin is a protease enzyme that convert angiotensinogen to angiotensin I, its produced as prorenin in the renal tubules. The prorenin is converted into active renin molecule, the body maintains balance between the prorenin and renin level. This homeostasis is found to be loss in patient with diabetes mellitus and this research looks into the possible roles of this in the pathogenesis of diabetic nephropathy by seeing the outcome of management of diabetic nephropathy target on restoring this homeostasis [2].
Alteration of Prorenin-Renin Homeostasis in Diabetes Mellitus
Many works have reported increase level of prorenin in patient with Diabetes Mellitus. The mechanism behind this increment has not been fully identified but some research as pointed out increase production of prorenin in some extra- renal sources such as vitreous humor in diabetic retinopathy has the cause of increase level of prorenin in patient with insulin-independent diabetes mellitus. Researches done by assaying the level of Antibody against Prorenin (ADD-PR) also reported the increase level of prorenin in diabetes and also linked this increase level to the development of diabetes retinopathy. Increase in prorenin level has been found to proceed manifestation of some microangiopathy such retinopathy and nephropathy in diabetes [3].
The increase in prorenin was found to be associated with reduction in active renin. One of the suggested mechanism for this by this review were that of Paul in which blockage of lysosomal glycosylation of prorenin reduces the transit time of conversation of prorenin to renin and also that of Chu, which reported not only increase in the rate but also increase in the amount of prorenin converted to renin by the blockage of lysosomal glycosylation. These actually suggested, presence of glucose moiety which are actually higher in diabetes; retarded the conversion of prorenin to renin thereby altering the homeostasis [4].
Researchers reported inhibitions of cathepsin B a proteolytic enzyme that catalyze cleavage of prosegment of prorenin to give active renin by high glucose concentration. This could also account for the increase in the level of prorenin seen in patient with diabetes [5].
Methodology
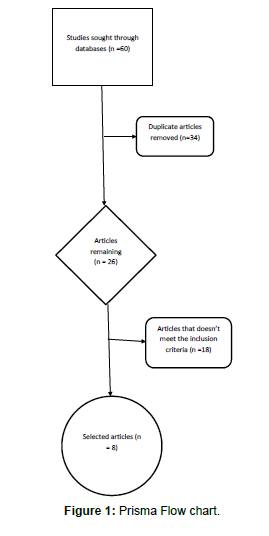
Literature search of articles on reports the therapeutic importance of lowering the level or effects of prorenin on diabetic nephropathy was made on different scientific databases; which about 60 articles were collected and about 52 were exclude because they don’t meet the inclusion criteria. Most of this articles are related directly to prorenin like articles which focus on the effects of Handle Region Peptide (HRP) which is an inhibitor of prorenin or indirectly like those articles that looked into effects of Angiotensin Receptor Blocker (ARB) on diabetic nephropathy. Inclusion criteria include that article is of statistically significance and shows the correlation of prorenin to diabetic nephropathy and that the article must be related to prorenin or reninangiotensin system and also the methodology of the research must be involved mechanism in this path not in combination with others. Some of the articles were discarded because they were duplicate of others or had similar findings to other selected articles [6].
The articles were reviewed under two categories, which are:
• Effects of HRP on diabetic nephropathy
• Effects of ARB on diabetic nephropathy
The Prisma flow chart showing the analysis of processes of articles selection is shown below (Figure 1).
Results
A total of 60 articles were retrieved from different databases and only 8 articles were eventually used in this studies.
Studies on Effects of Handle Region Peptide (HRP) on Diabetic Nephropathy
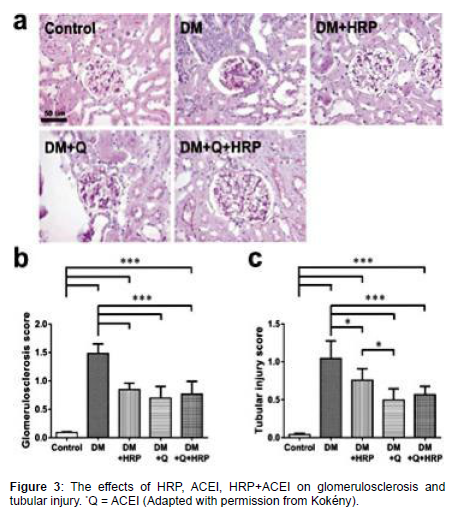
Kökény compared the effects of HRP, ACE inhibitor (Quinapril) and both on diabetic nephropathy in rats, it was observed that none has effects on renal hypertrophy, HRP has no effect on proteinuria but ACEI does and also ACEI + HRP does and Summary of their observation are recorded in the Table 1 below.
| Effects/ Parameters |
HRP | ACEI | HRP + ACEI |
|---|---|---|---|
| Renal Hypertrophy | No effect | No effect | No effect |
| Proteinuria | No effect | Reduction | Reduction |
| Glomerulosclerosis | Reduction | Reduction | Reduction (not addictive) |
| Tubular Injury | Mild effect | Better effect | Better effect |
| Plasma Renin Activity | Increase | Markedly increase | Markedly increase |
| Glomerular TGF Expression | Slightly reduced | Reduced | Markedly Reduced |
| Tubular TGF Expression | No effect | Reduced | Markedly Reduced |
| Renal MMP Expression | No effect | Restored function | Restored function |
| Renal TIMP Expression | Reduced | Reduced | Reduced (additive) |
| ERK(1/2) Phosphorylation | No effect | Reduced | Reduced |
| Podocyte Injury | Reduced | Reduced | Reduced |
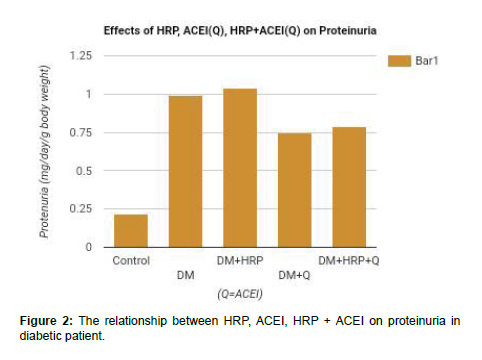
The quantitative analysis of the of HRP, ACEI and HRP + ACEI on proteinuria and protein: Creatinine ratio deducted from a study are reported in the Table 2 and Figures 2 & 3 below.
| Proteinuria (mg/day/g body weight) | Protein/ Creatinine (mg/mg) | |
|---|---|---|
| Control | 0.22 ± 0.03 | 6.4 ± 0.6 |
| Diabetic | 0.99 ± 0.23 | 51.3 ± 0.8 |
| Diabetic + HRP | 1.04 ± 0.1 | 54.7 ± 12.5 |
| Diabetic + ACEI | 0.75 ± 0.12 | 31.4 ± 10.3 |
| Diabetic +HRP+ ACEI | 0.79 ± 0.11 | 38.7 ± 6.5 |
Studies on Effects of Angiotensin II Receptor Blocker (ARB) on Diabetic Nephropathy
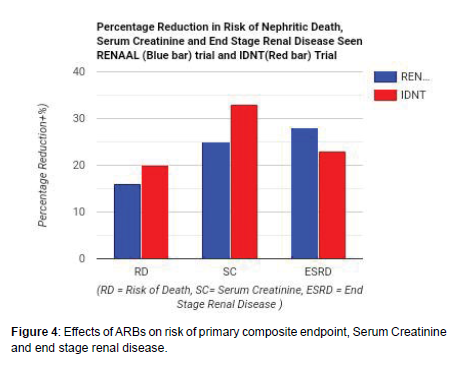
Three trials done on the effects of Angiotensin II Receptor Blocker (ARB) were analyzed. In a trial, the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) which was done on 1513 subjects reveals the effectiveness of losartan in reducing the relative risk of primary composite end-point of nephritic death, serum creatinine, end stage renal disease and blood pressure regulation. Similarly, in the Irbesartan Diabetic Nephropathy Trial (IDNT) which was done on 1715 subjects with type II diabetes reveals the effectiveness of irbesartan in reducing relative risk of primary composite end point nephritic death, serum creatinine level, end stage renal disease and blood pressure regulation. The Irbesartan Microalbuminuria in hypertensive patients with type 2 diabetes (IRMA 2) study, irbesartan was noticed to causes persistent microalbuminuria with normal renal function and reduces the risk of diabetic nephropathy development by 39% (p < 0.001) when compared with placebo [7-10].
The results of both RENAAL and IDNT trials were analyzed and reported in the Tables and Figures below.
Discussion
As mentioned earlier, there’s distortion in the prorenin-renin homeostasis in diabetic mellitus, this distortion causes prorenin accumulation at the expense of renin thereby facilitating binding of prorenin to (pro)renin receptor [11].
The binding of prorenin to (pro)renin receptor causes nonproteolytic activation of the receptor leading to increase expression of profibrotic molecules which leads to glomerulosclerosis. This could serve as explanation to the reduction in glomerulosclerosis seen with usage of HRP in Table 1.
Also, activation of (pro)renin receptor by prorenin causes activation of Mitogen Activated Protein (MAP) Kinase and Extracellular Signal-Regulated Kinase (ERK)1/2. ERK(1/2) activation causes upregulation of transforming growth factor β1 gene expression, subsequently upregulating the genes coding for profibrotic molecules, such as plasminogen-activator inhibitor-1, fibronectin and collagens, and inducting the mesangial cell proliferation [12, 13].
Blockage of binding of angiotensin II to AT1 receptor by ARB makes it available for binding receptor which produces anti-inflammatory, antifibrotic, anti-proliferative, antihypertrophic and vasodilatory effects. The antifibrotic effects antagonize the effects of those profibrotic molecules activated by prorenin (Table 3 and Figure 4).
| Studies | Achieved Blood Pressure (mm Hg) |
Reduction in RR of primary composite end-point nephritic death Percentage p value | Reduction in Serum Creatinine Percentage p value | Reduction in End Stage Renal Disease Percentage p value | |||
|---|---|---|---|---|---|---|---|
| RENAAL (Losartan) [10] | < 140/90 | 16% | p < 0.02 | 25% | p < 0.006 | 28% | p < 0.02 |
| IDNT (Irbesartan) [11] | < 135/85 | 20% | p < 0.02 | 33% | p < 0.003 | 23% | p < 0.07 |
Table 3: Showing the effects of ARB on relative risk of nephritic death, Serum Creatinine level and end stage Renal disease.
Conclusion
The increase in profibrotic molecules as a result of non-proteolytic activation of (pro)renin receptor by prorenin as a contributory role in the pathogenesis of diabetic nephropathy and this gives explanation to the usefulness of Handle Region Peptide (HRP) which is a competitive antagonist in the management of diabetic nephropathy. Also this effect can be antagonized by the antifibrotic effects of Angiotensin Receptor Blocker (ARB) and selective AT2 agonist, Compound 21 (C21); hence their importance in the management of diabetic nephropathy [14, 15].
Acknowledgement
None
Conflict of Interest
None
References
- Mederos MA, Reber HA, Girgis MD (2021) Acute Pancreatitis: A Review. JAMA 325: 382-390.
- Konok GP, Thompson AG (1969) Pancreatic ductal mucosa as a protective barrier in the pathogenesis of pancreatitis. Am J Surg 117: 18-23.
- Dalbec KM, Max Schmidt C, Wade TE, Wang S, Swartz-Basile DA, et al. (2010) Adipokines and cytokines in human pancreatic juice: unraveling the local pancreatic inflammatory milieu. Dig Dis Sci 55: 2108-2112.
- Yuan X, Wu J, Guo X, Li W, Luo C, et al. (2021) Autophagy in Acute Pancreatitis: Organelle Interaction and microRNA Regulation. Oxid Med Cell Longev 2021: 8811935.
- Wang H, Li C, Jiang Y, Li H, Zhang D (2020) Effects of Bacterial Translocation and Autophagy on Acute Lung Injury Induced by Severe Acute Pancreatitis. Gastroenterol Res Pract 2020: 8953453.
- Yang H, Ma S, Guo Y, Cui D, Yao J (2019) Bidirectional Effects of Pyrrolidine Dithiocarbamate on Severe Acute Pancreatitis in a Rat Model. Dose Response 17: 1559325819825905.
- Kong L, Deng J, Zhou X, Cai B, Zhang B, et al. (2021) Sitagliptin activates the p62-Keap1-Nrf2 signalling pathway to alleviate oxidative stress and excessive autophagy in severe acute pancreatitis-related acute lung injury. Cell Death Dis 12: 928.
- Yue J, López JM (2020) Understanding MAPK Signaling Pathways in Apoptosis. Int J Mol Sci 21.
- Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, et al. (2013) Regulation of autophagy by stress-responsive transcription factors. Semin Cancer Biol 23: 310-322.
- Cao W, Li J, Yang K, Cao D (2021) An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer 108: 304-322.
- Zhang L, Chen Y, Wang L, Chen XP, Zhang WG, et al. (2013) Chloroquine relieves acute lung injury in rats with acute hemorrhagic necrotizing pancreatitis. J Huazhong Univ Sci Technolog Med Sci 33: 357-360.
- Wang X, Zhou G, Liu C, Wei R, Zhu S, et al. (2016) Acanthopanax versus 3-Methyladenine Ameliorates Sodium Taurocholate-Induced Severe Acute Pancreatitis by Inhibiting the Autophagic Pathway in Rats. Mediators Inflamm 8369704.
- Roux C, Lesueur C, Aligny C, Brasse-Lagnel C, Genty D, et al. (2014) 3-MA inhibits autophagy and favors long-term integration of grafted Gad67-GFP GAB Aergic precursors in the developing neocortex by preventing apoptosis. Cell Transplant 23: 1425-1450.
- Wang XX, Zhang B, Xia R, Jia QY (2020) Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci 24: 9601-9614.
- Barrera K, Stanek A, Okochi K, Niewiadomska Z, Mueller C, et al. (2018) Acinar cell injury induced by inadequate unfolded protein response in acute pancreatitis. World J Gastrointest Pathophysiol 9: 37-46.
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Citation: Alare K, Taiwo O, Akindele Z, Olaniyi P, Adeyemo R (2022) The Contributory Mechanism of Alteration in Prorenin-Renin Homeostasis in the Pathogenesis of Diabetic Nephropathy. Cell Mol Biol, 68: 242. DOI: 10.4172/1165-158X.1000242
Copyright: © 2022 Alare K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1447
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1126
- PDF downloads: 321