Research Article Open Access
The Cognitive Impairment of Elderly Living with Human Immunodeficiency Virus (HIV): A Cross-Sectional Study about the Role of Viral Neurotoxicity
João Luiz Cioglia Pereira Diniz, Unaí Tupinambás, Ludimila Labanca, Sheila Melo Barbara, Oliveira Souza and Denise Utsch-Gonçalves*Faculty of Medicine, Federal University of Minas Gerais, Brazil
- *Corresponding Author:
- Denise Utsch Gonçalves
Tropical Medicine Post Graduation Program, Faculty of Medicine, Federal University of Minas Gerais
Av. Prof. Alfredo Balena, Sala 190, Belo Horizonte, Minas Gerais, CEP 30100130, Brazil
Tel: + 55 31 34099767
Fax: 55 31 34099767
E-mail: deniseg@medicina.ufmg.br
Received date: July 30, 2016; Accepted date: August 31, 2016; Published date: September 02, 2016
Citation: Diniz JLCP, Tupinambs U, Labanca L, Barbara SM, Souza O, et al. (2016) The Cognitive Impairment of Elderly Living with Human Immunodeficiency Virus (HIV): A Cross-Sectional Study about the Role of Viral Neurotoxicity. J Neuroinfect Dis 7:224. doi: 10.4172/2314-7326.1000224
Copyright: © 2016 Diniz JLCP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Aging with HIV has been associated with a higher frequency of neurocognitive disorders. The auditory evoked potentials P300 evaluate cognitive function. In a cross-sectional study, we analyzed the auditory cognition of elderly living with HIV through P300. We compared 34 HIV-infected elderly undergoing regular treatment to 76 HIV-negative elderly (controls) according to P300 latency and the scores of neuropsychological tests. The groups were stratified into age subgroups: 50 ≥ 59, 60 ≥ 69 and ≥70 years. Each age subgroup infected with HIV was compared to the age subgroup of controls immediately older. HIV group consisted of 20 (61%) men, mean age 61 ± 7 years and controls of 24 (32%) men, mean age 67 ± 4 years. Years of schooling were seven (interquartile range 4/8) in HIV group against four (4/11) in controls (p=0.044). P300 latency was similar between genders in the groups. P300 latency was 353 ± 35 ms in HIV group and 331 ± 29 ms in controls (p=0.006). In within-analysis, P300 latency augmented with the increase of age in HIV group (p=0.001) and remained stable in controls (p=0.252). In between-analysis, P300 latency was delayed in HIV subgroup 60 ≥ 69 as compared to controls ≥70 years (p=0.033) and delayed in HIV subgroup ≥70 years as compared to controls ≥70 years (p<0.001). In neuropsychological tests, HIV group presented poor performance in Nine Hole test (p=0.029) and correlation was found between an altered P300 and poor performance to execute the task with the dominant hand (p=0.043). We concluded that even under regular treatment, HIV infection may accelerate the cognitive impairment in the aging.
Keywords
HIV evolution; Anti-retroviral therapy; Escape from immune responses; Persistent
Introduction
The overall prevalence of human immunodeficiency virus (HIV) infection has been stable in this decade, although statistics have shown an increase in the absolute number of HIV-infected individuals due to the reduction in mortality allied to the increase in the survival rate [1]. Consequently, the demographic curve of age has changed over the past ten years. The proportion of individuals living with HIV over the age of 50 increased from 13.3% in 2001 to 16% in 2010 [2]. The largest relative increase in this population occurred among those aged 65 or more [2]. In Brazil, a significant increase in the AIDS number of cases occurred in both sexes in the individuals aged 60 or more [3].
The determinant factor for the demographic augmentation of elderly people living with HIV was the introduction in 1996 of the combined antiretroviral therapy (cART) [2,4]. However, despite the advance in HIV treatment and control, the life expectancy continuous reduced in about ten years when compared to uninfected people [4,5]. The consequences for the brain of the long-term infection and extensive exposure to cART are still unknown. In general, after the advent of cART, the incidence of HIV-associated dementia has been reduced from 30% to 10% [6]. But even after an effective cART and a consequent undetectable HIV plasma viremia, HIV-associated neurocognitive disorder (HAND) persists more frequently than the expected [4-6]. A challenge is the access to the HIV viral reservoir in central nervous system (CNS) to complete eradication of the virus, which involves epigenetic studies, including the virus gene expression and the mechanisms of transcriptional latency [7].
The HIV infection seems to accelerate the cognitive alterations associated with aging and to predispose to HAND even under regular cART [8-13]. HAND causes cognitive, motor, behavioral abnormalities and the degree to which aging can alter performance in neuropsychological tests is not uniform [6]. In addition, coexisting morbidities are common in this population and can independently cause impact to the neuropsychological performance [8]. The current diagnosis of HAND has been based on clinical criteria, associated with neuropsychological tests and exclusion of other causes of dementia [14,15]. In the USA, about 50% of individuals with HIV demonstrate performance in neuropsychological tests that is below expectations in terms of age, education and ethnicity when compared to the pattern of uninfected individuals of the same age group [8,9].
Electrophysiological tests have been studied for the diagnosis of HAND and auditory evoked potential P300 is accurate, noninvasive, safe, easy to perform, not expensive and clinically reliable for an early diagnosis of neurodegenerative diseases such as Alzheimer’s disease [16-21].
P300 is an electrophysiological potential generated by the recognition of auditory stimuli [19-21]. It represents the cortical activity involving skills of discrimination, integration and attention, being an indicator of the cortical processing speed [21]. Therefore, P300 is an endogenous potential that reflects the cognitive skills [19]. In the electrophysiological wave, the components N100, P200 and N200 precede P300 [20]. Both early components N100 and P200 reflect the sensorial processing and are of low value in diagnosing and monitoring cognitive impairment; N200 and P300 reflect cognitive processing, but P300 is more useful than N200 in diagnosing and monitoring cognitive deficit [21]. P300 starts in several areas of the cortex, mainly in the temporoparietal area [19-22].
People living with HIV have a decline in their mental skills that may be related to aging, comorbidities, viral neurotoxicity and cART [23-27]. The alteration in P300 has been shown to be the earliest sign in the process of cognitive loss [26-29]. The present study aimed at analyzing P300 and the neuropsychological tests in older adults with HIV by comparing them to a group of seronegative elderly people.
Materials and Methods
In this cross-sectional study, the group of elderly people infected with HIV consisted of patients aged 50 years or more followed at the referral center for infectious and parasitic diseases of Minas Gerais state (Southeast Brazil). The geriatric age for this population starts ten years earlier when compared to that of uninfected people (60 years) due to the senility effect caused by the HIV infection [4-6,8-9]. The group of individuals not infected with HIV (controls) consisted of healthy people followed at the referral center for geriatrics of Minas Gerais state that takes part in a multidimensional evaluation program for elderly people. The individuals from both groups received the invitation to participate in 2014 when they attended the scheduled medical consultation in each referral center. The inclusion of participants in each group followed the schedule of programmed consultation in each service so that the gender in each group was not controlled.
The exclusion criteria were severe or profound hearing loss, use of psychoactive drugs, psychiatric and/or neurological diseases, which were considered in the anamnesis and clinical examination. During the medical interview, depression was excluded based on the Brazilian version of the original geriatric depression scale of 30 items, considering the cut-off of 10/11 [30,31]. We used also the review of the medical records to guarantee the exclusion of neurologic diseases, psychotic disorders and mood disorders. In HIV group, the inclusion criterion was regular use of medication with adherence to follow-up. Moreover, the variables CD4+ cells and years of infection were considered to guarantee the control of HIV infection. In the control group, the inclusion criterion was the negative test for HIV. The participants received the orientation to sleep at least six hours in the night before the tests since sleep deprivation is associated with alteration in P300 latency and amplitude [32]. No one received monetary compensation for their participation. The selected participants underwent clinical and neurological exams and were submitted to neuropsychological tests to evaluate specific skills: Raven Colored Matrices (general intelligence), Rey Auditory Verbal Learning Test (verbal memory and learning), Five Digits Test (processing speed), Nine Hole Test (motor ability, speed and attention), Frontal Assessment Battery (concept formation, abstraction, mental flexibility, motor programming, inhibitory control and autonomy) [33].
The Auditory Evoked Potential-P300
The exam was conducted in an electrical and acoustic isolated environment with the individual in the seated position. The position of the electrodes was the frontal, vertex and parietal midline in relation to the bi-auricular reference using the auditory oddball paradigm [19,20,32]. The stimulus was the tone burst, using the frequencies of 1000 Hz as the frequent stimulus and 2000 Hz as the rare stimulus (MASBE model Contronic®, Brazil). The auditory intensity was 90 dB hearing level. The protocol was 300 stimuli delivered every 1 second, being 20% at 2000 Hz (stimuli rarely identified by the subject) and 80% at 1000 Hz. The individual identified and counted silently the rare stimuli. The sign of the electroencephalogram was augmented 50.000 times. The examiners measured the latency of waves N100, P200, N200 and P300 and the amplitude of the complex N200-P300. Two independent examiners performed the measurements of the latencies and amplitudes.
After the rare stimulus, N100 was the highest negative peak between 75-150 ms, P200 followed as the highest positive peak between 150-270 ms and N200 followed as the highest negative peak between 150-350 ms; P300 occurred as the highest positive peak between 250-500 ms preceded by N100, P200 and N200 [19,20,32]. The amplitudes were calculated using the subtraction of the measure of the peaks of the waves N200 and P300.
Parameters
The delayed P300 latency defined the auditory processing disorder (dependent variable). The independent variable was the presence of HIV infection. The latency of P300 was compared within-groups, between-groups and also with the scores of the neuropsychological tests, that were measured by the time spent to complete the task. The comparative analysis considered the stratification of age in subgroups of 50-59 years (G1), 60-69 years (G2) and 70 years or more (G3). Each age subgroup infected with HIV was compared to the age subgroup of controls immediately older in order to counterbalance the effect of early aging caused by HIV infection [8,9].
Statistical analysis
The database was built in Epidata®. We conducted the analysis in the free software R version 3.0.3. The descriptive analysis classified the variables of central tendency and defined variability. We used the Shapiro-Wilks test to assess the distribution of the continuous variables. Normally distributed variables were reported as mean ± standard deviation (SD) and compared with analysis of variance (ANOVA). Data that were not normally distributed were reported as median with interquartile range (IR) and compared with Mann Whitney test. Categorical variables were reported as number and percentage and compared with chi-square test. The significance level was 5% and 95% confidence intervals (CI) were built.
Ethical aspects
Approval was obtained by the Ethics Committee of the Federal University of Minas Gerais, number 0733.0.203.000-12. All participants gave voluntary written consent and declared they were aware of the procedures and their freedom to participate. All the procedures have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Results
During the study, 120 elderly people infected with HIV received the invitation to participate, of whom 86 were excluded because of either refusing to participate or fulfilling the exclusion criteria.
Therefore, 34 elderly people in regular use of HAART were the selected population of the HIV group. For the group of elderly people without HIV (controls), 90 received the invitation to participate, of which 80 accepted and they were tested HIV negative.
Table 1 shows the demographic variables considered in the study. In the HIV group, mean time of diagnosis was 13 ± 6 years, mean CD4+ cells were 628 ± 238, mean CD4+ nadir was 282 ± 156. Correlation was not observed between P300 latency and time of diagnosis (p=0.538), CD4+ cells (p=0.575) and CD4+ nadir (p=0.327). The viral load was undetectable for all the participants.
| Variablea | HIV group (n=34) | Controls (n=76) | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Gender, Male | 20 (61) | 24 (32) | 0.005 | |||||
| Education, years | 7 (4/11) | 4 (4/8) | 0.044 | |||||
| Hours of sleep | 7 (6/8) | 6 (5/8) | 0.172 | |||||
| Age, years | 60.7 ± 7.1 | 66.8 ± 3.8 | <0.001 | |||||
| HIV Subgroups (n)b | ||||||||
| Age, years | G1-pos (17) | G2-pos (10) | G3-pos (7) | G2-neg (52) | G3-neg (24) | |||
| 55.0 ± 2.9 | 62.6 ± 2.3 | 71.9 ± 1.6 | 65.0 ± 2.4 | 71.8 ± 1.7 | ||||
| aData are expressed as mean value ± SD; median (interquartile range), or absolute numbers (percentage); bG1=50 = 59 years; G2=60 = 69 years; G3 = 70 years; pos=positive; neg=negative | ||||||||
Table 1: Demographic data of 34 elderly people with HIV and 76 elderly people without HIV (controls).
The evoked potentials related to events P300
P300 was similar between genders; it was delayed in HIV group (p=0.006). Analysis of variance compared the P300 latency according to age subgroups (Table 2). The latencies of N100 (p=0.262), P200 (p=0.419), N200 (p=0.753), and amplitude of N200-P300 (p=0.784) were similar between-groups.
| P300 Latency (ms)a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comparisons Mean ± SDb |
Gender (n) | p-value | ||||||
| Male (44) | Female (66) | |||||||
| HIV | 355.74 ± 21.84 (20) | 342.53 ± 35.55 (14) | 0.338 | |||||
| Controls | 332.23 ± 23.43 (24) | 330.91 ± 15.01(52) | 0.873 | |||||
| HIV status (n) | ||||||||
| Positive (34) | Negative (76) | 0.006 | ||||||
| 353.64 ± 35.15 | 331.33 ± 29.53 | |||||||
| Sub groupsc | ||||||||
| G1-pos | G2-pos | G3-pos | G2-neg | G3-neg | ||||
| Within-groups Analysisd | ||||||||
| HIV | 339.43 ± 26.95 | 355.19 ± 13,71 | 382.97 ± 17.66 | - | - | 0.001 | ||
| Controls | - | - | - | 332.06 ± 29.93 | 340.32 ± 18.57 | 0.252 | ||
| Between-groups Analysise | ||||||||
| G1-pos X G2-neg |
339.43 ± 26.95 | - | - | 332.06 ± 29.93 | - | 0.356 | ||
| G2-pos X G3-neg |
- | 355.19 ± 13.71 | - | - | 340.32 ± 18.57 | 0.033 | ||
| G2-pos X G2-neg |
- | 355.19 ± 13.71 | - | 332.06 ± 29.93 | - | 0.020 | ||
| G3-pos X G3-neg |
- | - | 382.97 ± 17.66 | - | 340.32 ± 18.57 | <0.001 | ||
| a Millisecond. b Standard deviation. c G1=50 = 59 years, G2=60 = 69 years and G3 = 70 years. d P300 latency augmented with the increasing of age in HIV group (p=0.001) and remained stable in the controls. e P300 latency was delayed in HIV group=60 = 69 years compared to controls = 70 years and also delayed in HIV group =70 years compared to controls =70 years. | ||||||||
Table 2: Comparison of P300 latency in the group with HIV (n=34) and without HIV (n=76) according to gender and age subgroups.
Neuropsychological tests and P300
Among the participants, 10 from the control group and two from the HIV group did not finish the battery of neuropsychological tests and so they were not considered in the forward analysis. Table 3 shows the results.
| Neuropsychological testing | HIV status | Mean (SD) | p-value* |
|---|---|---|---|
| Raven Colored Matrices | Negative | 21.51 (6.22) | 0.329 |
| Positive | 22.88 (7.21) | ||
| Rey Auditory Verbal Learning | Negative | 40.18 (8.41) | 0.385 |
| Positive | 38.42 (11.35) | ||
| Five digits | Negative | 89.37 (37.17) | 0.677 |
| Positive | 84.95 (52.64) | ||
| Nine Hole (dominant hand) | Negative | 21.22 (2.75) | 0.007 |
| Positive | 23.42 (5.01) | ||
| Nine Hole (non-dominant hand) | Negative | 22.09 (3.17) | 0.029 |
| Positive | 23.86 (4.41) | ||
| Frontal Assessment Battery | Negative | 15.15 (2.18) | 0.554 |
| Positive | 15.47 (2.82) | ||
| The Nine Hole test evaluates attention and the HIV group executed this test worse than control group using either the dominant hand or the non-dominant hand. | |||
Table 3: Comparative analysis of the neuropsychological tests of the group with HIV (n=32) and without HIV (n=66).
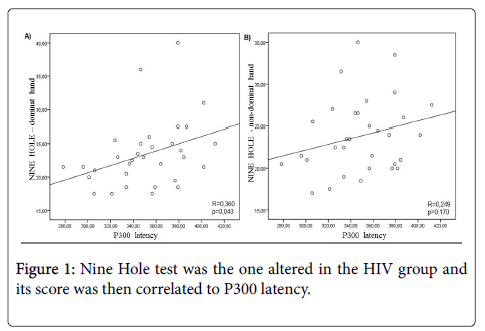
Among the neuropsychological tests, Nine Hole test was the one altered in the HIV group and its score was then correlated to P300 latency (Figure 1).
Discussion
P300 alteration in the HIV-infected people was found here and had already been previously reported [23,34-36]. In our study, older seronegative controls were compared to a group of younger and properly treated people living with HIV with confirmed undetectable viral load. The compared groups were different in the proportion of gender, since they represented the demographic features of the elderly in each population. Among those living with HIV, men predominate and among the healthy elderly, women predominate. In this study and according to the literature, P300 was similar in both genders (Table 2) [37-39].
P300 latency increases with aging [37-39]. The value of P300 latency in individuals between 45 and 79 years of age can vary between 331.71 ms and 407.50 ms and an increase of 1.8 ms to 2.9 ms per year of age has been observed [37-39]. In this study, the average P300 latency in both groups was within the normal range; however, it should be noted that despite the relative younger age of the HIV group compared to controls, the mean latency of P300 in the infected group was delayed in the age subgroup of 60 ≥ 69 years and ≥70 years (Table 2). The analysis by age subgroups showed the effect of early aging in case of HIV infection (Table 2). P300 latency delay reflects the degree of cognitive decline during the process of dementia [40].
The latencies of N100, P200 and N200 were similar in the groups. Chao et al. analyzed 15 HIV-infected individuals, mean age 44 years, and agreed that the P300 wave is the main target of alteration in this population [35]. Longitudinal comparative study of patients with Alzheimer's disease did not show alteration in the latencies of N100, P200 and N200, which indicates that the patients are able to keep intact the start of the sensory processing [17].
The years of schooling can interfere in P300. The higher the level of education, the abler the person is to perform the task that generates the P300 wave [19-21]. Valcour et al. evaluated the number of years of schooling in a cohort of people aging with HIV and pointed out to the fact that maybe the aging did not influence the performance in neuropsychological tests when the analysis considers seronegative controls consisting of individuals from similar socioeconomic backgrounds [41]. Additionally, the comorbid illnesses can also modulate frequency of cognitive disorders in the elderly living with HIV [8,13]. Prolonged exposure to cART augments the comorbidities such as dyslipidemia, coronary artery disease, diabetes mellitus, all recognized as risk factors for dementia [8,42]. A chronic inflammation related to HIV infection predisposes to vascular events and the guidelines have indicated cART as earliest as possible [42,43]. The studies about the treatment with statins, regardless of cholesterol levels, are underway [42]. Both strategies aim at reducing the inflammatory process. Furthermore, it is highly recommended that this population ceases smoking and engages into a healthy lifestyle.
In the current study, although the individuals of the HIV group had more years of schooling and were younger comparatively to controls (Table 1), P300 latency was worse among them (Table 2). This data supports the theory of progressive neurotoxicity in CNS related to HIV aging even under regular cART and adequate viral control [33,35,44]. A chronic form of CNS-immune reconstitution inflammatory syndrome (CNS-IRIS) may occur [44,45]. The occurrence of CNS-IRIS in the absence of opportunistic infections may be due to an exaggerated immune response to HIV, auto-antigens, poor CNS antiretroviral drug distribution or even a drug-induced neurotoxicity [46,47].
Antiretroviral regimens with good CNS penetration are presumably more effective in controlling viral replication than regimens with poorer penetration [46]. However, even under undetectable viral load in the peripheral blood, the viral replication may persist in the CNS [35]. Interestingly, cART with good CNS penetration was already associated with poorer neurocognitive performance in neuropsychological tests [48]. The explanation may be associated with the epigenetic mechanisms such as the DNA methylation and the histone modification that can promote alterations in genes expression when influenced by the environment [49-51]. Many epigenetic modifiers play an essential role for the maintenance of a latent infection and the long term cART might favor aberrant epigenetic modifications that alter the genes expression that induces cognitive dysfunction, being one more possible risk factor to the cognitive impairment related to HIV [7,52-54].
The cognitive effects of HIV on the CNS involve alteration in attention, working memory, speed of information processing, learning efficiency, executive functions and abstraction, whose pattern of alterations is consistent with the involvement of subcortical or frontostriatal brain systems [12,55,56]. The neuropsychological tests that include multiple domains are sensitive to detect cognitive impairment related to HIV infection [14]. However, they are not able to determine HAND or light motor cognitive disorders [11]. In this study, comparing to controls, the elderly living with HIV took longer to execute the Nine Hole test, which depends on motor skills, and P300 delay was correlated with a longer time to execute the task (Table 3). Possibly, this finding may be an indication of an early cognitive impairment. Subcortical dementia associated with HIV causes alterations in motor skills at an early stage of the disease [5,6]. Brain damage caused by HIV occurs frequently at the temporoparietal junction and includes posterior hippocampus, posterior temporal plane, superior temporal sulcus, anterior and medial temporal lobe and injuries in these areas alter P300 [55,56].
Aging in general population is associated with an increase in P300 latency and longer time to execute Nine Hole test [37-39]. In the present study, the negative correlation of P300 and Nine Hole test in HIV group could not be explained only by the aging effect, since the HIV population was younger than the controls (Table 1). In fact, aging and HIV neurotoxicity might be additive factors in the expression of cognitive decline. Perhaps, the people living with HIV aged more than 50 years should be screened for cognitive impairment in order to allow early interventions. For example, several studies have suggested that auditory training has positive consequences to cognitive and social function of the elderly [57,58].
Finally, we showed that cognitive decline in people living with HIV seems to occur earlier than the expected. P300 and Nine Hole test might be considered in short form protocols for screening of cognitive disorders since these tests are quick and easy to perform, non-invasive and low-cost. Neuropsychological and electrophysiological tests are established to assess cognition and are complementary [14,18-21]. Nine Hole test evaluates sustained attention, information processing speed, motor functioning and working memory while P300 evaluates skills of discrimination, integration and attention. Recent longitudinal studies demonstrate that the mild cognitive impairment progression to HAND within several years, so that the screening of asymptomatic neurocognitive impairment is important [14-21, 59,60].
The most important limitation of this study was the cross-sectional design. It cannot be determined whether the exposure to HIV caused the cognitive decline. Due to this, we planned to work with a control group of older uninfected people in order to minimize the age effect as a possible confounding factor in the association between HIV infection and cognitive impairment.
Conclusion
Aging with HIV was shown to be associated with a subclinical decline in the cognition. The mild impairment could not be explained either by the aging effect or by the persistent of viral load and it occurred in people living with HIV with higher educational level. Taking into account the available treatment for cognitive disorders, a universal screening for asymptomatic neurocognitive impairment might be considered in HIV-infected people with more than 50 years.
Acknowledgments
This work received Grant for the students of the National Council for Scientific and Technological Development (CNPq) and of the Foundation to support the research of the state of Minas Gerais (FAPEMIG).
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Lo S, Horton R (2015) AIDS and global health: the path to sustainable development. Lancet 386: 106-108.
- http://www.cdc.gov/hiv/pdf/statistics_2011_hiv_surveillance_report_vol_23.pdf
- http://www.aids.gov.br/sites/default/files/anexos/page/2011/49056/_p_boletim_2010_ingles_pdf_p__14798.pdf
- Erlandson KM, Schrack JA, Jankowski CM (2014) Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS 11: 279-290.
- Gannon P, Khan MZ, Kolson DL (2011) Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr Opin Neurol 24: 275-283.
- Valcour V, Sithinamsuwan P, Letendre S, Ances B (2011) Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep 8: 54-61.
- du Chéné I, Basyuk E, Lin YL, Triboulet R, Knezevich A, et al. (2007) Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J 26: 424-435.
- Tedaldi E, Minniti N, Fischer T (2015) HIV-Associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int Biomed Res Int 2015: 1-12.
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ (2014) Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS 181: 11-18.
- Kaul M (2004) HIV-1 associated dementia: update on pathological mechanisms and therapeutic approaches. Curr Opin Neurol 22: 315-320.
- Heaton RK, Grant I, Butters N, White DA, Kirson D, et al. (1995) The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc 1: 231-251.
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, et al. (1993) Dementia in AIDS patients: Incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 43: 2245-2252.
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, et al. (2004) Higher frequency of dementia in older HIV-1 individuals: the Hawaii aging with HIV-1 Cohort. Neurology 63: 822-827.
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, et al. (2007) Updated research nosology for HIV associated neurocognitive disorders. Neurology 69: 1789-1799.
- Clifford DB, Ances BM (2013) HIV-Associated Neurocognitive Disorder (HAND). Lancet Infect Dis 13: 976-986.
- Bonanni L, Franciotti R, Onofrj V, Anzelloti M, Mancino E, et al. (2010) Revisiting P300 cognitive studies for dementia diagnosis: early dementia with Lewy bodies (DLB) and Alzheimer disease (AD). Neurophysiol Clin 40: 255-265.
- Lai CL, Lin RT, Liou LM, Liu CK (2010) The role of event-related potentials in cognitive decline in Alzheimer’s disease. Clin Neurophys 121: 194-199.
- Katada E, Sato K, Ojika K, Ueda R (2004) Cognitive event-related potentials: useful clinical information in Alzheimer’s disease. Curr Alzheimer Res 1: 63-69.
- Amin HU, Malik AS, Kamel N, Chooi WT, Hussain M (2015) P300 correlates with learning & memory abilities and fluid intelligence. J Neuroeng Rehabil 12: 87.
- Polich J, Herbst KL (2000) P300 as a clinical assay: rationale, evaluation, and findings. Intern J Psychophys 38: 3-19.
- Gironell A, García-Sánchez C, Estévez-González A, Boltes A, Kulisevsky J (2005) Usefulness of P300 in subjective memory complaints: a prospective study. J Clin Neurophysiol 22: 279-284.
- Katayama J, Polich J (1998) Stimulus context determines P3a e P3b. Psychophysiology 35: 23-33.
- Ollo C, Johnson R, Grafman J (1991) Signs of cognitive change in HIV disease: an event-related brain potential study. Neurology 41: 209-215.
- Schroeder MM, Handelsman L, Torres L, Dorfman D, Rinaldi P, et al. (1994) Early and late cognitive event-related potentials mark stages of HIV-1 infection in the drug-user risk group. Biol Psychiat 35: 54-69.
- Cysique L, Vaida F, Letendre S, Gibson S, Cherner M, et al. (2009) Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology 73: 342-348.
- Birchall M, Wight R, French P, Cockbain Z, Smith S (1992) Auditory function in patients infected with the human immunodeficiency virus. Clin Otolaryngol Allied Sci 17: 117-121.
- Nielsen-Bohlman L, Fein G, Boyle D, Ezekiel F (2002) N400 event-related potential reduction indexes: early central nervous system impairment in HIV. J NeuroAIDS 2: 51-65.
- Bankaitis AE, Keith RW (1995) Audiological changes in associated with HIV infection. Ear Nose Throat J 74: 353-359.
- Farnarier G, Somma-Mauvais H (1990) Multimodal evoked potentials in HIV infected patients. Electroencephalogr Clin Neurophysiol Suppl 41: 355-369.
- Castelo MS, Coelho-Filho JM, Carvalho AF, Lima JW, Noleto JC, et al. (2010) Validity of the Brazilian version of the Geriatric Depression Scale (GDS) among primary care patients. Int Psychogeriatr 22: 109-113.
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, et al. (1983) Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 17: 37-49.
- Danos P, Kasper S, Scholl HP, Kaiser J, Ruhrmann S, et al. (1994) Clinical response to sleep deprivation and auditory-evoked potentials – preliminary results. Pharmacopsychiatry 27: 70-71.
- Bloch M, Kamminga J, Jayewardene A, Bailey M, Carberry A, el al. (2016) A screening strategy for HIV-associated neurocognitive disorders that accurately identifies patients requiring neurological review. Clin Infect Dis 63:687-693.
- Stenklev NC, Laukli E (2004) Cortical Cognitive Potentials in Elderly Persons. J Am Acad Audiol 15: 401-413.
- Chao LL, Lindgren JA, Flenniken DL, Weiner MW (2004) ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clin Neurophysiol 115: 1583-1591.
- Polich J, Ilan A, Poceta JS, Mitler MM, Darko DF (2000) Neuroelectric assessment of HIV: EEG, ERP, and viral load. Int J Psychophysiol 38: 97-108.
- Fein G, Turetsky B (1989) P300 latency variability in normal elderly: effects of paradigm and measurement technique. Electroencephalogr Clin Neurophysiol 72: 384-394.
- Pfefferbaum A, Ford J, Roth W, Hopkins W, Kopell B (1979) Event-related potential changes in healthy aged females. Electroencephalogr Clin Neurophysiol 46: 81-86.
- Brown W, Marsh J, La Rue A (1983) Exponential electrophysiological aging: P300 latency. Eletroencephalogr Clin Neurophysiol 55: 277-285.
- Polich J, Ehlers CL, Otis S, Mandell AJ, Bloom FE (1986) P300 latency reflects the degree of cognitive decline in dementing illness. Electroencephalogr Clin Neurophysio 63: 138-144.
- Valcour V, Paul R, Neuhaus J, Shikuma C (2011) The effects of age and HIV on neuropsychological performance. J Intern Neuropsych Soc 17: 190-195.
- Gilbert JM, Fitch KV, Grinspoon SK (2015) HIV-related cardiovascular disease, statins, and the REPRIEVE trial. Top Antivir Med 23: 146-149.
- Rodger AJ, Sabin CA (2016) How have guidelines on when to start antiretroviral therapy affected survival of people living with HIV infection? Curr Opin HIV AIDS 11: 487-491.
- Johnson TP, Nath A (2014) New Insights into immune reconstitution inflammatory syndrome of the central nervous system. Curr Opin HIV AIDS 9: 572-578.
- Barber DL, Andrade BB, Sereti I, Sher A (2012) Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol 10: 150–156.
- Nightingale S, Winsto A, Letendre S, Michael BD, McArthur JC, et al. (2014) Controversies in HIV-associated neurocognitive disorders. Lancet Neurol 13: 1139-1151.
- Etherton MR1, Lyons JL, Ard KL (2015) HIV-associated neurocognitive disorders and antiretroviral therapy: current concepts and controversies. Curr Infect Dis Rep 17: 485.
- Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, et al. (2009) Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS 23: 1359-1366.
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737-749.
- Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, et al. (2002) G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev 16: 1779-1791.
- Zhang T, Termanis A, Özkan B, Bao XX, Culley J, et al. (2016) G9a/GLP Complex maintains imprinted DNA methylation in embryonic stem cells. Cell Rep 15: 77-85.
- Coull JJ, Romerio F, Sun JM, Volker JL, Galvin KM, et al. (2000) The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol 74: 6790-6799.
- Imai K, Togami H, Okamoto T (2010) Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. Biol Chem. 285: 16538-16545.
- Penner MR, Roth TL, Barnes CA, Sweatt JD (2010) An epigenetic hypothesis of aging-related cognitive dysfunction. Front Aging Neurosci. 2: 9.
- Deeks SG (2009) Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 17: 118-123.
- Ciccarelli N, Fabbiani M, Baldonero E, Fant L, Calda R, et al. (2012) Effect of aging and human immunodeficiency virus infection on cognitive abilities. J Am Geriatr Soc 60: 2048-2055.
- Anderson S, Kraus N (2013) Auditory training: evidence for neural plasticity in older adults. Perspect Hear Hear Disord Res Res Diagn 17: 37-57.
- Anderson S, White-Schwoch T, Choi HJ, Kraus N (2014) Partial maintenance of auditory-based cognitive training benefits in older adults. Neuropsychologia 62: 286-296.
- Grant I, Franklin DJ, Deutsch R, Woods SP, Vaida F, et al. (2014) Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 10: 2055-2062.
- Obermeit LC, Beltran J, Casaletto KB, Franklin DR, Letendre S, et al. (2016) Evaluating the accuracy of self-report for the diagnosis of HIV-associated neurocognitive disorder (HAND): defining "symptomatic" versus "asymptomatic" HAND. J Neurovirol 9-12.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 12886
- [From(publication date):
September-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 11917
- PDF downloads : 969