Research Article Open Access
The Continuum of Metabolic Stress According to the Gas Model of Alzheimer's Disease
Pierre A. Denis*
SOS Médecins, 50 rue Ville-Pépin, Saint-Malo, France
- Corresponding Author:
- Pierre A. Denis
SOS Médecins, 50 rue Ville-Pépin
35400 Saint-Malo, France
E-mail: pierredenisfr@yahoo.fr
Received date: February 18, 2014; Accepted date: March 28, 2014; Published April 30, 2014
Citation: Denis PA (2014) The Continuum of Metabolic Stress According to the Gas Model of Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 4:149. doi:10.4172/2161-0460.1000149
Copyright: © 2014 Denis PA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
The present article focuses on the model metabolic stress in AD. This novel model proposes that nitrogen nanobubbles, having invaded the brain interstitial fluid from the blood through glucose transporter-1 (GLUT-1), cause the pressure to increase in the close surroundings, before being embedded in amyloid-β fibrils in the form of cerebral amyloid angiopathy that fixes the pollutant. Following the huge increase in the surrounding pressure, molecular oxygen, the regular form of oxygen in a low PO2 solute, gather into oxygen nanobubbles, leading to various normal responses, albeit damaging. Therefore, nanobubbles trigger the NADPH oxidase-NO antibubble biomachinery that produces superoxide and peroxynitrite. The high NADPH/NADP+ turnover is supported by the pentose phosphate pathway. Oxygen nanobubbles in mitochondria could explain the impairment of complexes I and IV. The amyloid percolation well model might resolve the issue of the coimmunoprecipitation of Aβ with the latter complexes, Aβ stabilizing oxygen bubbles, as it might stabilize nitrogen bubbles in the ISF. The permeabilization of the mitochondrial membrane by unspecific pores fixes the overpressure on the mitochondria. The bubble-induced crowding of the respiratory chains causes energy depletion due to the disruption of oxidative phosphorylation, leading to the irreversible injury of respiration, also known as Warburg effect. The main consequence is a deficit in the cholinergic system. Last, the peroxynitrite/CO2 system is deciphered as a CO2 antibubble buffer, rescuing the impaired carbonic anhydrase in AD. Sometimes, CO2 is not released from peroxynitrite, and then produces nitrite anions and carbonyl radicals after intermediate reactions. These respectively lead to the nitrotyrosination and carbonylation of numerous proteins but hold the cells free from a CO2 bubble-induced disruption of the cytoplasmic and the mitochondrial membranes. The AD Gas Model paves the way to new approaches to address the pathophysiology of the most devastating brain disease in human beings.
Keywords
Alzheimer’s disease; Nanobubble; Warburg effect; Oxidative stress and nitrosative stress; Amyloid; Carbonic anhydrase; Acetylcholine
Abbreviations
Aβ: β-amyloid; AcCoA: Acetyl Coenzyme A; Ach: Acetylcholine; AD: Alzheimer’s Disease; APP: Amyloid Precursor Protein; BBB: Blood Brain Barrier; CAA: Cerebral (or congophilic) Amyloid Angiopathy; ChAT: Choline Acetyltransferase; CRB: Chemical Representation of Bubbles; CSF: Cerebrospinal Fluid; FAD: Flavin Adenine Dinucleotide; GLUT: Glucose Transporter; ISF: Interstitial Fluid; LDH: Lactate Dehydrogenase; MPTP: Mitochondrial Permeability Transition Pore; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; NO: Nitric Oxide; NOS: Nitric Oxide Synthase; NOX = NADPH Oxidase; PET: Positron Emission Tomography; PDC: Pyruvate Dehydrogenase Complex; ROS: Reactive Oxygen Species; SOD: Superoxide Dismutase; TCA: Tricarboxylic Acid Cycle
“Free radical biology and medicine: it’s a gas, man!” Pryor et al. [2]
Foreword: the main features of the Gas Model of Alzheimer’s disease (AD) [1] will be reminded step by step in the Discussion section, which means that the reader doesn’t need to read the first article in Medical Hypothesis.
Introduction
The present article focuses on the previous model [1] of metabolic stress in AD. The Gas Model of AD stated that the pathological lesions that define the sporadic forms of “Alzheimer’s disease would be linked to the insidious accumulation of nitrogen, having invaded the brain interstitial fluid (ISF) from the blood via the physiological cycling pool of vascular glucose transporters (GLUT-1). According to this hypothesis, the nitrogen nanobubbles, being chemically inert and actually indestructible for human beings, cannot escape from the ISF anymore. They would exert a huge and deleterious pressure against cellular components, especially in microglia and in astrocytes. They could enhance the existing cell oxygen anisotropy, which might enhance the natural bubble nucleation of 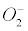 − 2O2 in cells or in mitochondria [1].”
− 2O2 in cells or in mitochondria [1].”
The classic problem of oxygen toxicity states that superoxide radical ion 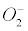 is one key understanding [2], superoxide being scavenged in the form of peroxynitrite. Peroxynitrite appears as a pivot molecule among all Reactive Oxygen Species (ROS). An extensive literature exists on ROS formation in AD, especially those released by microglia [3], the resident macrophages in the brain. However, the literature on the subject does not deal with bubble occurrence when gases found in cells are O2, NO,
is one key understanding [2], superoxide being scavenged in the form of peroxynitrite. Peroxynitrite appears as a pivot molecule among all Reactive Oxygen Species (ROS). An extensive literature exists on ROS formation in AD, especially those released by microglia [3], the resident macrophages in the brain. However, the literature on the subject does not deal with bubble occurrence when gases found in cells are O2, NO,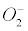 and CO2. Indeed, I believe that the oxygen and also the CO2 toxicity depend mainly on the ability of any gas to generate bubbles in a solute, and not only on their respective chemical reactivities.
and CO2. Indeed, I believe that the oxygen and also the CO2 toxicity depend mainly on the ability of any gas to generate bubbles in a solute, and not only on their respective chemical reactivities.
The Results section lists a few major pathological hallmarks regarding the metabolic stress in AD, such as the ROS formation and nitrosative stress, the main mitochondrial abnormalities, and the shift in the metabolic pathway. Obviously, the presentation is not as neat as it could be and the paragraphs will probably appear unconnected. Last, the discussion section will connect all the latter observations into an intelligible framework, from the point of view of dissolved gases. The AD Gas Model will throw light on their meanings, leading to an expected conclusion: these could be normal and desirable reactions against the presence of an indestructible (=inert) pollutant having contaminated the ISF
Materials and Methods
The scientific method is chosen according to the Inventor’s Paradox from G. Pólya (1887-1985) and its heuristic approach [4]. The heuristic approach has been complemented by Bayesian statistics [5] that are already under way in many other areas of science.
Results
The reactive oxygen species (ROS)
An extensive literature demonstrated an enhanced NADPHoxidase (also known as NOX) activity in AD [6,7], especially within microglia [8], and the strong association between NOX activity and the individual’s cognitive scores [6]. NADPH oxidase leads to ROS formation such as superoxide and peroxynitrite.
The nitrosative stress
The pathological hallmarks related to nitrite (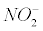 )or nitrate (
)or nitrate (  ) molecules are called nitrosative stress. There is increasing evidence that nitrosative stress is involved in the pathogenesis of AD [9-11].
) molecules are called nitrosative stress. There is increasing evidence that nitrosative stress is involved in the pathogenesis of AD [9-11].
The nitrite formation: Keith and Powell pioneered the study of peroxynitrite with bicarbonate buffers [12]. According to Squadrito and Pryor [13], Calabrese et al. [9], nitrating species arise in vivo mainly from the reaction of peroxynitrite on carbon dioxide, which is a fast and efficient reaction, and do not involve nitric oxide itself as a nitrating agent. Meli et al. [14] determined the extent of radical formation by mixing peroxynitrite, carbon dioxide and NO. Nitrite anions and a CO3- carbonyl radical result from the reaction of peroxynitrite with carbon dioxide as follows. The chemical representation of bubbles (CRB) [1] is helpful to represent CO2 as a gas in the following equation. The CRB is a novel chemical notation for gas particles in a solute, where a circle depicts a quantum bubble (which is the true representation of a gas particle in a solute).
ONOO. +CO2 → ONOOCO2 → CO3. + NO2. → 3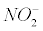 (1)
(1)
Immunohistochemistry shows the presence in the neurons of protein carbonyl immunoreactivity in β-tubulin, β-actin and creatine kinase BB in AD but also in control brain extracts [15]. Hippocampus in AD shows an increase in protein carbonylation [16].
The nitrotyrosination: Nitrotyrosination is one of the major events in the neurotoxicity induced by Aβ [17]. Tyrosine residues partially trap intermediates that arise from the CO2 - peroxynitrite reaction [18] (Equation 1). The nitro group. NO2 reacts with a phenol compound such as tyrosine producing 3-nitrotyrosine.
tyrosine +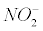 .− >NO2Tyr (2)
.− >NO2Tyr (2)
Equation 2 is an oversimplification since reactions undergo a radical mechanism Squadrito and Pryor [13]. The discussion section will reduce the involvement of nitrosative stress in AD, which seems at first sight a complicated task.
The mitochondrial abnormalities
The mitochondrial dysfunction: “Mitochondrial dysfunction has been observed as characteristic of many neurodegenerative diseases, even before other distinctive disease features and symptoms appear’’ [19]. We shall study the results of some experiment carried out in AD samples.
The Complex IV dysfunction: A variety of studies have demonstrated that complex IV dysfunction can increase ROS generation. Complex IV (also referred to as cytochrome c oxidase) is the last enzyme in the respiratory transport chain of mitochondria (or bacteria). It receives an electron from each of the four cytochrome c molecules, and transfers them to one oxygen molecule, converting molecular oxygen into two molecules of water.
There is evidence that the mitochondria are a direct site of Aβ accumulation in AD neurons [20] and that Aβ can penetrate into mitochondria through mitochondrial outer membrane import receptors [21]. β-amyloid can directly disrupt mitochondrial function and inhibits key enzymes [22]. In Aβ-rich synaptic mitochondria in brains of transgenic mice, coimmunoprecipitation assays show the binding of Aβ with cytochrome c [23]. However, the current amyloid hypothesis does not explain why β-amyloid is toxic for mitochondria, as in a model of AD transgenic mouse [20].
The Complex I dysfunction: Complex I (also referred to as NADH: ubiquinone oxidoreductase or NADH dehydrogenase) is a potent source of reactive oxygen species [24]. Complex I can produce ROS such as superoxide and hydrogen peroxide [25]. In normal conditions, during forward electron transfer, a premature electron leakage to oxygen occurs, resulting in a very small amount of superoxide being produced.
In vitro studies showed that β-amyloid caused a selective defect in Complex I activity. Furthermore, the defect in Complex I was associated with an increase (5 fold) of intracellular reactive oxygen species [26].
The permeabilization of the mitochondrial membrane: The permeability transition denotes an increase of the mitochondrial inner membrane permeability to solutes with molecular weights up to about 1,500 Da [27]. It is assumed to be mediated by the opening of a channel, the permeability transition pore (MPTP), “whose molecular nature remains a mystery’’ [27].
There is evidence that the opening of the MPTP leads to calcium overload in the cytoplasm [28]. Intracellular Ca2+ alters the lipid organization of the inner mitochondrial membrane by interacting with cardiolipin, the major phospholipid of this membrane. Calcium complexation with cardiolipin is one of the early steps, which sets off additional inner mitochondrial membrane permeabilization [29].
The abnormalities in the neuron metabolism
The astrocyte-neuron lactate shuttle model: Within the neuron, the energy supply mainly involves lactate, and not glucose [30]. Lactate is provided through specific transporters by the nearby astrocyte, as a by-product of glucose-6-P (G6P) hydrolysis. At first, in astrocyte, G6P is metabolized through the glycolytic pathway to pyruvate.
G6P(astrocyte) + 2NAD + +2ADP + 2Pi− > [enzymes]2 pyruvate + 2NADH + 2H + +2H2 O (3)
then pyruvate is converted into lactate
pyruvate + NADH < − > [lactatedehydrogenaseLDHA]lactate(astrocyte) + NAD+ (4)
Lactate is exported out of the astrocyte by the monocarboxylate transporter 1 or 4 (MCT1/4) and carried into neuron via MCT2 [30].
lactate (astrocyte)− > [MCT1/4]lactate(ISF)− > [MCT2]lactate(neuron)
Once in the neuron, lactate dehydrogenase B converts lactate to pyruvate which is converted into ATP through Krebs cycle within mitochondria.
lactate (neuron) < − > [lactatedehydrogenaseLDHB]pyruvate
Some glycolytic enzyme samples from several brains from AD patients at autopsy increase and correlate with increased contents of lactate dehydrogenase [31].
The whole process is depicted as the astrocyte-neuron lactate shuttle model [30,32].
The carbonic anhydrase: The main reaction that scavenges CO2 coming from the Krebs cycle (see Appendix B) involves the enzyme carbonic anhydrase (CA). CAs (comprised of at least five distinct families) catalyze the interconversion of CO2 and water into bicarbonate and protons [33].
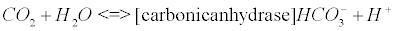 (5)
(5)
CA, especially isoenzyme I is an extraordinarily efficient catalyst [34]. The catalytic mechanism of CA II, which is also a potent catalyst in the brain tissue, has been studied in great detail [35,36]. Impaired CA may lead to pH imbalance in AD [16]. There is evidence that oxidative modification of CA II could explain its diminished activity that has been reported in AD brain compared to age-matched control brain [16,37].
The change of metabolism (Warburg effect): The main consequences of mitochondrial dysfunction is that aerobic metabolism (Krebs cycle within mitochondria) is turned into aerobic glycolysis, a lower energetic pathway. There is evidence that in presence of Aβ in brain cell cultures, the decrease in glucose transport was followed by a decrease in cellular ATP levels [38]. Despite an apparently normal oxygen content, oxidative reactions are prevented from properly recycling NAD+. It complies with aerobic glycosysis in AD, also known as Warburg effect [30]. The Warburg effect is one of the most important characteristics of cancer cells [39]. It is defined as a high rate of glycolysis followed by lactate accumulation in the cytosol in the presence of oxygen [40], rather than as a comparatively low rate of glycolysis followed by oxidation of pyruvate in mitochondria during normal oxidative phosphorylation [41]. In other words, despite an apparently sufficient oxygen concentration, ATP generation does not occur within mitochondria but through glycolysis within the cytoplasm.
Acetylcholine (Ach)
The deficiency in ACh synthesis [42] and the finding that acetylcholinesterase (AChE) is lowered in the cerebral cortex and hippocampus of patients with AD [43,44] are prominent features of AD.
Pyruvate is the most important precursor of the acetyl group of Ach [45]. AcetylCoA arises from the following reaction that occurs within mitochondria
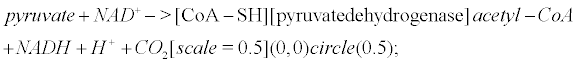 (6)
(6)
Pyruvate dehydrogenase is lowered in post mortem brain tissue from cases of AD [46]. The reaction that leads to Ach involves choline acetyltransferase (ChAT)
acetylCoA+ choline− > [ChAT]acetylcholine (7)
The pentose phosphate pathway: There is evidence that the activity of pentose phosphate pathway increases in AD [47]. The change of metabolism from mitochondrial oxidative phoshorylation to glycolysis is accompanied by the engagement of the pentose phosphate pathway. The activity of G6PD in AD is almost double the one of normal controls [48] and is involved in the following step during the PPP
Glucose − 6 − P + NADP+ <=> [G6PD]6 − phospho − glucono −1,5 − lactone + NADPH + H+ (8)
Discussion
This paper aims to decipher all the above observations to the light of the Gas Model of AD. Some propositions are really speculative. They should be understood as directions for future research, as novel ideas rather than as a vain desire to completely unravel the disease.
Conclusion
New insights into ROS in AD
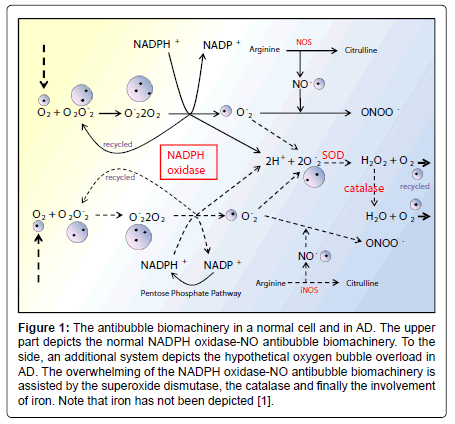
Currently, NADPH oxidase is believed to have the primary purpose of producing ROS [6]. The phagocytic NADPH oxidase plays an essential role in innate immunity by catalyzing the formation of superoxide, which facilitates the destruction of invading microorganisms during phagocytosis [8], however the authors cannot link an aseptic disease such as AD with this role. Recently, NADPH-oxidase, SOD and NOS have been linked to an antibubble biomachinery [1]. An antibubble enzyme is involved during a 3-stage pathway: nanobubble fission, implosion and scavenging. Last, molecular oxygen is recycled, allowing further oxidative phosphorylations within mitochondria. For example SOD is one of the most typical bubble fission enzyme, catalyzing the fission of the 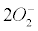 bubble into hydrogen peroxide and molecular oxygen (Figure 1). A low oxygen level in cells is common [49-51] and might prevent bubble nucleation. Sometimes, molecular oxygen, the regular form of oxygen in a low
bubble into hydrogen peroxide and molecular oxygen (Figure 1). A low oxygen level in cells is common [49-51] and might prevent bubble nucleation. Sometimes, molecular oxygen, the regular form of oxygen in a low  solute, gather into oxygen nanobubbles, leading to various normal responses, albeit damaging: the antibubble biomachinery prevents further bubble enlargement but produces harmful molecules (=ROS).
solute, gather into oxygen nanobubbles, leading to various normal responses, albeit damaging: the antibubble biomachinery prevents further bubble enlargement but produces harmful molecules (=ROS).
Figure 1: The antibubble biomachinery in a normal cell and in AD. The upper part depicts the normal NADPH oxidase-NO antibubble biomachinery. To the side, an additional system depicts the hypothetical oxygen bubble overload in AD. The overwhelming of the NADPH oxidase-NO antibubble biomachinery is assisted by the superoxide dismutase, the catalase and finally the involvement of iron. Note that iron has not been depicted [1].
Oxidative stress plays a crucial role in the pathophysiology of AD, but the origin of ROS is not clearly established, and authors note that most abnormalities are already found in the normal aging brain [52]. The Gas Model of AD proposes that nitrogen nanobubbles, having invaded the brain ISF from the blood through GLUT1, increase the pressure in the close surroundings. The overpressure is assumed to enhance the gathering of gas particules in the cytoplasm or in mitochondria within neighbouring cells, leading to oxygen nanobubble formation, and finally the overwhelming of the antibubble biomachinery and the related peroxidative stress (e.g. lipid peroxidation [53], cholesterol oxidation to oxysterols [54], mitochondrial DNA oxidation [55]).
The β-amyloid
Involvement of pore-like structures into the regulation of the pressure: From the perspective of engineering, in the AD Gas Model, “the action of APP has been reduced to an electromechanical pressure sensor. The infra-nanometric checking of the APP levels in the membrane occurs through the action of different membrane-spanning enzymes (e.g. α-, β- and γ-secretases); it is transduced with specific protein fragments, such as Aβ. It logically leads to a 3-D mapping of the surrounding pressure. The cell integrates APP oscillations from various locations around the cell, giving rise to elongation or migration’’ [1].
Inert gas bubbles in the ISF are expected to exert a huge pressure on neurons and astrocytes. Laplace’s Law indicates that nanobubbles exert a pressure of several hundred atmosphere (Appendix A) which results in shear stress or crushing, depending on the bubble situation. When the cell membrane is crushed by a nearby structure, APP is also pushed down, sliding through the membrane since its frail C-terminal domain does not anchor it, and there is evidence that Aβ is sometimes released beside the membrane after its enzymatic cleavage in AD [56]. Aβ forms the annular pore-like protofibrils found in the cytoplasmic membrane [57,58]. The pores could work as a safety valve against the pressure increase. This could explain the poor selectivity of these channels toward smaller ions [59]; then Aβ, following the cytoplasmic leak, could be expelled out of the neuron through the pore, then toward the bubbles.
Following the same logic as above, MPTP could rescue the overpressure in the mitochondrial matrix. The opening of the MPTP could depress the matrix, which is a kind of rescue solution that allows mitochondria to produce further energy and prevents metabolic stress.
The cytotoxicity by Aβ as a direct consequence of its action as foam-stabilizing agent: The Gas Model of AD proposed that in the ISF, amyloid fibrils could entrap any minute bubbles in a mixed hydrophobic and water soluble protein web, enclosing progressively the amyloid pores through epitaxial growth (amyloid percolation well model). The soluble Aβ (1-40) would solubilize the amyloid plaque in the ISF, while the insoluble Aβ (1-42) would directly be in contact with the hydrophobic nitrogen bubbles (what is currently called the central hydrophobic cluster of Aβ). The Cerebral Amyloid Angiopathy (CAA) stabilizes the pollutant, since nitrogen as an inert gas is an indestructible component in the humain brain. The pressure is also stabilized. The amyloid percolation well model resolves the issue of the mitochondrial dysfunction and the metabolic/energetic stress related to the Warburg effect and to the pentose phosphate pathway activation (see below). The Gas Model of AD predicts that oxygen nanobubbles are generated within the mitochondria.Therefore, and according to the amyloid percolation well model, Aβ should interact with oxygen bubbles found close to the cytochrome c. This is consistent with Du’s findings of Aβ -rich synaptic mitochondria in the brain of transgenic mice. Coimmunoprecipitation assays carried out for Aβ 1-42 with this subunit confirmed the binding to cytochrome c [23]. Additionnaly, the interaction between Aβ 1-42 and subunit 1 of the cytochrome c oxidase (complex IV) explains the decline in the activity of the complex IV enzyme [60]. This binding is more likely to be linked to a crowding effect (for crowding effect, Ellis [61] and Minton [62]).
Further, I propose that NO is trapped in β-amyloid deposits, because we have described β-amyloid as a foam-stabilizing agent. There is evidence of an increased expression of NO-synthase in the presence of Aβ peptide [63]. In addition, the possible entrapment of NO in amyloid deposists may have enhanced the conditions for a guanylyl cyclase-mediated inflammatory cascade. This is consistent with the activation of microglia after infusion of Aβ [64].
To summarize, Aβ as a foam-stabilizing agent is supposed to stabilize the air bubbles in the ISF (it gives rise to CAA) or having nucleated on respiratory chain proteins (Figures 2 and 4). Aβ prevents further enlargement of bubbles and the danger of a membrane disruption, though it prevents the respiratory chains from working properly. All these hypotheses need further experiments to be confirmed.
Issues related to the amyloid hypothesis: The amyloid percolation well model could put an end to the current confusion surrounding the disease [65] or the disillusionment with the amyloid hypothesis [66], by redefining all the features that define the pathophysiology from the point of view of foam physics. In 1992, the amyloid hypothesis [67] proposed that Aβ accumulation is the primary event in AD pathogenesis, and that AD represents the effects of a chronic imbalance between Aβ production and Aβ clearance. However, some authors consider the hypothesis to be a controversial theory, “primarily because there is a poor correlation between the concentrations and distribution of amyloid depositions in the brain and several parameters of AD pathology, including degree of dementia, loss of synapses, loss of neurons and abnormalities of the cytoskeleton’’ [68]. In the AD Gas Model, APP processing is triggered by the pressure oscillation on the membrane. Since the theoretical invasion of nitrogen through the GLUT nitrogen noria [1] seems to be a normal senile process, Aβ is constitutionally produced and stabilizes bubbles, buffering inert gas and fixing the pressure rise. It complies with previous evidence that Aβ is a normal product of APP metabolism throughout life [69,70] and some humans without symptoms of AD have many cortical Aβ deposits, the latter being diffuse forms of amyloid plaques, consistent with a slow and usual diffuse process of inert gas from the blood to the ISF, in short: brain aging (?)
Figure 2: The antibubble biomachinery in a normal cell and in AD. The upper part depicts the normal NADPH oxidase-NO antibubble biomachinery. To the side, an additional system depicts the hypothetical oxygen bubble overload in AD. The overwhelming of the NADPH oxidase-NO antibubble biomachinery is assisted by the superoxide dismutase, the catalase and finally the involvement of iron. Note that iron has not been depicted [1].
Mutations of APP in familial Alzheimer’s disease (FAD) increase β-protein production [71,72]. Cultured cells which express a β-APP complementary DNA bearing a double mutation found in a Swedish FAD family produce 6-8 times more Aβ than cells expressing normal β-APP [71]. As a matter of fact, cultured cells are exposed to a very low pressure, as a soft tissue. Since the tissue architecture is destroyed, cohesion forces have to disappear. Then, APP slides faster and more easily in the membrane, given rise to enhanced APP processing, and obviously all the more since the protein is mutated. Then Aβ stabilizes bubbles found in the bulk, according to the limitless amount of oxygen and nitrogen.
Twenty years after the amyloid hypothesis, and despite extensive researches, therapies based on this hypothesis have failed to prove any benefits, which is consistent with the idea that the disease is not triggered by APP processing, but by something else (inert gas?), and consistent with the arguments of rare declared opponents of the amyloid hypothesis [73].
To summarize, the Gas Model of AD is consistent with ``the possibility that Aβ deposition may be a common bystander, not an agent provocateur’’ [73]. In such a way, Aβ stabilizes bubbles, preventing their further natural enlargement (Figure 2).
The change of metabolism
Issues related to the Warburg effect: The Warburg effect has been defined as a high rate of glycolysis followed by lactate accumulation in the cytosol in the presence of oxygen [Issues related to the Warburg effect: The Warburg effect has been defined as a high rate of glycolysis followed by lactate accumulation in the cytosol in the presence of oxygen [40]. There is evidence that activities of some glycolytic enzyme sampling from several brains of AD patients at autopsy increase [31]. In an earlier study [37] (1984), the finding that the activities of glycolytic enzymes were lower in AD and in Pick’s disease could be linked to the severity of the disease. The fact that ATPases, acetylcholinesterase and protein kinase were also reduced in the whole temporal lobe is an indication that the patients were extremely ill and finally that biopsy samples contained less living cells than the controls.]. There is evidence that activities of some glycolytic enzyme sampling from several brains of AD patients at autopsy increase [31]. In an earlier study [37] (1984), the finding that the activities of glycolytic enzymes were lower in AD and in Pick’s disease could be linked to the severity of the disease. The fact that ATPases, acetylcholinesterase and protein kinase were also reduced in the whole temporal lobe is an indication that the patients were extremely ill and finally that biopsy samples contained less living cells than the controls.
As a consequence of the above analysis, the Warburg effect is believed to be the consequence of the oxygen bubble toxicity, the bubbles being a kind of respiratory poison. There would just be a physical shift in the oxygen distribution, the oxygen content being the same when comparing normal and AD brains. The normal diffusion process of oxygen in cells [74] involves oxygen monoparticles whereas the same diffusion process seems disturbed according to the AD Gas Model. Nanobubbles take the place of oxygen monoparticules after the huge (though local) increase in the surrounding pressure, especially within mitochondria.
Mitochondial Respiration<=>[ Oxygen nanobubbles within mitochondria][Oxygen monoparticles within mitochondria] Aerobic Glycolysis
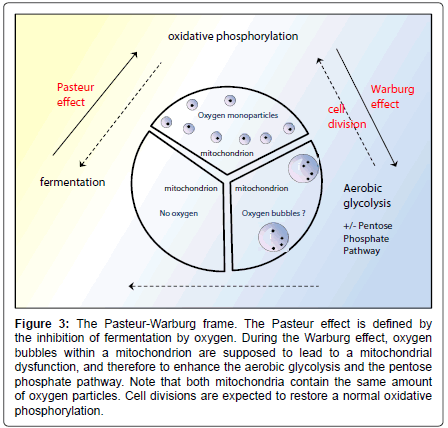
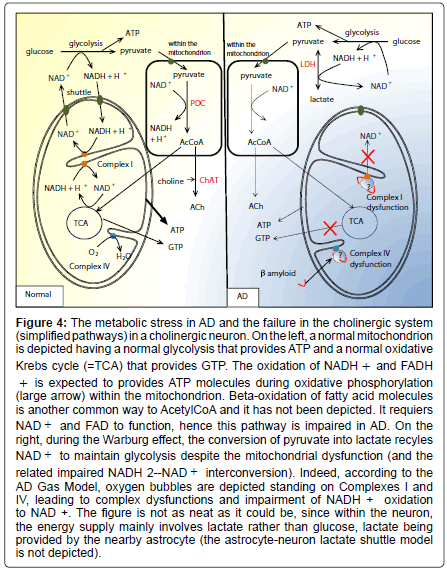
The latter equation comes from the ``Pasteur-Warburg frame’’ (Figure 3), which is a novel picture that summarizes the process shifts. The “Pasteur-Warburg frame”, although hypothetical, shows the link between the physical appearance of oxygen within mitochondria and the predominant metabolism pathway which will occur in the cell. This is in agreement with the Warburg effect in cancer cells, the coupling between respiration and the formation of adenosine triphosphate having been broken [75]. This is also in agreement with the irreversible injury of respiration [75], since once a bubble has grown from a smaller one, there is no possibility to cut it. It seems that there is no “big bubble’’ fission enzyme but just nanobubble fission enzymes such as NOX and SOD that break 2- or 3-oxygen particle bubbles. The bubble-induced Warburg effect complies with the amyloid percolation well model: when Aβ stabilizes an oxygen bubble having nucleated on the respiratory chains, the latter bubble becomes isolated from the bulk and cannot be broken, therefore producing an irreversible inhibition of the respiratory chains (Figure 4). However, in cancer, a cell division might decrease the number of bubbles per mitochondrion and restore a normal respiration process.
Figure 3: The Pasteur-Warburg frame. The Pasteur effect is defined by the inhibition of fermentation by oxygen. During the Warburg effect, oxygen bubbles within a mitochondrion are supposed to lead to a mitochondrial dysfunction, and therefore to enhance the aerobic glycolysis and the pentose phosphate pathway. Note that both mitochondria contain the same amount of oxygen particles. Cell divisions are expected to restore a normal oxidative phosphorylation.
Figure 4: The metabolic stress in AD and the failure in the cholinergic system (simplified pathways) in a cholinergic neuron. On the left, a normal mitochondrion is depicted having a normal glycolysis that provides ATP and a normal oxidative Krebs cycle (=TCA) that provides GTP. The oxidation of NADH + and FADH + is expected to provides ATP molecules during oxidative phosphorylation (large arrow) within the mitochondrion. Beta-oxidation of fatty acid molecules is another common way to AcetylCoA and it has not been depicted. It requiers NAD + and FAD to function, hence this pathway is impaired in AD. On the right, during the Warburg effect, the conversion of pyruvate into lactate recyles NAD + to maintain glycolysis despite the mitochondrial dysfunction (and the related impaired NADH 2--NAD + interconversion). Indeed, according to the AD Gas Model, oxygen bubbles are depicted standing on Complexes I and IV, leading to complex dysfunctions and impairment of NADH + oxidation to NAD +. The figure is not as neat as it could be, since within the neuron, the energy supply mainly involves lactate rather than glucose, lactate being provided by the nearby astrocyte (the astrocyte-neuron lactate shuttle model is not depicted).
Further investigations are needed to assert the mechanistic role of macrobubbles in the Warburg effect. Foams being known to increase optical density, it would be of interest to measure and compare the optical densities of mitochondrial fractions from normal cultured cells, cancer cell lines and cells found in AD brain.
The failure of the astrocyte-neuron lactate shuttle model leads to memory dysfunction in AD: PET studies show a glucose hypometabolism in AD [76,77]. The competition for carbohydrate substrate, such as lactate between astrocytes and healthy neurons, could explain why the said neurons become dysfunctional [78]. In other words, the astrocyte-neuron pair competes to retain lactate, which is consistent with the glucose starvation. The best way to retain lactate within astrocytes is to engage it in another chemical pathway, which prevents lactate from being carried by MCT to the neuron, and prevents further lactate spoliation. This could explain why activities of some glycolytic enzyme samples from several brains of AD patients at autopsy increase [31], which also complies with the Warburg effect in astrocytes and neurons.
Astrocyte-neuron lactate transport is required for long-term memory formation [79], and disrupting the expression of the neuronal lactate transporter MCT2 also leads to amnesia. These observations are helpful to understand memory dysfunction in AD. During the Warburg effect, the conversion of pyruvate into lactate recyles NAD+ (see Equation (4) and Figure 4) to maintain glycolysis despite the mitochondrial dysfunction (and the related impaired NADH2- NAD+ interconversion). It complies with the elevated level of lactate dehydrogenase [31]. However, as seen previously, the energy supply within the neuron mainly involves lactate, and not glucose. Eventually, since the Warburg effect provides much lactate, metabolic exchanges between astrocytes and neurons fail, since neurons become independent from the supply of lactate by astrocytes. The main consequence is logically the impairment of the astrocyte-neuron lactate shuttle, and hence a memory impairment.
The pentose phosphate pathway (PPP) rescues the antibubble biomachinery: According to the AD Gas Model, the coenzyme nicotinamide adenine dinucleotide phosphate (NADPH), is a key molecule during the process of bubble fission and is involved in the following chemical reaction
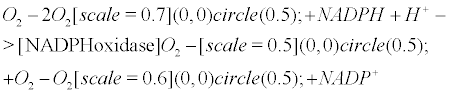
The second (and fourth) step of the pentose phosphate pathway recycles NADPH. Indeed, glucose-6-phosphate could be reduced by glucose-6-phosphate dehydrogenase (G6PD), an enzyme in the pentose phosphate pathway, as it releases NADPH (Equation 8). The triggering of the pentose phosphate pathway is believed to assist the mitochondrial dysfunction, therefore preventing the premature death of neurons and astrocytes from oxygen bubble overload. Eventually, the high NADPH/ NADP+ turnover, resulting from of the overwhelming of the NADPH oxidase-NO antibubble biomachinery, is supported by the PPP.
Issues related to the cholinergic hypothesis: The finding that AChE is lowered in AD has led to the hypothesis that AD is a consequence of a deficit in the cholinergic system. The AD Gas Model is in agreement with this conclusion. The cholinergic hypothesis has led to the development of cholinesterase inhibitors such as donepezil [80,81]. Unfortunately, clinical benefits show neither extensive improvement nor stabilization of the patients [82]. Donepezil, rivastigmine, galantamine and memantine are symptomatic and do not decelerate or prevent the progression of the disease [83]. This is consistent with the irreversibility of the nitrogen leak though the BBB, once it has begun. As a matter of fact, the mitochondrial dysfunction impairs the whole cholinergic system, in reducing the amount of substrate for ACh. Mitochondrial pores and nanobubble-induced crowding of the respiratory chains cause energy depletion due to the disruption from oxidative phosphorylation to aerobic glycolysis (Warburg effect). The early consequences of the mitochondrial dysfunction is that NADH molecules (resulting from the glycolysis (Equation 3), the oxidative decarboxylation of pyruvate to Acetyl-CoA (Equation 6) and the Krebs cycle (Equation 12) are not oxidized anymore to NAD+ in Complex I (NADH dehydrogenase). Therefore Krebs cycle reactions stop, leading to lactate accumulation [84], a metabolic pathway close to the fermentation pathway. The decrease in pyruvate dehydrogenase in post mortem brain tissue from cases of AD [46] appears to be a normal down-regulation process of the enzyme synthesis. Another study concluded that lowered pyruvate dehydrogenase reflected neuronal loss [84]. Hence, within mitochondria, pyruvate cannot be converted into acetylCoA anymore since Reaction (6) requires NAD+ (Figure 4). It leads to reduce the amount of substrate for ACh. Finally, the lowered levels in AChE seem to be the consequence of a normal down-regulation process. On the whole, the mitochondrial dyfunction explains the failures in the cholinergic system.
New insights into the nitrosative stress
The current literature seems confused about the role (if any) of nitrotyrosination and carbonylation in AD [9].
In fact, nitrosative stress raises the issue of why CO2 reacts with ONOO., rather than its putative role.
There is a general acceptance of the role of carbonic anhydrase (CA) in biology: it interconverts carbon dioxide and bicarbonate to maintain acid-base balance. However, to my knowledge, despite the extensive literature on the subject, there is no paper dealing with the fact that CO2 is a gas, and that it should generate nanobubbles within cells or mitochondria if it is not scavenged into bicarbonate or another form. Eventually, the production of this gas seems extremely regulated, and once one CO2 molecule is formed, CA catalyzes its implosion. This could be the main purpose of the celerity of the enzyme, in other words, to prevent the conditions for a bubble enlargement. The CRB is a potent and easy way to understand how CA is involved as an antibubble enzyme (like SOD and NADPH-oxidase), showing that a quantum bubble vanishes.
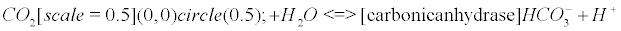 (9)
(9)
However, it is logical to propose that impaired CA may lead to CO2 bubble overload or delay the implosion process. Carbonic anhydrase dysfunction impairs cognition and is associated with mental retardation, AD and aging [85]. When carbonic anhydrase is impaired, the delayed implosion of CO2 is expected to favour the reaction with peroxynitrite, all the more since the rate of reaction of peroxynitrite with CO2 is fast (rate constant pH-independent k = 5.8.104 ) squadrito1998oxidative. Hereafter I recall Equation [1].
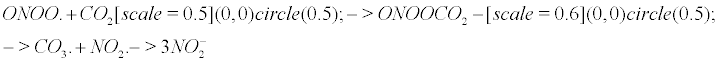
However, the above reaction is not accurate becauseCO2 could be rapidly regenerated after reaction with ONOO. according to the following reaction [13,18,86].
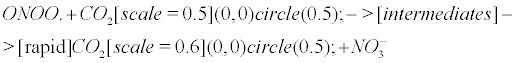 (10)
(10)
Eventually, the peroxynitrite/CO2 system (and incidentally the NO/CO2 system) is deciphered as a CO2 buffer, rescuing the impaired carbonic anhydrase in AD. This complies with the comprehensive statement of the NO antibubble biomachinery, and more generally complies with the previous discovery of several unidentified antibubble pathways. (i) When CO2 is released from the buffer-like peroxynitrite (Equation 10), it can correctly implode through CA, though numerous CAs may be impaired. (ii) WhenCO2is not released from peroxynitrite (Equation 1), it produces nitrite anions and carbonyl radicals after intermediate reactions. These respectively lead to the nitrotyrosination and carbonylation of numerous molecules, but hold the cells free from a CO2 bubble-induced disruption of the cytoplasmic and the mitochondrial membranes.
Nitrotyrosination of proteins may interfere with tyrosine phosphorylation, thereby affecting the activity of the proteins (enzymes). Proteins such as α -actin, α -tubulin [87], tau protein [88] and various other molecules show posttranslational nitrotyrosination in AD, probably leading to impaired axonal transport, or to alterations in the glucose metabolism (nitrotyrosination of triosephosphate isomerase [89]).
Conclusion and Future Directions
All the latter reactions occur during a continuum of oxidative, nitrosative stress and glucose starvation that begins with neuronal and synaptic dysfunction, but that finally leads to the cell death after the complete insulation of the blood brain barrier by the cerebral amyloid angiopathy. The Gas Model of AD has been built by an integrated approach. It proposes that β-amyloid is involved in normal and desirable reactions triggered by the presence of nitrogen in the brain ISF and oxygen bubbles within mitochondria; just as ROS are involved in normal and desirable reactions triggered by the possible pressureinduced gathering of molecular oxygen into nanobubbles; reactive nitrogen species and carbonylation seem able to rescue an impaired carbonic anhydrase. Altogether, they lead to the impairment of specific areas in the brain. On the whole and for the first time since the discovery of the disease one century ago [90], the AD Gas Model can decipher the pathophysiology, and even though many points remain speculative and need further experimentation, I believe it is time to confirm or refute the presence of nitrogen in amyloid deposits.
The AD Gas Model paves the way for new diagnostic and therapeutic approaches to address the most devastating brain disease. It expands the frontiers of neurodegenerative diseases to facilitate understanding of the complexity of cancer
Appendix A: The Law Of Laplace
The Law of Laplace gives the pressure difference between a bubble and the solution:
ΔP = 2.γ / R (11)
non where ΔP is the pressure difference across the fluid interface, γ the surface tension (or wall tension) and R the radius.
Mathematically, the presence of a 15 to 100 diameter bubble would exert a pressure difference of: 300 to 2 000 atm. However, Equation 11 is formally exact, provided the bubble is large enough for γ to be treated macroscopically, and that the thickness of the interface is much smaller than the radius of the bubble goldman 2009 generalizations. The boundary condition for the radius R ( 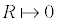 ) demonstrates that a quantum bubble has an almost infinite pressure difference, which in reality represents the repulsive forces originating from the electron cloud of the atom toward the solvent molecules; no particle may penetrate beside this infinitesimal radius. Therefore, the latter calculi about the pressure difference in minute bubbles are not physically granted, they are just an order of magnitude that depicts the huge pressure exerted by nanoscaled bubbles.
) demonstrates that a quantum bubble has an almost infinite pressure difference, which in reality represents the repulsive forces originating from the electron cloud of the atom toward the solvent molecules; no particle may penetrate beside this infinitesimal radius. Therefore, the latter calculi about the pressure difference in minute bubbles are not physically granted, they are just an order of magnitude that depicts the huge pressure exerted by nanoscaled bubbles.
Appendix B: The Krebs Cycle
Within mitochondria of astrocytes, neurons and microglia, the Krebs cycle transforms pyruvate arising from the glycolysis (within the cytoplasm) into CO2, water and ATP.
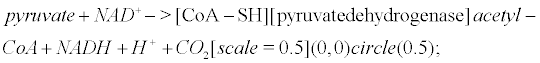
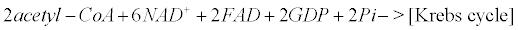
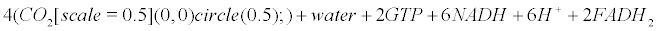 (12)
(12)
Acknowledgements
I am grateful to Cécile Jones for her relevant comments, her technical support concerning the English language, as well as for her tireless availability.
References
- Denis PA (2013) Alzheimer's disease: a gas model. The NADPH oxidase-Nitric Oxide system as an antibubble biomachinery. Med Hypotheses 81: 976-987.
- Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, et al. (2006) Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291: R491-511.
- Block ML (2008) NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci 9 Suppl 2: S8.
- Pólya G (1957) How to solve it. England: Penguin Books; 2nd. ed.
- Bayes T (1991) An essay towards solving a problem in the doctrine of chances. 1763. MD Comput 8: 157-171.
- Ansari MA, Scheff SW (2011) NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med 51: 171-178.
- Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, et al. (2010) NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal 12: 1371-1382.
- Wilkinson BL, Landreth GE (2006) The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer's disease. J Neuroinflammation 3: 30.
- Calabrese V, Sultana R, Scapagnini G, Guagliano E, Sapienza M, et al. (2006) Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer's disease. Antioxid Redox Signal 8: 1975-1986.
- Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, et al. (2007) Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of alzheimer's disease. Brain Res 1148: 243-248.
- Vural H, Sirin B, Yilmaz N, Eren I, Delibas N (2009) The role of arginine-nitric oxide pathway in patients with Alzheimer disease. Biol Trace Elem Res 129: 58-64.
- Keith W, Powell R (1996) Kinetics of decomposition of peroxynitrous acid. Journal of the Chemical Society A: Inorganic, Physical, Theoretical 90-90.
- Squadrito GL, Pryor WA (1998) Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med 25: 392-403.
- Meli R, Nauser T, Latal P, Koppenol WH (2002) Reaction of peroxynitrite with carbon dioxide: intermediates and determination of the yield of CO3*- and NO2*. J Biol Inorg Chem 7: 31-36.
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR (2001) Protein oxidation in the brain in Alzheimer's disease. Neuroscience 103: 373-383.
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, et al. (2006) Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging 27: 1564-1576.
- Guix FX, Ill-Raga G, Bravo R, Nakaya T, de Fabritiis G, et al. (2009) Amyloid-dependent triosephosphate isomerase nitrotyrosination induces glycation and tau fibrillation. Brain 132: 1335-1345.
- Zhang H, Squadrito GL, Pryor WA (1997) The mechanism of the peroxynitrite-carbon dioxide reaction probed using tyrosine. Nitric Oxide 1: 301-307.
- Pohanka M (2013) Alzheimer´s disease and oxidative stress: a review. Curr Med Chem 21: 356-364.
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, et al. (2006) Mitochondria are a direct site of a_ accumulation in alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 15: 1437-1449.
- Devi L, Anandatheerthavarada HK (2010) Mitochondrial trafficking of APP and alpha synuclein: Relevance to mitochondrial dysfunction in Alzheimer's and Parkinson's diseases. Biochim Biophys Acta 1802: 11-19.
- Casley CS, Canevari L, Land JM, Clark JB, Sharpe MA (2002) Beta-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J Neurochem 80: 91-100.
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, et al. (2010) Early de_cits in synaptic mitochondria in an alzheimer's disease mouse model. Proceedings of the National Academy of Sciences 107: 18670-18675.
- Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417: 1-13.
- Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7: 1140-1149.
- Bobba A, Amadoro G, Valenti D, Corsetti V, Lassandro R, et al. (2013) Mitochondrial respiratory chain Complexes I and IV are impaired by β-amyloid via direct interaction and through Complex I-dependent ROS production, respectively. Mitochondrion 13: 298-311.
- Bernardi P (2013) The mitochondrial permeability transition pore: a mystery solved? Front Physiol 4: 95.
- Luque-Contreras D, Carvajal K, Toral-Rios D, Franco-Bocanegra D, Campos-Peña V (2014) Oxidative stress and metabolic syndrome: Cause or consequence of alzheimer's disease? Oxidative Medicine and Cellular Longevity 1-11.
- Grijalba MT, Vercesi AE, Schreier S (1999) Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry 38: 13279-13287.
- Newington JT, Harris RA, Cumming RC (2013) Reevaluating metabolism in alzheimer's disease from the perspective of the astrocyteneuron lactate shuttle model. Journal of Neurodegenerative Diseases 1-13.
- Bigl M1, Brückner MK, Arendt T, Bigl V, Eschrich K (1999) Activities of key glycolytic enzymes in the brains of patients with Alzheimer's disease. J Neural Transm 106: 499-511.
- Guzmán M, Blázquez C (2001) Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab 12: 169-173.
- Badger MR, Price GD (1994) The role of carbonic anhydrase in photosynthesis. Annual review of plant biology 45: 369-392.
- Silverman DN, Lindskog S (1998) The catalytic mechanism of carbonic anhydrase: implications of a rate-limiting protolysis of water. Accounts of Chemical Research 21:30-36.
- Lindskog S (1997) Structure and mechanism of carbonic anhydrase. Pharmacol Ther 74: 1-20.
- Supuran CT, Scozzafava A (2007) Carbonic anhydrase activators as potential anti-alzheimer's disease agents. Protein Misfolding in Neurodegenerative Diseases: Mechanisms and Therapeutic Strategies 265-288.
- Meier-Ruge W, Iwangoff P, Reichlmeier K (1984) Neurochemical enzyme changes in Alzheimer's and Pick's disease. Arch Gerontol Geriatr 3: 161-165.
- Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP (1997) Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci 17: 1046-1054.
- Kim HH, Joo H, Kim T, Kim E, Park SJ, et al. (2009) The mitochondrial warburg effect: A cancer enigma. Interdisciplinary Bio Central 1:1-7.
- Walenta S, Mueller-Klieser WF (2004) Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 14: 267-274.
- Feron O (2009) Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol 92: 329-333.
- White HL, Scates PW (1990) Acetyl-L-carnitine as a precursor of acetylcholine. Neurochem Res 15: 597-601.
- Shinotoh H, Namba H, Fukushi K, Nagatsuka S, Tanaka N, et al. (2000) Progressive loss of cortical acetylcholinesterase activity in association with cognitive decline in Alzheimer's disease: a positron emission tomography study. Ann Neurol 48: 194-200.
- Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, et al. (1999) In vivo mapping of cerebral acetylcholinesterase activity in aging and Alzheimer's disease. Neurology 52: 691-699.
- Tucek S, Cheng SC (1974) Provenance of the acetyl group of acetylcholine and compartmentation of acetyl-CoA and Krebs cycle intermediates in the brain in vivo. J Neurochem 22: 893-914.
- Perry EK, Perry RH, Tomlinson BE, Blessed G, Gibson PH (1980) Coenzyme A-acetylating enzymes in Alzheimer's disease: possible cholinergic 'compartment' of pyruvate dehydrogenase. Neurosci Lett 18: 105-110.
- Palmer AM (1999) The activity of the pentose phosphate pathway is increased in response to oxidative stress in Alzheimer's disease. J Neural Transm 106: 317-328.
- Martins RN, Harper CG, Stokes GB, Masters CL (1986) Increased cerebral glucose-6-phosphate dehydrogenase activity in alzheimer's disease may reflect oxidative stress. Journal of neurochemistry 46: 1042-1045.
- Wittenberg JB, Wittenberg BA (2003) Myoglobin function reassessed. J Exp Biol 206: 2011-2020.
- Koren K, Dmitriev RI, Borisov SM, Papkovsky DB, Klimant I (2012) Complexes of Ir(III)-octaethylporphyrin with peptides as probes for sensing cellular O2. Chembiochem 13: 1184-1190.
- Wittenberg BA, Wittenberg JB (1989) Transport of oxygen in muscle. Annu Rev Physiol 51: 857-878.
- Benzi G1, Moretti A (1995) Are reactive oxygen species involved in Alzheimer's disease? Neurobiol Aging 16: 661-674.
- de Bruin V, Vale M, Viana G, et al. (2000) Lipid peroxidation and nitrite plus nitrate levels in brain tissue from patients with alzheimer's disease. Gerontology 46: 179-184.
- Othman G (2012) Do increased levels of oxysterols underlie alzheimer's disease/parkinson's disease overlap? Journal of Alzheimer's Disease & Parkinsonism 2012.
- ;Shoffner JM, Brown MD, Torroni A, Lott MT, Cabell MF, et al. (1993) Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics 17: 171-184.
- ;Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, et al. (2000) Intraneuronal Abeta42 accumulation in human brain. Am J Pathol 156: 15-20.
- ;Lashuel HA, Hartley DM, Petre BM, Wall JS, Simon MN, et al. (2003) Mixtures of wild-type and a pathogenic (E22G) form of Abeta40 in vitro accumulate protofibrils, including amyloid pores. J Mol Biol 332: 795-808.
- ;Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT Jr (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418: 291.
- ;Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC (2002) The channel hypothesis of Alzheimer's disease: current status. Peptides 23: 1311-1315.
- Shoffner JM, Brown MD, Torroni A, Lott MT, Cabell MF, et al. (1993) Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics 17: 171-184.
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, et al. (2000) Intraneuronal Abeta42 accumulation in human brain. Am J Pathol 156: 15-20.
- ;Minton AP (1998) Molecular crowding: analysis of effects of high concentrations of inert cosolutes on biochemical equilibria and rates in terms of volume exclusion. Methods Enzymol 295: 127-149.
- ;Baltrons M, Pedraza C, Heneka M, Garcia A (2002) Beta-amyloid peptides decrease soluble guanylyl cyclase expression in astroglial cells. Neurobiology of disease 10: 139-149.
- ;Frautschy S, Cole G, Ard M (2002) Beta amyloid protein clearance and microglial activation. In: Streit, W., editor. Microglia in the regenerating and degenerating central nervous system. Spinger 245.
- George P (2012) A pleotrophic age for alzheimer and parkinson disease. Journal of Alzheimer's Disease & Parkinsonism 2012.
- ;Kumar S (2012) Can the failure of alzheimer's disease clinical trials be stepping stones to ultimate success: Is the amyloid hypothesis discredited by animal models. Journal of Alzheimer's Disease & Parkinsonism.
- ;Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356.
- ;Neve RL, Robakis NK (1998) Alzheimer's disease: a re-examination of the amyloid hypothesis. Trends Neurosci 21: 15-19.
- ;Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, et al. (1992) Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature 359: 325-327.
- ;Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, et al. (1992) Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258: 126-129.
- Lashuel HA, Hartley DM, Petre BM, Wall JS, Simon MN, et al. (2003) Mixtures of wild-type and a pathogenic (E22G) form of Abeta40 in vitro accumulate protofibrils, including amyloid pores. J Mol Biol 332: 795-808.
- Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT Jr (2002) Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature 418: 291.
- Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC (2002) The channel hypothesis of Alzheimer's disease: current status. Peptides 23: 1311-1315.
- Hernandez-Zimbron LF1, Luna-Muñoz J, Mena R, Vazquez-Ramirez R, Kubli-Garfias C, et al. (2012) Amyloid-Beta peptide binds to cytochrome C oxidase subunit 1. PLoS One 7: e42344.
- Warburg O, et al. (1956) On the origin of cancer cells. Science 123: 309-314.
- Ellis RJ (2001) Macromolecular crowding: obvious but underappreciated. Trends Biochem Sci 26: 597-604.
- Minton AP (1998) Molecular crowding: analysis of effects of high concentrations of inert cosolutes on biochemical equilibria and rates in terms of volume exclusion. Methods Enzymol 295: 127-149.
- Baltrons M, Pedraza C, Heneka M, Garcia A (2002) Beta-amyloid peptides decrease soluble guanylyl cyclase expression in astroglial cells. Neurobiology of disease 10: 139-149.
- Kumar S (2012) Can the failure of alzheimer's disease clinical trials be stepping stones to ultimate success: Is the amyloid hypothesis discredited by animal models. Journal of Alzheimer's Disease & Parkinsonism
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356.
- Neve RL, Robakis NK (1998) Alzheimer's disease: a re-examination of the amyloid hypothesis. Trends Neurosci 21: 15-19.
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, et al. (1992) Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature 359: 325-327.
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, et al. (1992) Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258: 126-129.
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, et al. (1992) A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet 1: 345-347.
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, et al. (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature 360: 672-674.
- Roses AD (1994) Apolipoprotein E affects the rate of Alzheimer disease expression: beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol 53: 429-437.
- Denis PA (2014) Heuristic consequences of a load of oxygen in microtubules. Biosystems 118C: 17-30.
- Mosconi L, Pupi A, De Leon MJ (2008) Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann N Y Acad Sci 1147: 180-195.
- Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178-201.
- Demetrius LA, Simon DK (2012) An inverse-Warburg effect and the origin of Alzheimer's disease. Biogerontology 13: 583-594.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14334
- [From(publication date):
June-2014 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 9805
- PDF downloads : 4529