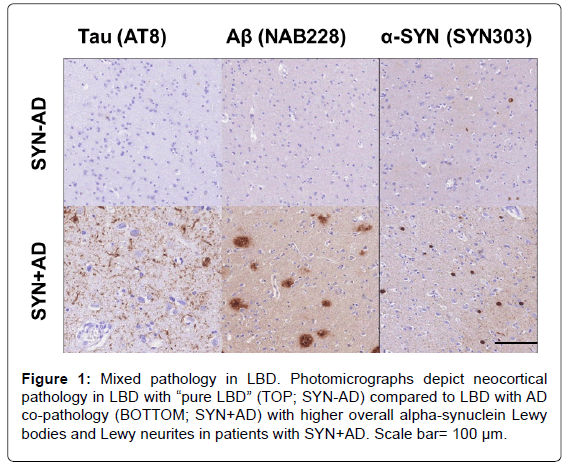
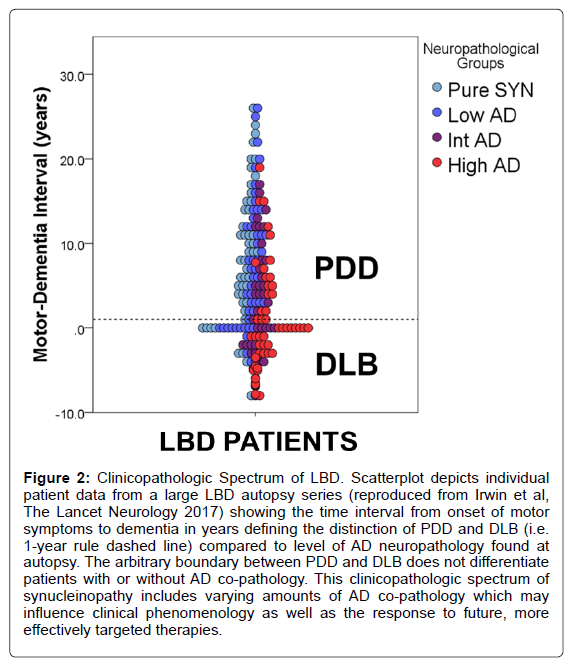
The Contribution of Tau, Amyloid-Beta and Alpha-Synuclein Pathology to Dementia in Lewy Body Disorders
Received: 25-Jul-2018 / Accepted Date: 03-Aug-2018 / Published Date: 10-Aug-2018 DOI: 10.4172/2161-0460.1000444
Keywords: Lewy body disorders; Parkinson disease; Alpha-synuclein; Amyloid plaques; Tau neurofibrillary tangles; Dementia
The Clinicopathological Spectrum of LBD and Overlap with AD
Parkinson’s Disease (PD) is a complex, progressive clinicopathological entity, clinically characterized by the variable presence of core extrapyramidal symptoms that include bradykinesia, tremor, postural instability and rigidity [1]. Non-motor features of the disease are increasingly recognized and include autonomic failure, constipation, cognitive impairment, ansomia, Rapid eye-movement sleep Behavior Disorder (RBD) the latter two features usually occurring during the preclinical or prodromal stage of disease evolution as biomarkers and predictors of later onset of clinical symptoms [2]. Cognitive impairment and dementia in PD are highly common complications of late stage disease [3,4] and correspond to poor prognosis [5] and more profound patient disability [6]. The natural history of PD is notable for its marked variability among patients in the mixture of symptoms and the rate of progression from estimated time of onset to death (average duration of illness15 years).
The pathological hallmark of PD is the presence of pathologically misfolded alpha-synuclein (SYN) protein in the form of Lewy Bodies (LBs) and Lewy Neurites (LNs) in neurons of the Central Nervous System (CNS) (Figure 1). Modern immunohistochemical staining of SYN pathology has shown SYN pathology, not only in PD patients but also in the cortex of a large number of patients with clinically diagnosed AD at autopsy [7]. Historically several terms have been used to describe these mixed post-mortem findings, including the “Lewy body variant of AD” [8], but current nomenclature has become more refined. Indeed, the first consensus meeting to define the clinical syndrome of Dementia with Lewy Bodies (DLB) to predict the presence of neocortical SYN pathology in dementia patients occurred in 1995 [9] and has been further revised [10,11] to improve ante-mortem diagnostic accuracy. DLB is characterized by bradykinesia and other parkinsonian features, wellformed visual hallucinations, periodic fluctuations in cognition and RBD. These clinical features are often shared with PDD, with the distinction that the cognitive impairment in DLB starts within a year of the onset of motor symptoms-the arbitrary “one-year rule” as delineated by the third consensus on the definition of DLB [10]. This clinical distinction of PDD and DLB is currently a matter of debate, [1,12] because there is no pathologic substrate found at autopsy that can reliably differentiate these clinically defined disorders [13,14] (Figure 2). Moreover, both PDD and DLB share genetic risk [15-18] and preclinical/prodromal features (e.g. anosmia, RBD) [19-21]. However, the DLB clinical syndrome is currently considered a distinct entity by many experts because it is useful for prognosis in patient care and in educating the lay public that it has features that differentiate it from clinical AD.
Figure 2: Clinicopathologic Spectrum of LBD. Scatterplot depicts individual patient data from a large LBD autopsy series (reproduced from Irwin et al, The Lancet Neurology 2017) showing the time interval from onset of motor symptoms to dementia in years defining the distinction of PDD and DLB (i.e. 1-year rule dashed line) compared to level of AD neuropathology found at autopsy. The arbitrary boundary between PDD and DLB does not differentiate patients with or without AD co-pathology. This clinicopathologic spectrum of synucleinopathy includes varying amounts of AD co-pathology which may influence clinical phenomenology as well as the response to future, more effectively targeted therapies.
Co-occurring AD associated amyloid-beta (Aβ) plaques and tau Neurofibrillary Tangles (NFTs) occur in 30-40% of patients with PD (Figure 1) and may contribute to clinical features of disease [22,23] Particularly Development of Dementia (PDD). Interestingly, PDD patients with greater levels of AD co-pathology may have a clinical phenotype that in some respects is more similar to DLB, with shorter time to dementia [13,22-24] and less prominent rest-tremor [25,26].
Thus, PD/PDD and DLB appear to exist on a clinicopathological spectrum of SYN pathology with varying degrees of AD co-pathology; hence the collective and more inclusive term Lewy body disorders (LBD) [8].
Attempts to cluster or sub-type PD based on clinical symptoms [27] may be helpful for prognosis but the underlying biological contributions to clinical heterogeneity of PD are currently unclear. Few studies include the full spectrum of LBD, since this often requires coordination of multimodal data collection across movement disorders and cognitive centers, where PD and DLB patients are often evaluated, respectively [22]. The current lack of a reliable method to detect and track SYN pathology in vivo makes detailed post-mortem clinicopathological work critical to elucidate the pathological substrates that contribute to cognitive impairment and clinical heterogeneity in LBD Here we review the recent literature on clinicopathological associations of dementia in LBD and include studies that have used in vivo biomarkers of AD pathology. The parkinsonian syndrome of multiple system atrophy, a related synculeinopathy, has a different neuropathological substrate of alpha-synuclein pathology primarily in oligodendrocytes [28] and is beyond the scope of this review.
Alpha-Synuclein in Disease Pathogenesis of LBD
In 1997 two near-simultaneous reports found pathogenic mutations in the alpha synuclein (SNCA) gene encoding SYN protein in patients with hereditary PD [29] and pathogenic SYN protein as the primary constituent of LB/LNs in both PD and DLB [30]. These two important studies demonstrated that abnormal aggregation of SYN protein in the CNS is central to the disease process in PD and DLB (i.e. LBD). Landmark work by Braak and colleagues [31] defined a non-random distribution of SYN pathology in a large cohort of cross-sectional autopsied patients with clinically defined PD1 (but not DLB) to develop a staging system of hypothesized disease spread within an individual. This model of spread of pathology from caudal brainstem regions in the medulla and the dopaminergic neurons of the substantia nigra of the midbrain to rostral subcortical and cortical regions largely maps to the clinical progression of disease in most patients from earliest symptoms to late stage dementia. Some deviations from this staging system have been described [32-35], including reports of the presence of postmortem SYN pathology in some patients who died without evidence of clinical dementia or parkinsonism [36], which could suggest SYN pathology does not always contribute to neurodegeneration. Most of these patients with asymptomatic SYN pathology at autopsy (i.e. Incidental Lewy Body Disease or ILBD) have mild deposits in lower brainstem regions and are thought to be in a prodromal or preclinical stage of PD [37]. More direct evidence for pathogenicity of SYN pathology has been described recently in animal [38,39] and cell [40-42] model systems that have shown that pathogenic “seeds” of misfolded SYN pathology may induce native SYN misfolding and a cell to cell, prion-like propagation of SYN pathology between neurons. Strikingly, intracerebral injections of recombinant SYN protein alone can cause a motor phenotype and reduced survival in both transgenic [39] and “wild-type” [38] murine models associated with a time- and dose-dependent accumulation of SYN pathology in anatomically connected regions of the brain to recapitulate human disease. These findings of trans-neuronal spread of SYN pathology are reminiscent of the histopathological staging model of PD by Braak and colleagues [31] and have been replicated in animal models [43-45]. Further, several PD patients who received experimental fetal tissue grafts into the basal ganglia were found to have low levels of SYN pathology in grafted tissue at autopsy several years after implantation [46]. It is unclear if these aggregations of SYN pathology are spread from host PD brain tissue or developed independently due to other disease-related factors.
DLB staging is thought to follow a pattern of caudal-rostral spread of SYN pathology similar to the stages Braak described in PD from the brainstem to limbic and neocortical areas in most patients [11], However, the findings of pathologic criteria for LBD at post mortem in some patients with dementia but no clinical parkinsonism [13] or striatal dopaminergic deficit on in vivo dopamine transporter imaging [47], could suggest that SYN pathology may have a different epicenter and pattern of spread during life in some DLB patients. Future work with more quantitative approaches to pathology staging and eventual in vivo markers of SYN pathology may further resolve these discrepancies.
Despite the tantalizing evidence of a prion-like mechanism of disease in PD and DLB, there is a sharp distinction between prion disease (i.e. caused by a proteinaceous infectious particle) [48] and SYN pathology, as there is currently no evidence that SYN pathology is infectious or can transmit between humans or animals, even in the setting of cadaver-derived human growth hormone recipients from pituitary samples which likely often contained SYN pathology as opposed to the rare contamination of prion protein which caused an epidemic of Cruetzfield-Jacob disease [49].
Observations of PD-like clinical symptoms and SYN pathology in adult patients with the multisystem lysosomal storage disease Gaucher’s disease with homozygous mutations in the Glucocerebrosidase A1 (GBA1) gene, led to the discovery of increased frequency of heterozygous GBA1 mutation carriers in LBD patients compared to the general population [50]. GBA1 mutations are associated with more rapidly progressive clinical parkinsonism [15,16], earlier onset of cognitive impairment [51,52] and advanced SYN pathology in the absence of AD co-pathology [13,17,53]. These findings have led to the consideration that autophagy (a lysosomal mechanism for disposing of altered proteins) and other mechanisms of protein homeostasis contribute to disease pathogenesis and an imperative to focus on GBA1 mutation carriers as an important biological subgroup of LBD.
Ongoing work to elucidate mechanisms of SYN pathology propagation and other potential downstream mechanisms of disease such as oxidative stress, synaptic dysfunction, disruption of axonal transport and inhibition of protein degradation pathways are vital to the development of disease-modifying therapies. Equally important for the success of clinical trials for these potential therapies is the ability to reliably detect biologically meaningful subgroups of LBD patients for homogenous patient recruitment when testing experimental therapies.
Clinicopathological Correlates of Dementia in PD (PDD)
Cognitive impairment and dementia are common in PD and will develop in the majority of patients usually late in the course of the disease [3,4] but with significant heterogeneity in the timing of onset [13,22,23] and the rate of progression. Roughly [24] percent of PD patients are found to have mild-cognitive impairment at diagnosis [54] and are at high risk of developing later incipient dementia [55].
The pathologic substrates linked to dementia in PD are diverse and include a range of histopathological findings. including the distribution of Lewy SYN pathology in the neocortex [56-58], subcortical cholinergic loss [14,59], Cerebrovascular Disease (CVD) [60], Argyrophilic Grain Disease (AGD) [61], TAR DNA-binding protein 43 (TDP-43) inclusions in limbic structures [62] and hippocampal sclerosis of aging (HpScl) [63]. With standardized neuropathological assessments for AD and related disorders [64,65] there is increasing recognition of mixed or multiple pathologies in LBD and other neurodegenerative diseases, along with the non-demented aging spectrum.
Large autopsy-series of PD find that the distribution of SYN pathology in a neocortical pattern is a strong correlate of dementia during life [23,56-58,66]. We previously performed a deep pathological phenotyping of a large cohort of PD patients (n=140), who died with or without dementia and included systematic evaluation of SYN, Tau NFTS, Aβ plaque, TDP-43, CVD and HpScl, as well as genotyping for Apolipoprotein E (APOE) and MAPT tau haplotype [23]. We found that each of these pathologies was associated with dementia independently. However, using a multivariate approach, we found the strongest correlate with dementia was the neocortical burden of SYN pathology, suggesting that propagation of caudal to rostral spread of SYN pathology [31] as proposed by Braak, is a main driver of the emergence of dementia in PD. Thus, patients with PDD at end-stage disease have indistinguishable pattern of wide-spread SYN pathology compared to DLB. We also found an association of the APOE Ɛ4 genotype with dementia that was independent of Aβ plaque and tau NFT pathology. This suggests that the APOE genotype may confer risk for SYN pathology in a manner distinct from AD pathology. APOE Ɛ4 has been found in a greater frequency in both patients with “pure” (no copathology) LBD and those with LBD and AD co-pathology compared to the general population [18], and some post-mortem studies find an independent association of APOE Ɛ4 with SYN pathology [58,67], which further reinforces a link between this common risk variant and LBD.
AD Co-Pathology across the Spectrum of LBD
Several other large-scale studies of autopsied PD brains have found a strong influence of co-existent AD pathology on cognitive status [4,23,24,60,66,68-70]. One study found that the combination of SYN with Aβ plaque and tau NFT pathology is the strongest correlate with dementia in PD [66], and another, using ante-mortem neuropsychological data to define cognitive status in PD70, similarly found a combination of SYN and AD co-pathology to be the most influential. We and others find that high levels of AD co-pathology in PD are nearly universally associated with PDD (i.e.<10% of PD without dementia has significant co-morbid AD at autopsy23) and also are associated with higher cortical SYN compared to PD patients without significant AD co-pathology [13,23,57,58,60,70,71]. Moreover, patients with PDD who have significant AD co-pathology tend to be older, have a shorter time interval to develop dementia after onset of parkinsonian motor symptoms and a shorter life span [23,68].
The Sydney longitudinal, multicenter study of PD followed a large cohort of patients with PD prospectively and found a similar subgroup of older PD patients with more aggressive disease [4,24,69]. These data suggest that age-related factors, including cerebrovascular disease and AD co-pathology, may increase the risk of earlier onset of dementia in the course of disease that more closely approximates DLB on the spectrum of LBD vs PD (Figure 2). Indeed, the majority of DLB patients (>70%) in a large, multi-center cohort were found to have a medium to high-level of AD neuropathologic change at autopsy [13]. A minority of those patients with DLB who had a pattern of “pure” SYN pathology (i.e. no Alzheimer co-pathology) were carriers of the GBA1 mutation or had other co-pathologies, including CVD, indicating that while AD co-pathology influences the DLB phenotype, it does not do so in every case.
In our study of patients with LBD spectrum disease (PDD/DLB), we found that tau NFT pathology is the strongest correlate of reduced survival and earlier time to dementia [13], while others find AB plaque or SYN pathology to be a strong correlate for the timing of dementia58 and survival [72]. Since all three pathologies (i.e. Tau, Aβ and SYN) are correlated in the neocortex in LBD [13,25], sample size and varying methodologies used to detect and quantify these pathologies could contribute to discrepancies in outcome. These limitations notwithstanding, AD co-pathology and associated neocortical spread of SYN appear to confer an overall worse prognosis in LBD.
Few data exists, relating post-mortem pathology to specific antemortem clinical features or cognitive profiles. Cognitive and motor features of LBD are heterogenous, [10,73,74] and there is currently no clear clinical phenotype to distinguish AD co-pathology in LBD. Datadriven clusters of PD patients have shown that a clinical subgroup with less prominent rest tremor and more prominent postural instability is linked to higher Aβ and SYN pathology in the neocortex [26] and to clinical biomarkers of AD [27]. Few autopsy-confirmed studies with ante-mortem quantitative neuropsychological testing data exist to test the cognitive domains that are impaired in LBD with and without AD co-pathology [25,75-77]. Some studies suggest that temporallobe mediated naming tasks may be worse in LBD with mixed AD co-pathology compared to pure LBD [25,76,77]. We find increasing tau pathology is a strong correlate of worsening cognitive scores in LBD patients with dementia, [25] and others find that a combination of pathology in the prefrontal cortex and temporal lobe is a strong correlate of cognitive decline [70].
Using digital methods to measure the burden of pathology parametrically, we have found similar levels of Aβ pathology but much lower levels of tau pathology in LBD with AD co-pathology than seen in autopsy-confirmed clinical AD, but the tau pathology has greater concentration in the temporal lobe [25]. Further, we have also found that overall SYN pathology in LBD with AD co-pathology is highest in the frontal and temporal lobes. This novel digital investigative methodology suggests that tau may accumulate in a manner that is distinct from AD in LBD and share a locus of pathology with SYN in the temporal lobe.
It is impossible to deduce the timing or mechanism for these observations from human post-mortem histology alone, but several strands of evidence suggest a link between tau and SYN pathology in LBD. First, the Contorsi kindred of autosomal dominant PD patients with the Ala53Thr pathogenic mutation in the SNCA gene was found to have high levels of tau pathology in addition to SYN [78]. Genetic variation in the H1 haplotype of the tau gene MAPT has been linked to increased risk for PD [79] and DLB [80], as well as the accumulation of cortical SYN pathology [81] and the risk of dementia in PD in some studies [82-84], but not others [23]. An in vitro cell model [85,86] and transgenic SYN murine models [86] suggest that tau and SYN pathology can accelerate co-polymerization of tau. More recently, novel experiments in a cell model have demonstrated two distinct strains of recombinant SYN fibril preparations, including one strain that can induce both tau and SYN pathology [42]. Moreover, the use of specific novel monoclonal antibodies to study these distinct strains of pathogenic SYN has detected unique patterns of pathology in human LBD samples [87]. This growing body of work provides compelling evidence to suggest synergy between tau and SYN pathology.
Some evidence suggests that increased AD co-pathology in LBD may mask the usual cognitive features in DLB of visual hallucinations and cognitive fluctuations [88,89]. Indeed, the clinical criteria for DLB are specific but less sensitive to detect neocortical SYN Lewy pathology at autopsy [90]. It is likely that the majority of patients with an AD clinical amnestic syndrome that have widespread neocortical SYN pathology at autopsy may represent a “limbic predominant” pattern originating from the amygdala [35], as brains from these patients have less subcortical and brainstem SYN pathology and extracranial SYN pathology in the peripheral nervous system [71] typical of LBD [91]. Roughly 50% of patients with sporadic and hereditary AD have SYN co-pathology in the amygdala and other limbic regions at autopsy [7,92]. Further, brains from patients with clinical AD and co-existent SYN pathology have higher hippocampal tau than LBD [93], suggesting these patients are biologically distinct from clinical LBD (i.e. PD, PDD, DLB). It is also possible that a subset of clinical AD patients with neocortical SYN pathology reported at autopsy could be misdiagnosed during life, since clinical diagnostic accuracy for DLB based on the one year rule is currently less than optimal [94]. Thus, the pathological spectrum of SYN pathology also includes a large proportion of AD patients, making clinical distinction of AD patients with and without SYN pathology challenging. Recent revised clinical criteria for DLB10 await validation and might improve the ante-mortem detection of SYN pathology in dementia of any type.
AD Biomarker Studies in LBD
In vivo biomarkers to detect signatures of Aβ plaque and tau tangle pathology in AD have been studied in LBD and provide converging evidence to the post-mortem data discussed above. Assays for pre-mortem cerebrospinal fluid (CSF) measurements of total-and phosphorylated forms of tau (t-tau, p-tau) and Aβ1-42 show direct associations with post-mortem tau NFT and Aβ plaque pathology in AD [95] where low Aβ1-42 and high t-tau and p-tau represent a signature of AD pathology. This pattern of CSF analytes can robustly differentiate between AD from non-demented controls [96] and predicts clinical progression to a diagnosis of AD in patients with Mild Cognitive Impairment (MCI) [97]. In PD, one prospective study found that low levels of CSF Aβ1-42 predict cognitive decline, [98] and cross-sectional studies have found that AD biomarkers in CSF are associated with cognitive impairment [99-102]. Similarly, in DLB, biomarkers for AD in CSF show association with poor prognostic clinical markers such as falls, institutionalization and shortened life span [103]. Across the LBD spectrum, the CSF biomarker signature of AD is found increasingly more common between groups of PD, PDD and DLB (reviewed in 22), which resemble frequencies of AD co-pathology seen in large autopsy studies (i.e. <10% PD, 40% PDD, >70% DLB) [13,22,23]. In early clinical PD, levels of t-tau and p-tau in CSF are lower than in control patients and are highly correlated with CSF measurements of total-alpha-synuclein [104], further suggesting that the accumulation of tau pathology and pathogenic species released into CSF may be distinct in LBD compared to AD and normal aging. Despite these differences in low t-tau/p-tau levels in early PD, cross-sectional samples of more advanced PD/PDD and DLB have found wide ranges of CSF AD biomarker values, with some overlap of individual data points with both controls and AD patients [105]. There is little autopsy confirmed data on the validity of AD CSF biomarkers in LBD. These few studies that do exist suggest that AD co-pathology may influence CSF biomarker level associated with autopsy proven AD [106]. We found a direct association with post-mortem measurement of CSF t-tau and Aβ with post-mortem severity of Aβ and tau pathology, as well as correlations with Aβ and the t-tau/Aβ ratio with SYN pathology [107]; further suggesting synergy between AD and SYN co-pathology. Preliminary data from this study suggest that clinical diagnostic accuracy to distinguish LBD with AD co-pathology from “pure” LBD ante-mortem using a cut-point of the t-tau/Aβ ratio may be in a range suitable for clinical trials (>80% sensitivity/specificity) [107]. Differentiating clinical AD with and without SYN co-pathology using CSF biomarkers is more challenging; however, some studies suggest CSF alpha-synuclein levels may improve diagnostic accuracy [105,108]. CSF alpha-synuclein assays are not yet fully reliable because of the risk that leaked blood during lumbar puncture can contaminate CSF measurements, requiring the need to account for hemoglobin levels in CSF [109]. Further, there is a large overlap of SYN levels between control and groups of PD patients with PD, making interpretation of the values for individual patients difficult [110]. Newer assays for phosphorylated 108 or oligomeric [111,112] forms of CSF synuclein are in development and may be more sensitive to disease-specific forms of alpha-synuclein. Finally, new approaches using patient CSF samples to seed and induce pathological misfolding of recombinant or native synuclein in a similar manner to prion disease testing (i.e. real-time quaking inversion; RT-QuIC assays) [113,114] are promising for a specific marker of SYN pathology in vivo. Future studies to replicate and validate these assays must be done before an authentic SYN-specific marker can be trusted for utility as a precise tool for selecting accurately diagnosed patients for clinical trials of more effective therapies in LBD
Neuroimaging is another technique for studying AD co-pathology in LBD. Hippocampal atrophy on structural MRI has been linked to tau pathology in AD [115] and predicts cognitive impairment [116] and reduced survival in DLB [117]. Positron Emission Tomography (PET) using tracers specific to Aβ plaque pathology finds a similar frequency of amyloid pathology in PDD and DLB [118] but less abundantly in PD without dementia, in keeping with post-mortem studies described above [13,22,23]. Further, PET amyloid tracer binding appears to relate to post-mortem AB neuritic plaque burden in PD [119]. Moreover, PET imaging to detect tau pathology is emerging and flortaucipir, a novel PET tracer directed at AD tau pathology has been examined in several studies of LBD. These studies have generated data to suggest that tau pathology is overall lower in LBD than in AD, but in a distribution distinct from the typical localization in AD in posterior temporoparietal [120,121] and primary motor/sensory cortices [122]. Further, PET tau binding correlates in general with cognitive impairments across the LBD spectrum of PD, PDD and DLB [121] but in focused studies of patients with early PD and MCI tau binding is negligible in those with the least amount of altered cognition [123,124]. Moreover, PET tau binding was found only in those patients with PET amyloid positivity [122,123], which conforms with post-mortem work showing advanced tau pathology in LBD largely in the setting of high-level Aβ pathology [22]. A small subset of patients with LBD evaluated by PET using a combination of amyloid and tau tracers has shown tau pathology but not amyloid plaque formation [120,121]. This discrepancy may be due to the insensitivity of the PET amyloid tracer to milder, more diffuse plaque pathology; or it could represent further evidence that tau pathology alone is a distinct form of co-pathology accruing in LBD. Additional studies of AD pathology in LBD with emerging biomarkers in prospective cohorts followed to autopsy is likely to validate these ante-mortem imaging results. Finally, there is an urgent need for a reliable way to detect and track SYN pathology during life to fully solve the timing and progression of SYN-associated neurodegeneration in both “pure” LBD and LBD with AD co-pathology.
Conclusion
The clinical components of the LBD spectrum make up a complex clinicopathological entity with diverse cognitive and motor features (Figure 2) and widespread distribution in the CNS of SYN pathology, often accompanied by AD co-pathology. When AD co-pathology is significant, it likely contributes to the clinical phenotype in ways not yet fully understood, including the timing of dementia and overall survival. Tau pathology, in particular is a strong correlate of cognitive impairment and survival and may be induced by the propagation of pathogenic strains of SYN pathology. While there is debate over the clinical distinction of PD and DLB, a more salient issue may be the ante-mortem detection and differentiation of LBD patients with AD co-pathology from those with “pure” SYN pathology. LBD patients with AD co-pathology and worse prognosis may influence clinical trial outcomes for both symptomatic therapies as well as emerging SYNtargeted disease-modifying therapies; thus, it is pertinent for clinical trial designs in LBD to consider stratification of enrollment based on AD and SYN biomarker profiles. In the final analysis, significant advances in meaningful therapies will depend on a more precise understanding of how the diverse spectrum of molecular pathologies in LBD interact to produce clinical neurodegeneration.
Acknowledgement
Research reported in this publication was supported by funding from TL1TR001880, NIA AG010124, and NINDS NS088341, NS053488.
References
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, et al. (2015) MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 30:1591-1601.
- Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, et al. (2015) MDS research criteria for prodromal Parkinson's disease. Mov Disord 30: 1600-1611.
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P (2003) Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Arch Neurol 60: 387-392.
- Halliday G, Hely M, Reid W, Morris J (2008) The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol 115: 409-415.
- Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ (2010) Relationships between age and late progression of Parkinson's disease: A clinico-pathological study. Brain 133: 1755-1762.
- Rosenthal E, Brennan L, Xie S, Hurtig H, Milber J, et al. (2010) Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord 25: 1170-1176.
- Hamilton RL (2000) Lewy bodies in Alzheimer's disease: A neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol 10: 378-384.
- Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, et al. (2007) DLB and PDD boundary issues: Diagnosis, treatment, molecular pathology, and biomarkers. Neurology 68: 812-819.
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, et al. (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): Report of the consortium on DLB international workshop. Neurology 47: 1113-1124.
- Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, et al. (2015) TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol 211: 897-911.
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, et al. (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65: 1863-1872.
- Boeve BF, Dickson DW, Duda JE, Ferman TJ, Galasko DR, et al. (2016) Arguing against the proposed definition changes of PD. Mov Disorders : official journal of the Mov Disord 31: 1619-1622.
- Irwin DJ, Grossman M, Weintraub D, Hurtig HW, Duda JE, et al. (2017) Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol 16: 55-65.
- Ballard C, Ziabreva I, Perry R, Larsen JP, O'Brien J, et al. (2006) Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 67: 1931-1934.
- Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, et al. (2013) A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol 70: 727-735.
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, et al. (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med 361: 1651-1661.
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, et al. (2012) GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 79: 1944-1950.
- Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, et al. (2013) APOE ε4 increases risk for dementia in pure synucleinopathies. JAMA Neurol 70: 223-228.
- Boeve BF, Silber MH, Ferman TJ, Kokmen E, Smith GE, et al. (1998) REM sleep behavior disorder and degenerative dementia: An association likely reflecting Lewy body disease. Neurology 51: 363-370.
- Jacobs ML, Dauvilliers Y, St Louis EK, McCarter SJ, Romenets SR, et al. (2016) Risk factor profile in Parkinson's disease subtype with REM sleep behavior disorder. J Parkinsons Dis 6: 231-237.
- Boeve BF, Dickson DW, Olson EJ, Shepard JW, Silber MH, et al. (2007) Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med 8: 60-64.
- Irwin DJ, Lee VM, Trojanowski JQ (2013) Parkinson's disease dementia: Convergence of α-synuclein, tau and amyloid- βpathologies. Nat Rev Neurosci 14: 626-636.
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, et al. (2012) Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 72: 587-598.
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 23: 837-844.
- Coughlin D, Xie X, Liang M (2018) Clinical and pathological influences of tau pathology in lewy body disorders. Under review.
- Selikhova M1, Williams DR, Kempster PA, Holton JL, Revesz T, et al. (2009) A clinico-pathological study of subtypes in Parkinson's disease. Brain 132: 2947-2957.
- Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB (2017) Clinical criteria for subtyping Parkinson's disease: Biomarkers and longitudinal progression. Brain 140: 1959-1976.
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, et al. (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: 670-676.
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science 276: 2045-2047.
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, et al. (1997) Alpha-synuclein in Lewy bodies. Nature 388: 839-840.
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, et al. (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197-211.
- Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116: 1-16.
- Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, et al. (2008) Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 18: 220-224.
- Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, et al. (2004) Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol 63: 742-749.
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, et al. (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117: 613-634.
- Parkkinen L, Kauppinen T, Pirttilä T, Autere JM, Alafuzoff I (2005) Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol 57: 82-91.
- Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, et al. (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol 115: 437-444.
- Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, et al. (2012) Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949-953.
- Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, et al. (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209: 975-986.
- Luk KC, Song C, O'Brien P, Stieber A, Branch JR, et al. (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A 106: 20051-20056.
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, et al. (2011) Exogenous alpha-synuclein fibrils induce lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72: 57-71.
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, et al. (2013) Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154: 103-117.
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, et al. (2013) Prion-like spreading of pathological α-synuclein in brain. Brain 136: 1128-1138.
- Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, et al. (2014) Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75: 351-362.
- Paumier KL, Luk KC, Manfredsson FP, Kanaan NM, Lipton JW, et al. (2015) Intrastriatal injection of pre-formed mouse alpha-synuclein fibrils into rats triggers alpha-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol Dis 82: 185-199.
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med 14: 504-506.
- Thomas AJ, Attems J, Colloby SJ, O'Brien JT, McKeith I, et al. (2017) Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 88: 276-283.
- Bolton DC, McKinley MP, Prusiner SB (1982) Identification of a protein that purifies with the scrapie prion. Science 218: 1309-1311.
- Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, et al. (2013) Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol 70: 462-468.
- Nichols WC, Pankratz N, Marek DK, Pauciulo MW, Elsaesser VE, et al. (2009) Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology 72: 310-316.
- Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, et al. (2016) GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson's disease. Mov disord 31: 95-102.
- Swan M, Doan N, Ortega RA, Barrett M, Nichols W, et al. (2016) Neuropsychiatric characteristics of GBA-associated Parkinson disease. J Neurol Sci 370: 63-69.
- Clark LN, Kartsaklis LA, Wolf Gilbert R, Dorado B, Ross BM, et al. (2009) Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol 66: 578-583.
- Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65: 1239-1245.
- Pigott K, Rick J, Xie SX, Hurtig H, Chen-Plotkin A, et al. (2015) Longitudinal study of normal cognition in Parkinson disease. Neurology 85: 1276-1282.
- Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, et al. (2000) Alpha-synuclein cortical lewy bodies correlate with dementia in Parkinson's disease. Neurology 54: 1916-1921.
- Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kovari E (2013) Neuropathology of dementia in a large cohort of patients with Parkinson's disease. Parkinsonism Relat Disord 19: 864-868.
- Ruffmann C, Calboli FC, Bravi I, Gveric D (2016) Cortical Lewy bodies and Aβ burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol Appl Neurobiol 42: 436-450.
- Hall H, Reyes S, Landeck N, Bye C, Leanza G, et al. (2014) Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson's disease. Brain 137: 2493-2508.
- Jellinger KA, Attems J (2008) Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol 115: 427-436.
- Ferrer I, Santpere G, van Leeuwen FW (2008) Argyrophilic grain disease. Brain 131: 1416-1432.
- Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, et al. (2007) Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol 114: 221-229.
- Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, et al. (2011) Hippocampal sclerosis in advanced age: Clinical and pathological features. Brain 134: 1506-1518.
- Montine TJ, Monsell SE, Beach TG, Bigio EH, Bu Y, et al. (2015) Multisite assessment of NIA-AA guidelines for the neuropathologic evaluation of Alzheimer's disease. Alzheimers Dement 12: 164-169.
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: A practical approach. Acta Neuropathol 123: 1-11.
- Compta Y, Parkkinen L, O'Sullivan SS, Vandrovcova J, Holton JL, et al. (2011) Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: Which is more important? Brain 134: 1493-1505.
- Tsuang DW, Wilson RK, Lopez OL, Luedecking-Zimmer EK, Leverenz JB, et al. (2005) Genetic association between the APOE*4 allele and Lewy bodies in Alzheimer disease. Neurology 64: 509-513.
- Jellinger KA, Seppi K, Wenning GK, Poewe W (2002) Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J Neural Transm (Vienna) 109: 329-339.
- Reid WG, Hely MA, Morris JG, Loy C, Halliday GM (2011) Dementia in Parkinson's disease: A 20-year neuropsychological study (Sydney Multicentre Study). J Neurol Neurosurg Psychiatry 82: 1033-1037.
- Howlett DR, Whitfield D, Johnson M, Attems J, O'Brien JT, et al. (2015) Regional multiple pathology scores are associated with cognitive decline in lewy body dementias. Brain Pathol 25: 401-408.
- Toledo JB, Gopal P, Raible K, Irwin DJ, Brettschneider J, et al. (2016) Pathological α-synuclein distribution in subjects with coincident alzheimer's and lewy body pathology. Acta Neuropathol 131: 393-409.
- Ferman TJ, Aoki N, Crook JE, Murray ME4, Graff-Radford NR, et al. (2018) The limbic and neocortical contribution of alpha-synuclein, tau, and amyloid beta to disease duration in dementia with Lewy bodies. Alzheimers Dement 14: 330-339.
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, et al. (2007) Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 22: 1689-1707.
- Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, et al. (2012) Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 27: 349-356.
- Sabbagh MN, Adler CH, Lahti TJ, Connor DJ, Vedders L, et al. (2009) Parkinson disease with dementia: Comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord 23: 295-297.
- Peavy GM, Edland SD, Toole BM, Hansen LA, Galasko DR, et al. (2016) Phenotypic differences based on staging of Alzheimer's neuropathology in autopsy-confirmed dementia with Lewy bodies. Parkinsonism Related Disord 31: 72-78.
- Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, et al. (2005) Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 64: 2069-2073.
- Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, et al. (2002) Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol 104: 7-11.
- Zabetian CP, Hutter CM, Factor SA, Nutt JG, Higgins DS, et al. (2007) Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson's disease. Ann Neurol 62: 137-144.
- Labbé C, Heckman MG, Lorenzo-Betancor O, Soto-Ortolaza AI, Walton RL, et al. (2016) MAPT haplotype H1G is associated with increased risk of dementia with Lewy bodies. Alzheimers Dement 12: 1297-1304.
- Colom-Cadena M, Gelpi E, Martà MJ, Charif S, Dols-Icardo O, et al. (2013) MAPT H1 haplotype is associated with enhanced α-synuclein deposition in dementia with Lewy bodies. Neurobiol Aging 34: 936-942.
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, et al. (2009) The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 132: 2958-2969.
- Morley JF, Xie SX, Hurtig HI, Stern MB, Colcher A, et al. (2012) Genetic influences on cognitive decline in Parkinson's disease. Mov Disord 27: 512-518.
- Goris A, Williams-Gray CH, Clark GR, Foltynie T, Lewis SJ, et al. (2007) Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann Neurol 62: 145-153.
- Waxman EA, Giasson BI (2011) Induction of intracellular tau aggregation is promoted by α-synuclein seeds and provides novel insights into the hyperphosphorylation of tau. J Neurosci 31: 7604-7618.
- Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, et al. (2003) Initiation and synergistic fibrillization of tau and alpha-synuclein. Science 300: 636-640.
- Covell DJ, Robinson JL, Akhtar RS, Grossman M, Weintraub D, et al. (2017) Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson's disease. Neuropathol Appl Neurobiol 43: 604-620.
- Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, et al. (2003) Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60: 1586-1590.
- Tiraboschi P, Attems J, Thomas A, Brown A, Jaros E, et al. (2015) Clinicians' ability to diagnose dementia with Lewy bodies is not affected by β-amyloid load. Neurology 84: 496-499.
- Lopez OL, Becker JT, Kaufer DI, Hamilton RL, Sweet RA, et al. (2002) Research evaluation and prospective diagnosis of dementia with Lewy bodies. Arch Neurol 59: 43-46.
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, et al. (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: 689-702.
- Lippa CF, Fujiwara H, Mann DM, Giasson B, Baba M, et al. (1998) Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153: 1365-1370.
- Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, et al. (2015) Neuropathologically mixed Alzheimer's and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol 129: 729-748.
- McKeith IG, Fairbairn AF, Bothwell RA, Moore PB, Ferrier IN, et al. (1994) An evaluation of the predictive validity and inter-rater reliability of clinical diagnostic criteria for senile dementia of Lewy body type. Neurology 44: 872-877.
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, et al. (2009) Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66: 382-389.
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, et al. (2009) Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 65: 403-413.
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, et al. (2010) Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 67: 949-956.
- Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, et al. (2010) CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology 75: 1055-1061.
- Leverenz JB, Watson GS, Shofer J, Zabetian CP, Zhang J, et al. (2011) Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson's disease. Parkinsonism Relat Disord 17: 61-64.
- Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, et al. (2010) CSF Abeta(42) and tau in Parkinson's disease with cognitive impairment. Mov Disord: official journal of the Movement Disord 25: 2682-2685.
- Compta Y, Marti MJ, Ibarretxe-Bilbao N, Junqué C, Valldeoriola F, et al. (2009) Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord 24: 2203-2210.
- Alves G, Brønnick K, Aarsland D, Blennow K, Zetterberg H, et al. (2010) CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry 81: 1080-1086.
- Lemstra AW, de Beer MH, Teunissen CE, Schreuder C, Scheltens P, et al. (2017) Concomitant AD pathology affects clinical manifestation and survival in dementia with lewy bodies. J Neurol Neurosurg Psychiatry 88: 113-118.
- Kang JH, Mollenhauer B, Coffey CS, Toledo JB, Weintraub D, et al. (2016) CSF biomarkers associated with disease heterogeneity in early parkinson's disease: The Parkinson's Progression Markers Initiative study. Acta Neuropathol 131: 935-949.
- Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, et al. (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69: 1445-1452.
- Toledo JB, Brettschneider J, Grossman M, Arnold SE, Hu WT, et al. (2012) CSF biomarkers cutoffs: The importance of coincident neuropathological diseases. Acta Neuropathol 124: 23-35.
- Irwin DJ, Xie SX, Coughlin D, Nevler N, Akhtar RS, et al. (2018) CSF tau and β-amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology 90: e1038-1038e1046.
- Shi M, Tang L, Toledo JB, Ginghina C, Wang H, et al. (2018) Cerebrospinal fluid alpha-synuclein contributes to the differential diagnosis of Alzheimer's disease. Alzheimers Dement.
- Mollenhauer B, Parnetti L, Rektorova I, Kramberger MG (2016) Biological confounders for the values of cerebrospinal fluid proteins in Parkinson's disease and related disorders. J Neurochem 139 Suppl 1: 290-317.
- Eusebi P, Giannandrea D, Biscetti L, Abraha I, Chiasserini D, et al. (2017) Diagnostic utility of cerebrospinal fluid alpha-synuclein in parkinson's disease: A systematic review and meta-analysis. Mov Disord 32:1389-1400.
- Majbour NK, Chiasserini D, Vaikath NN, Eusebi P, Tokuda T, et al. (2017) Increased levels of CSF total but not oligomeric or phosphorylated forms of alpha-synuclein in patients diagnosed with probable Alzheimer's disease. Sci Rep 7: 40263.
- Hansson O, Hall S, Ohrfelt A, Zetterberg H, Blennow K, et al. (2014) Levels of cerebrospinal fluid alpha-synuclein oligomers are increased in Parkinson's disease with dementia and dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Res Ther 6: 25.
- Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, et al. (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3: 812-818.
- Groveman BR, Orru CD, Hughson AG, Raymond LD, Zanusso G, et al. (2018) Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol Commun 6:7.
- Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, et al. (2008) MRI correlates of neurofibrillary tangle pathology at autopsy: A voxel-based morphometry study. Neurology 71: 743-749.
- Kantarci K, Lesnick T, Ferman TJ, Przybelski SA, Boeve BF, et al. (2016) Hippocampal volumes predict risk of dementia with Lewy bodies in mild cognitive impairment. Neurology 87: 2317-2323.
- Graff-Radford J, Lesnick TG, Boeve BF, Przybelski SA, Jones DT, et al. (2016) Predicting survival in dementia with lewy bodies with hippocampal volumetry. Mov Disord 31: 989-994.
- Petrou M, Dwamena BA, Foerster BR, MacEachern MP, Bohnen NI, et al. (2015) Amyloid deposition in parkinson's disease and cognitive impairment: A systematic review. Mov Disord 30: 928-935.
- Akhtar RS, Xie SX, Brennan L, Pontecorvo MJ, Hurtig HI, et al. (2016) Amyloid-beta positron emission tomography imaging of alzheimer's pathology in parkinson's disease dementia. Mov Disord Clin Pract 3: 367-375.
- Kantarci K, Lowe VJ, Boeve BF, Senjem ML, Tosakulwong N, et al. (2017) AV-1451 tau and β-amyloid positron emission tomography imaging in dementia with Lewy bodies. Ann Neurol 81: 58-67.
- Gomperts SN, Locascio JJ, Makaretz SJ, Schultz A, Caso C, et al. (2016) Tau positron emission tomographic imaging in the lewy body diseases. JAMA Neurol 73: 1334-1341.
- Lee SH, Cho H, Choi JY, Lee JH2, Ryu YH, et al. (2018) Distinct patterns of amyloid-dependent tau accumulation in Lewy body diseases. Mov Disord 33: 262-272.
- Winer JR, Maass A, Pressman P, Stiver J, Schonhaut DR, et al. (2018) Associations Between Tau, β-Amyloid, and Cognition in Parkinson Disease. JAMA Neurol 75: 227-235.
- Hansen AK, Damholdt MF, Fedorova TD, Knudsen K1, Parbo P, et al. (2017) In Vivo cortical tau in Parkinson's disease using 18F-AV-1451 positron emission tomography. Mov Disord 32: 922-927.
Citation: Irwin DJ, Hurtig HI (2018) The Contribution of Tau, Amyloid-Beta and Alpha-Synuclein Pathology to Dementia in Lewy Body Disorders. J Alzheimers Dis Parkinsonism 8: 444. DOI: 10.4172/2161-0460.1000444
Copyright: © 2018 Irwin DJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8361
- [From(publication date): 0-2018 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 7443
- PDF downloads: 918