The Clinical and Histopathological Significance of Performing Surgical Cavity Random Biopsy in Breast Conserving Surgery on Disease Course and Local Recurrence
Received: 25-Jan-2016 / Accepted Date: 16-Feb-2016 / Published Date: 25-Feb-2016 DOI: 10.4172/2572-4118.1000104
Abstract
Objective: Surgical treatment of breast cancer has changed dramatically in recent years. The National Institutes of Health Consensus Panel confirmed that breast-conservation surgery (BCS) is as effective as mastectomy in overall patients’ survival. Our aim was to assess the clinical and pathological value of performing surgical cavity random biopsies (SCRB) in BCS and their impact on the subsequent management.
Methods: A retrospective study was conducted on 494 patients who had BCS in our firm between 2001 and 2006. Outcome measures were examined at 3,4,5 years follow up period for each patient. Tumour immunohistochemistry, adjuvant therapy, recurrence rate and demographic data were collected and represented with Pearson’s chi-squared test.
Results: The median age was 59 years. Out of 494, 23 patients (4.65%) had positive SCRB whom all had subsequent surgical intervention plus adjuvant therapy. 7 patients had total mastectomy, 13 had re-excision of positive margins and only 3 had axillary node clearance. Recurrence rate was reported in 7 patients only (1.41%) and the overall mean survival time for all patients was 74.585 months (95% C.I 73.839-75.332).
Conclusion: Our practice of performing SCRB had changed the management of 23 patients and revealed an excellent recurrence rate of only 1.41% which is below the national figures reported in the literature (7-9%). SCRB is a reliable method of determining margin status, minimising re-excision and reducing overall recurrence rate.
Keywords: Breast neoplasm; Breast conserving surgery; Mastectomy
35603Abbrevations
BCS: Breast Conserving Surgery; BCT: Breast Conserving Therapy, IOR: Intra-Operative Radiology; DCIS: Ductal Carcinoma in situ ; LCIS: Lobular Carcinoma in situ ; IDC: Invasive Ductal Carcinoma; ILC: Invasive Lobular Carcinoma; TBP: Tumour Bed Positivity
Introduction
Surgical treatment of breast cancer has changed significantly in recent years. The preferred method of treatment for many women with early breast cancer is conservative surgical therapy (principally lumpectomy and axillary dissection) followed by breast irradiation i.e. without total or radical mastectomy [1].
Sentinel node biopsy is being investigated as an alternative to standard axillary node dissection. This could decrease morbidity following standard axillary dissection [2].
In 1992, the National Institutes of Health Consensus Panel confirmed that breast-conservation treatment (BCT) was as effective as mastectomy in terms of overall and disease-free survival in patients with early-stage breast cancer [3].
If cancer cells are found in the margins of the removed specimen then an additional surgery is needed to clear the residual cavity from any tumour cells, this could be either re-excision or total mastectomy. Subsequently, most of the patients will receive five to seven weeks of radiotherapy to eliminate any cancer cells that may be present in the remaining breast tissue [4]. The combination of partial mastectomy and radiation is commonly called Breast Conserving Therapy/Surgery.
Absolute contraindications can arise when two or more primary tumours are located in different quadrants of the breast, associated diffuse microcalcifications which appear malignant and previous breast irradiation [5]. Breast irradiation cannot be given during pregnancy, but it may be possible to perform breast-conserving surgery in the third trimester and administer irradiation after delivery [6].
Relative contraindications are a history of collagen vascular disease and the presence of a large tumour in a small breast [7].
The most important factor in reducing the risk of local recurrence is to ensure that no residual disease remains present at the excision margin. Shavings can be taken from the wall of the cavity following wide excision to assess for any remaining tumour involvement [8]. According to the most recent definition proposed by the American Society of Breast Surgeons, a margin of normal tissue greater than or equal to 2 mm is considered negative. A close margin of less than 2mm is considered as positive [9]. Standard practice in surgical pathology dictates that random biopsies from the four quadrants of the residual surgical cavity should be taken after breast conserving surgery [10].
Methods
A retrospective study was conducted in the Breast Care Unit of our institution over five years, from July 2001 to July 2006. A total number of 494 patients were recruited to the study, all of them had Breast Conserving Surgery (BCS). Study protocol was written based on available relevant literature and specified aims were set to achieve useful results to clinical practice. The local hospital database was utilised to identify patients who had BCS (partial mastectomy, segmental resection, lumpectomy, wide local excision).
The Inclusion criteria was all female patients undergoing breast conserving surgery under the care of one consultant. Exclusion criteria were any female patient who had total mastectomy as an initial surgical intervention, cases where surgical cavity random biopsies were not performed for any reason (performed by trainee unaware of the practice, wrong patient details resulting in destroyed sample, no data present on subsequent follow up after random biopsies), and finally any male patients with breast cancer.
Surgical technique
All patients underwent a wide local excision with a 1-2 cm macroscopic clearance. The deep margin of excision was the Pectoralis Major Muscle fascia. Following excision of the primary tumour, four bed biopsies were taken with tissue forceps and knife from the superior, inferior, medial and lateral walls of the residual cavity.
These were labelled as surgical cavity random biopsies and were submitted separately for histopathological analysis.
Further surgery
A proportion of patients with positive tumour bed underwent further surgery (re-excision or mastectomy). A small number of patients opted not to have further surgery and were treated by radiotherapy alone. A bed biopsy is regarded as positive irrespective of the histology of the primary tumour (i.e. whether DCIS or invasive).
Statistical analysis
Patients were analysed with respect to disease-free, distant disease free and overall survival. The subgroups analysed were patient demographics, tumour grade, tumour histology, positive biopsies, reexcision rates, recurrence rates, oestrogen receptors status, follow up, post operative adjuvant therapy and Lymph Nodes status. Data were represented in an Excel sheet and were processed using the SPSS statistical programme (SPSS limited, 2006, UK) to get tables as well as statistical factual numbers. A 5% significance level was used in this analysis.
The Kaplan-Meier technique was used to produce a curve of the incidence of local recurrence. The log-rank test was performed in order to determine underlying differences in the incidence of local recurrence between these patients. Pearson’s chi-square test was used to correlate clinico-pathological factors with cavity margin shaving positivity. The independent sample t-test was used for a comparison of means.
Follow up
All patients were followed up at 3 months for the first year, then every six months for the second year and then once a year for the rest of the three years. The median follow up for this study was 37 months (range 13-60 months) years.
Results
494 patients were successfully recruited to this retrospective study from July 2001 to July 2006. Authors searched each patient’s 1st histology report in the pathology department after the initial BSC.
Out of the total number, only 23 (4.65%) patients had one or more positive biopsy (biopsies) from one or more of the four quadrants of the residual breast cavity wall which were sampled during the initial wide local excision. All of those patients who had positive random biopsies had a subsequent surgical intervention with or without adjuvant therapy.
Among the 23 patients with positive margins, only 7 patients had total mastectomy after the initial breast conserving surgery while 13 patients had re-wide local excision or re-excision of positive margins. The other 3 patients had only axillary node clearance for disease staging and further adjuvant therapy. As part of BCS definition, about 91% of patients had post operative adjuvant radiotherapy after the second operation with or without chemotherapy or hormonal therapy.
Within the positive biopsy group (23 patients), 12 patients had invasive breast carcinoma (9 ductal and 3 lobular) while 8 of them had mixed invasive and in-situ cancer. Only 3 patients had carcinoma in situ (2 ductal and 1 lobular). Histology reports after the second operation revealed 12 residual carcinoma in situ (DCIS and LCIS), 9 invasive carcinoma (IDC and ILC) and 2 mixed (invasive and in situ ) from the positive cavity wall biopsies (Table 1).
| Patients | Histology Report -1 | Histology Report -2 | Positive Biopsy | Further Management | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sup | Inf | Med | Lat | Surgery | Chemo | Radio | Hormonal | |||
| 1 | DCIS | Residual DCIS | 1 | Mastectomy | + | |||||
| 2 | DCIS+IDC | Residual DCIS | 1 | ANC | + | + | ||||
| 3 | IDC | Residual DCIS+IDC | 1 | 1 | Mastectomy | + | + | |||
| 4 | ILC | Residual ILC | 1 | Mastectomy | + | |||||
| 5 | DCIS | Residual DCIS | 1 | Re-WLE | + | + | ||||
| 6 | IDC | Residual DCIS | 1 | Re-WLE | + | + | + | |||
| 7 | IDC+LCIS | Residual IDC | 1 | Re-WLE | + | + | ||||
| 8 | IDC | Residual LCIS | 1 | Re-WLE | + | + | ||||
| 9 | IDC+DCIS | Residual DCIS | 1 | Re-WLE | + | + | + | |||
| 10 | DCIS | Residual DCIS | 1 | 1 | 1 | Mastectomy | + | |||
| 11 | IDC | Residual DCIS | 1 | Re-WLE | + | + | ||||
| 12 | IDC | Residual IDC | 1 | Mastectomy | + | + | + | |||
| 13 | IDC+ILC | Residual IDC | 1 | Re-WLE | + | + | + | |||
| 14 | IDC+ILC | Residual DCIS | 1 | ANC | + | |||||
| 15 | IDC+DCIS | Residual IDC | 1 | Mastectomy | + | + | ||||
| 16 | IDC+DCIS | Residual IDC | 1 | Re-WLE | + | + | ||||
| 17 | DCIS+IDC | Residual DCIS | 1 | Mastectomy | + | + | ||||
| 18 | IDC | Residual DCIS | 1 | Mastectomy | + | + | ||||
| 19 | DCIS+ILC | Residual DCIS | 1 | Re-WLE | + | + | ||||
| 20 | IDC+DCIS | Residual IDC+DCIS | 1 | Re-WLE | + | + | ||||
| 21 | IDC | Residual IDC | 1 | 1 | Re-WLE | + | + | + | ||
| 22 | IDC | Residual IDC | 1 | Re-WLE | + | + | ||||
| 23 | ILC | Residual ILC | 1 | Re-WLE | + | + | ||||
Table 1: Histology reports after the second operation (IDC: Intraductal carcinoma, ILC: Intralobular carcinoma, DCIS: Ductal carcinoma in situ, LCIS: Lobular carcinoma in situ , ANC: Axillary nodes clearance, WLE: Wide local excision).
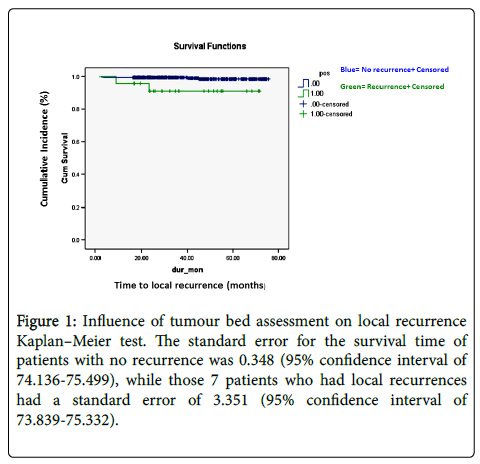
There were 7 patients in total (1.41%) who had local recurrence of breast neoplasm after BCS after complete removal of the tumour with one of more surgical interventions (Table 2). The Kaplan–Meier test demonstrates the influence of tumour bed assessment on the overall survival in months for patients who had breast conserving surgery (Figure 1).
| Value | df | Asymp. Sig. (2- sided) | |
|---|---|---|---|
| Pearson Chi- Square | 11.420* | 2 | 0.003 |
| Likelihood Ratio | 4.945 | 2 | 0.084 |
| Linear- by- Linear Association | 11.047 | 1 | 0.001 |
| Nopf valid cases | 494 |
*4 cells (66.7%) have expected count less than 5. The minimum expected count is 10.
Table 2: Chi-Square tests.
Figure 1: Influence of tumour bed assessment on local recurrence Kaplan–Meier test. The standard error for the survival time of patients with no recurrence was 0.348 (95% confidence interval of 74.136-75.499), while those 7 patients who had local recurrences had a standard error of 3.351 (95% confidence interval of 73.839-75.332).
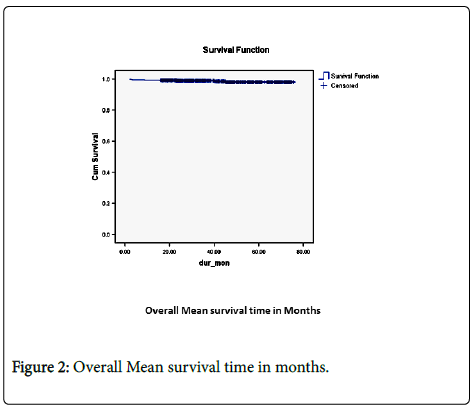
The overall mean survival time was 74.585 months (95% confidence interval 73.839-75.332) (Figure 2).
The mean follow up of all 494 patients was 40.63 months (95% confidence interval for mean 38.99-42.27) while the median was 35.68 months (Table 3). The mean age for the 494 patients is 59.11 years with 95% confidence interval between 58.10- 60.12. The median age was 59 years (standard deviation 11.39) and the range was 62 years (interquartile range=16).
| Descriptives | Stastic | Std. Error | ||
|---|---|---|---|---|
| dur_mon | Mean | 40.6339 | 0.83657 | |
| 95% Confidence Interval for mean | Lower Bound | 38.9903 | ||
| Upper Bound | 42.2776 | |||
| 5% trimmed Mean | 40.2175 | |||
| Median | 35.6833 | |||
| variance | 345.724 | |||
| Std. Deviation | 18.59365 | |||
| Minimum | 1.97 | |||
| Maximum | 75.6 | |||
| Range | 73.63 | |||
| Interquartile Range | 33.38 | |||
| Skewnwss | 0.339 | 0.11 | ||
| Kurtosis | -1.182 | 0.219 | ||
Table 3: Follow up Descriptive (months).
Discussion
Invasive breast carcinoma and its precursor, ductal carcinoma in situ (DCIS) have traditionally been treated by mastectomy. However, the more widely used approach today is local excision with adjuvant radiotherapy. Long term follow-up studies have shown no difference in overall survival between these two approaches, thus both can be considered oncologically sound [11].
Breast conservation therapy has become the preferred treatment for many Stage I and II breast cancers. Local recurrence after conservation therapy is reportedly dependent upon a number of pathological, clinical, and treatment factors, and ranges between 9% and 43% [12,13]. In a detailed analysis of mastectomy specimens, Holland et al. showed that in 42% of cases, residual disease could be found at a distance of 2 cm from the tumour edge [14,15]. Although recurrence rates drop significantly with the addition of local radiotherapy, the likely presence of residual tumour in a large proportion of these patients makes regular follow up assessment essential [13].
Breast conserving surgery aims to achieve cure while achieving the best cosmetic result for the patient. This can often present a fine balance, however, as the local recurrence rate increases as the extent of the excision decreases [16]. A method of taking shavings from the resultant cavity wall has shown encouraging early results from the Southampton and Glasgow breast units [17,18].
During our literature review, we have identified around 13 papers with a total of 2862 patients recruited from 1980 to 2006. All of those studies have confirmed the clinical and pathological importance of performing surgical cavity random biopsies in breast conserving surgery as a method of detecting any residual disease. About 770 patients (26.89%) out of the total number included in all studies had positive cavity wall biopsies and 365 patients of them (47.46%) had further surgery as a result of their random biopsy status. This is clinically important as a significant number of patients had further management after their initial breast conserving surgery.
Macmillan et al. performed their first study in 1994 when the entire wall of the cavity from which the lumpectomy specimen had been taken was excised. This was involved with disease in 38% of patients. Additional random biopsies of the secondary cavity were performed in 130 patients and were involved with disease in 13%. Residual disease was detected in the tumour bed of 37% of patients with screen-detected tumours [18]. Subsequently, Macmillan et al. have concluded that the incidence of tumour bed positivity was 39.3%, the local recurrence rate was 2.0% and distant recurrence rate was 10.4% [19]. Moreover, and in 1999 Malik et al. analysed cavity shaving as a method of assessing completeness of surgical excision after breast-conserving surgery in 543 women. Tumour bed positivity (TBP) was found in 37% of patients (16% with invasive disease). TBP was significantly associated with high tumour grade, presence of an extensive intraductal component, young age and large tumour diameter. It was also associated with a significantly shorter overall survival when compared to patients who were tumour bed negative [20,21].
Additionally, Huston et al. in 2006 had revealed that the complete resection of 4 to 6 additional margins during the initial BCT resulted in the lowest subsequent reoperation rate, and the largest total volume specimen excised among the techniques studied [22]. Furthermore, Taylor et al. suggested that positive bed biopsy is associated with an increase in local recurrence rates but has no effect on overall survival following wide excision of breast cancer [23]. Barthelmes et al. demonstrated that shaving the margin of the cavity as a method to ensure completeness of excision has achieved an acceptable rate of local control [24].
Importantly to note, was the study by Beck et al., who showed that margin analysis of wide local excision specimens is a poor predictor of completeness of excision [25]. Gupta et al. have concluded that the histological examination of random sections from breast quadrants yielded important information about the presence of multi-focality, multi-centricity, vascular invasion, and margin involvement by carcinoma [26].
Patients receiving breast conservation therapy have a lifelong risk of local recurrence. It is absolutely unacceptable to have tumour cells directly at the cut edge of the excised specimen, regardless of the type of post-surgical adjuvant therapy [27]. The sampling of at least one random biopsy from each quadrant of a residual partial mastectomy cavity has been the traditional method of selection and has been recommended in textbooks of surgical pathology. In this age of cost containment, an evaluation of this approach seems timely and of a proper value [28].
Negative surgical margins minimize the risk of local recurrence after breast-conserving surgery. Intra-operative frozen section analysis allows resection of suspicious or positive margins at the time of lumpectomy and results in low rates of local recurrence and reexcision [29,30]. Most recurrences arise at or close to the site of the previous excision. In spite of its common usage as a surgical technique, methods for assessment of the excision margin after conservation surgery continue to be a source of debate. Thus far, however, management strategies have tended to focus principally on the breast specimen itself [31].
A certain proportion of local recurrence appears to be inevitable, even in spite of a wide margin of excision (e.g. quadrantectomy). As a result of this, radiotherapy is routinely required to reduce such chance of recurrence to an acceptable level. Randomised studies bear testimony to the efficacy of using radiotherapy, with a suggested local recurrence rate of 6-12% vs 29-43% when radiotherapy in combination with wide local excision (with confirmed negative margins) is compared with wide local excision alone [32].
In our retrospective study, we have noticed that all patients who had positive cavity wall biopsies were scheduled for further subsequent surgical operation with adjuvant therapy (mostly Radiotherapy in 91.3% of patients). Furthermore, this study showed a satisfactory completion of tumour resection in the breast conserving surgery. This is clearly demonstrated by the incidence of having positive surgical cavity random biopsies, which was 4.65% (23 out of 494 patients). These results are better than the available data form the reviewed literature, which showed an incidence of 9-42.85% of having residual tumour (invasive or in-situ) in the cavity shaved biopsies. With respect to local recurrence, we had only 7 patients (1.41%) among the total of 494 who developed a neoplastic recurrence after having breast conserving surgery.
Systematic cavity shaves of these margins are a reliable method of determining margin status, reducing close margins, and reducing re-excision. Our study indicates that in a significant percentage of random sections derived from each of the four quadrants disclose clinically important new findings. On this basis we would recommend the continued use of the traditional random quadrant bloc submissions after breast conserving surgery.
References
- Apantaku LM (2002) Breast-conserving surgery for breast cancer. Am Fam Physician 66: 2271-2278.
- Forner VC, Climent GJ, Peris FMV, Diana FC, Arcas MC, et al. (2000) [Locating the sentinel node in breast cancer by gamma probe and staining agent. Preliminary study]. Rev Esp Med Nucl 19: 207-210.
- [No authors listed] (1992) Consensus statement: treatment of early-stage breast cancer. National Institutes of Health Consensus Development Panel. J Natl Cancer Inst Monogr 1-5.
- Veronesi U, Saccozzi R, Greco M, Luini A, Sultan L (1977) [Conservative treatment of breast cancer. A therapeutic trial in progress at the Cancer Institute of Milan]. Bull Cancer 64: 619-625.
- Nixon AJ, Troyan SL, Harris JR (1996) Options in the local management of invasive breast cancer. Semin Oncol 23: 453-463.
- White JR, Halberg FE, Rabinovitch R, Green S, Haffty BG, et al. (2008) American College of Radiology appropriateness criteria on conservative surgery and radiation: stages I and II breast carcinoma. J Am Coll Radiol 5: 701-713.
- Weber B, Demange L, Rigaud C, Fernandes-Valoni A (1998) Remaining indications for total mastectomy in breast carcinoma. Bull Cancer 85: 755.
- Humphrey LL, Helfand M, Chan BK, Woolf SH (2002) Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 137: 347-360.
- Vicini FA, Beitsch PD, Quiet CA, Keleher A, Garcia D, et al. (2005) First analysis of patient demographics, technical reproducibility, cosmesis, and early toxicity: results of the American Society of Breast Surgeons MammoSite breast brachytherapy trial. Cancer 104: 1138-1148.
- Rietjens M, Urban CA, Rey PC, Mazzarol G, Maisonneuve P, et al. (2007) Long-term oncological results of breast conservative treatment with oncoplastic surgery. Breast 16: 387-395.
- Bethke KP (1996) Breast conservation: predictors and treatment of local recurrence. Semin Surg Oncol 12: 332-338.
- [No authors listed] (1995) Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. Early Breast Cancer Trialists' Collaborative Group. N Engl J Med 333: 1444-1455.
- Holland R, Veling SH, Mravunac M, Hendriks JH (1985) Histologic multifocality of Tis, T1-2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer 56: 979-990.
- Frazier TG, Wong RW, Rose D (1989) Implications of accurate pathologic margins in the treatment of primary breast cancer. Arch Surg 124: 37-38.
- Ghossein NA, Alpert S, Barba J, Pressman P, Stacey P, et al. (1992) Breast cancer. Importance of adequate surgical excision prior to radiotherapy in the local control of breast cancer in patients treated conservatively. Arch Surg 127: 411-415.
- Umpleby HC, Herbert A, Royle GT, Taylor I (1988) Wide excision of primary breast cancer: the incidence of residual carcinoma at the site of excision. Ann R Coll Surg Engl 70: 246-248.
- Macmillan RD, Purushotham AD, Mallon E, Ramsay G, George WD (1994) Breast-conserving surgery and tumour bed positivity in patients with breast cancer. Br J Surg 81: 56-58.
- Macmillan RD, Purushotham AD, Mallon E, Love JG, George WD (1997) Tumour bed positivity predicts outcome after breast-conserving surgery. Br J Surg 84: 1559-1562.
- Malik HZ, George WD, Mallon EA, Harnett AN, Macmillan RD, et al. (1999) Margin assessment by cavity shaving after breast-conserving surgery: analysis and follow-up of 543 patients. Eur J Surg Oncol 25: 464-469.
- Malik HZ, Purushotham AD, Mallon EA, George WD (1999) Influence of tumour bed assessment on local recurrence following breast-conserving surgery for breast cancer. Eur J Surg Oncol 25: 265-268.
- Huston TL, Pigalarga R, Osborne MP, Tousimis E (2006) The influence of additional surgical margins on the total specimen volume excised and the reoperative rate after breast-conserving surgery. Am J Surg 192: 509-512.
- Taylor I, Mullee MA, Carpenter R, Royle G, McKay CJ, et al. (1998) The significance of involved tumour bed biopsy following wide local excision of breast cancer. Eur J Surg Oncol 24: 110-113.
- Barthelmes L, Al Awa A, Crawford DJ (2003) Effect of cavity margin shavings to ensure completeness of excision on local recurrence rates following breast conserving surgery. Eur J Surg Oncol 29: 644-648.
- Beck NE, Bradburn MJ, Vincenti AC, Rainsbury RM (1998) Detection of residual disease following breast-conserving surgery. Br J Surg 85: 1273-1276.
- Gupta D, Nath M, Layfield LJ (2003) Utility of four-quadrant random sections in mastectomy specimens. Breast J 9: 307-311.
- Singletary SE (2002) Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 184: 383-393.
- Lowe DG, Klisak I, Sparkes RS, Mohandas T, Goeddel DV (1990) Chromosomal distribution of three members of the human natriuretic peptide receptor/guanylyl cyclase gene family. Genomics 8: 304-312.
- Olson TP, Harter J, Munoz A, Mahvi DM, Breslin T (2007) Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol 14: 2953-2960.
- Cendán JC, Coco D, Copeland EM (2005) Accuracy of intraoperative frozen-section analysis of breast cancer lumpectomy-bed margins. J Am Coll Surg 201: 194-198.
- Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, et al. (1995) Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomised trials on 1,973 patients. Eur J Cancer 31A: 1574-1579.
- Whelan T, Clark R, Roberts R, Levine M, Foster G (1994) Ipsilateral breast tumor recurrence postlumpectomy is predictive of subsequent mortality: results from a randomized trial. Investigators of the Ontario Clinical Oncology Group. Int J Radiat Oncol Biol Phys 30: 11-16.
Citation: Rahmani S, Brown J, Gendy R (2016) The Clinical and Histopathological Significance of Performing Surgical Cavity Random Biopsy in Breast Conserving Surgery on Disease Course and Local Recurrence. Breast Can Curr Res 1: 104. DOI: 10.4172/2572-4118.1000104
Copyright: ©2016 Rahmani S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11500
- [From(publication date): 6-2016 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 10813
- PDF downloads: 687