The Association of Vaccination and the Incidence of New Cases of COVID-19 among Health Care Workers, December 16, 2020, through May 4, 2021
Received: 02-Aug-2021 / Accepted Date: 16-Aug-2021 / Published Date: 23-Aug-2021 DOI: 10.4172/2161-0681.1000394
Abstract
Background: Evidence is limited regarding the effectiveness of the two-dose messenger RNA (mRNA) vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) in preventing symptomatic severe acute respiratory coronavirus 2 (SARS-CoV-2) infection when vaccinated and unvaccinated groups are similar in size.
Aim: New research is needed to demonstrate the Vaccine Effectiveness (VE) in health care settings with relatively low vaccination rates and where the follow-up periods are more extended than prior research. This study uses a "Real-World" cohort of HCWs and provides insight into the effectiveness of the mRNA vaccines against symptomatic SARS-CoV-2 infection.
Methods: This was a single-center retrospective cohort study evaluating association between vaccinations with BNT162b2 and mRNA-1273 vaccines and incidence of new cases of symptomatic SARS-CoV-2 infections among HCWs between December 16, 2020, and May 4, 2021. Daily screening for symptoms was done using electronic “Fast Pass” and nasopharyngeal (NP) Polymerase chain reaction (PCR) confirmed all positive lateral-flow antigen tests. Incidence rate of COVID-19 among vaccinated and unvaccinated HCWs was calculated and VE was defined as 1-Incidence Rate Ratio (IRR). Statistical analysis was done using t-tests for normally distributed continuous variables and χ2 tests for categorical variables.
Results: Of the group of HCWs studied, only 52.1% received the mRNA vaccines with 91% of participants receiving the BNT162b2 vaccine. The incidence rate of symptomatic SARS-CoV-2 infection in the fully vaccinated group was 4.14 per 100 000 person-days compared to 95.53 per 100000 person-days in the unvaccinated group (IRR, 0.0433 [95% CI, 0.018-0.1]; VE=95.67%).
Conclusion: Receipt of the authorized mRNA vaccines, essentially BNT16b2, was associated with a significantly lower incidence of symptomatic SARS-CoV-2 infection in the absence of highly vaccination rates and for a median follow-up of 139 days.
Keywords: COVID-19; SARS-CoV-2; BNT162b2; mRNA-1273; Health care workers; Vaccine effectiveness
Introduction
The dynamics of the coronavirus disease 2019 (COVID-19) remain unprecedented. It has become clear that the response to COVID-19 is more like a marathon than a sprint. Data on Messenger Ribonucleic Acid (mRNA) Vaccine Effectiveness (VE) for Health Care Workers (HCW), who are frequently at risk of exposure to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have been consistent with the results from the phase 3 randomized clinical trials, which showed efficacy against symptomatic SARS-CoV-2 infection around 95% [1-11]. However, most studies among HCWs involved a highly vaccinated study population.
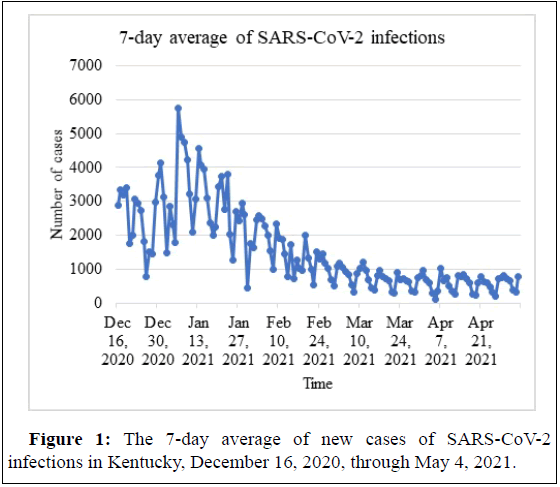
On December 16, 2020, a large-scale campaign was launched at Pikeville Medical Center, in close collaboration with the State of Kentucky and the Pike County Public Health Department, to vaccinate its HCWs, along with individuals at increased risk of COVID-19 complications and death. Both mRNA vaccines, BNT162b2 and mRNA-1273, were administered upon State-derived allocations. Concurrently with the vaccination campaign, the number of new COVID-19 cases surged in Kentucky, with the highest daily new SARS-CoV-2 cases was 5,742 reported on January 6, 2021, and mentioned in Figure 1 [12,13]. This study was conducted to assess the VE with the two authorized mRNA vaccines against symptomatic SARS-CoV-2 infections in HCWs over almost five months in the absence of highly vaccinated rates.
Materials and Methods
Study design
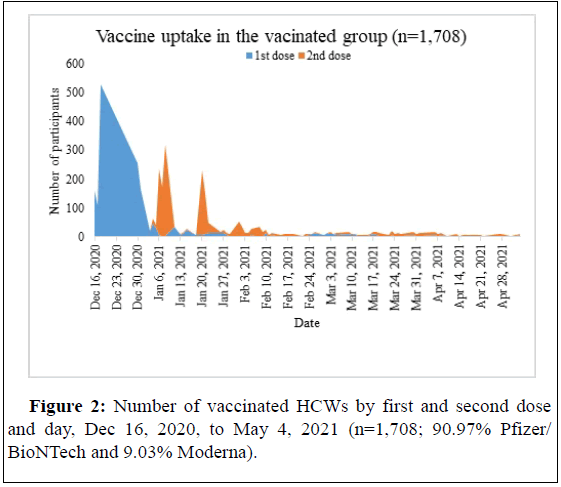
This retrospective cohort study examined the association between vaccination with the BNT162b2 and m-RNA-1273 vaccines and the incidence of new cases of symptomatic SARS-CoV-2 infections among HCWs. The study was conducted at the Pikeville Medical Center, a tertiary medical center that employs over 3,000 HCWs. All HCWs were eligible to receive the vaccine from the first day of the vaccination campaign on December 16, 2020, and throughout the study period until May 4, 2021, clearly observed in Figure 2.
Employment status, personal demographics, vaccination dates, and SARS-COV-2 molecular test results were retrieved from the Employee Health information system database. Participants who contracted SARS-CoV-2 infection before study enrollment were excluded. All HCWs were screened daily with an electronic "Fast Pass," a questionnaire which identified those who self-reported the most common COVID-19 symptoms.
Employees who did not pass the "Fast Pass" were sent directly to nasopharyngeal SARS-CoV-2 testing. Polymerase Chain Reaction (PCR) confirmed all positive lateral-flow antigen tests. Participants infected with SARS-CoV-2 were defined as symptomatic if they had any of the following: Temperature greater than 37.6˚C (100.4˚F), headache, sore throat, cough, dyspnea, rhinorrhea, diarrhea, nausea/ vomiting, myalgia, or loss of sense of taste or smell. All HCWs who had laboratory-confirmed COVID-19 by PCR were isolated under the Centers for Disease Control and Prevention (CDC) protocols [14].
The study sample also excluded symptomatic SARS-CoV-2 infections between days 0 through 13 of enrollment from the final effectiveness analysis. All records were de-identified before analysis, and Ethics approval was obtained from the institutional review board and a waiver of written informed consent.
Group assignment and definition of the follow-up period
Health care workers who received at least one vaccine dose between December 16, 2020, and May 4, 2021, were assigned to the vaccinated group. The unvaccinated control group was composed of HCWs who did not receive any vaccine doses during this period. The follow-up period for each vaccinated HCW was defined as starting on the day of receiving the first vaccine dose and ending either on May 4, 2021, or at the termination date of employment if it occurred before May 4, 2021. The follow-up period for each unvaccinated HCW was defined as starting either on December 16, 2020, or the date of hiring if it happened after December 16, 2020, and before May 4, 2021, and ending either on May 4, 2021, or at the termination date of employment if occurred before May 4, 2021. All participants, vaccinated and unvaccinated, were censored at the first positive SARS-CoV-2 PCR test result. The number of risk days for each employee was defined as any day in the appropriate follow-up period. During this timeframe, the 7-day average of new SARS-CoV-2 infections, dropped significantly over time in the state of Kentucky in Figure 1.
Definitions
Fully vaccinated: Participants were considered to be fully vaccinated; 14 or more days (≥ 14 days) after the second dose of the mRNA vaccine.
Partially vaccinated: Participants were considered partially vaccinated between 14 or more days (≥ 14 days) following the first dose till 13 or less days (<14 days) after the second dose of the vaccine or to the end of the follow-up period if the second dose was never administered.
Incidence rate: Number of positive SARS-CoV-2 cases divided by the cumulative follow-up time of the HCWs in the group within the examined time frame.
Incidence rate ratio: Ratio of incidence rate of symptomatic SARS-CoV-2 infections between the vaccinated (fully and partially) group and unvaccinated group.
Study outcomes and statistical analysis
The primary outcome was the effectiveness of the mRNA vaccines in HCWs against symptomatic SARS-CoV-2 infection when fully vaccinated and no prior documented infection. These HCWs were compared with unvaccinated HCWs for a follow-up period of twenty weeks. The secondary outcome was the effectiveness of partial vaccination against symptomatic SARS-CoV-2 infection in comparison with unvaccinated HCWs. We calculated the incidence rate ratio (IRR) and exact 95% confidence interval (CI) to define vaccine effectiveness as 1-IRR [15]. The groups were compared using t-tests for normally distributed continuous variables and χ2 tests for categorical variables. All reported tests were two sided, and a P value of less than 0.05 was considered significant. All statistical analyses were performed using R software version 4.0.3 (R Foundation for Statistical Computing).
Results
A total of 2904 HCWs without prior documented SARS-CoV-2 infection (median [S.D.] age, 38 [12] years; 2134 [73.48%] women) comprised the retrospective cohort. Of this cohort, 1513 HCWs (51.10%) received at least 1 dose of the BNT162b2 or mRNA-1273 vaccine, 1473 (50.72%) received 2 doses, and 1391 (47.90%) were not vaccinated. For the recipients of 2 doses of the BNT162b2 vaccine, the median days between doses was 21 (IQR, 21-21); For mRNA-1273, the median days between doses was 28 (IQR, 28-28). Most participants (91%) received the BNT162b2 vaccine. The median follow-up duration was 139 days (Dec 16, 2020, to May 4, 2021).
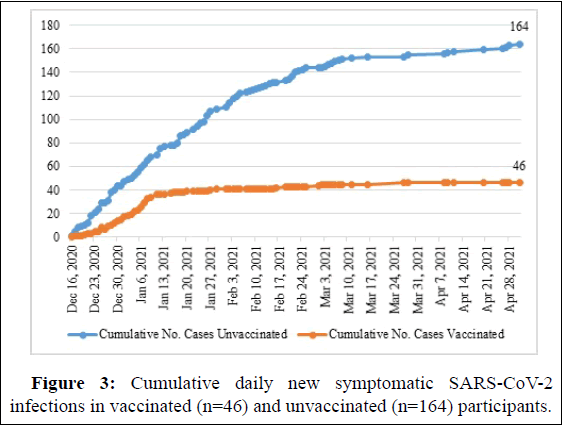
The mean follow-up period for vaccinated individuals 116.24 (SD=33.36, IQR=113-137). The unvaccinated group had a mean follow up of 106.83 days, (SD=46.93, IQR=19-139). The baseline characteristics of the participants are summarized in Table 1. Vaccinated HCWs were older compared with unvaccinated participants (median age, 42 vs. 33 years, respectively; P<0.001). The estimated risk of work-related exposure to SARS-CoV-2 was interestingly similar in health care providers in both groups. Overall, symptomatic SARS-CoV-2 infection was detected in 210 participants (7.2%), of whom 46 received at least one vaccine dose and 164 unvaccinated in Figure 3.
| Variable | Vaccinated | Unvaccinated | P-value |
|---|---|---|---|
| No. of participants | 1513 | 1391 | <0.001 |
| Received 2 doses of vaccine, No. (%) | 1473 (97.36) | 0 | N/A |
| Age, median [SD], y | 42 [12.09] | 33 [11.65] | <0.001 |
| Age group, No. (%) | N/A | N/A | N/A |
| ≤ 39 y | 656 (43.36) | 925 (66.50) | <0.001 |
| 40-59 y | 726 (47.98) | 422 (30.34) | <0.001 |
| ≥ 60 y | 131 (8.66) | 44 (3.16) | <0.001 |
| Sex, No. (%) | N/A | N/A | N/A |
| Female, No (%) | 1088 (71.91) | 1046 (75.20) | <0.001 |
| Male, No (%) | 425 (28.09) | 320 (23.01) | <0.001 |
| Employment sector, No. (%) | N/A | N/A | N/A |
| Administrationa | 517 (34.17) | 426 (30.63) | <0.001 |
| Providersb | 187 (12.36) | 68 (4.89) | 0.99 |
| Nursingc | 396 (26.17) | 530 (38.10) | <0.001 |
| Other health professionsd | 413 (27.30) | 367 (26.38) | <0.001 |
| Active COVID-19, No. (%) | 46 (3.04%) | 164 (11.79%) | <0.001 |
| Mean follow-up time, days [SD; IQR] | 116.24 [33.37;113-137] | 106.83 [46.94;69-139] | N/A |
| No. hospitalizations for severe COVID-19 | 0 | 5 | N/A |
| Death | 0 | 0 | N/A |
Abbreviations: IQR: Interquartile Range; SD: Standard Deviation.
No direct patient contact
Included doctors, nurse practitioners, and physician assistants
Included nurse aids, registered nurses, and licensed practical nurses
Included rehabilitation, speech, and respiratory therapists, phlebotomists, and other paramedical staff.
Table 1: Baseline characteristics of study participants.
For the primary outcome analysis, the incidence rate of symptomatic SARS-CoV-2 infection was 4.14 vs. 95.53 per 100 000 person-days in the fully vaccinated and unvaccinated cohorts, respectively, corresponding with an IRR of 0.0433 (95% CI, 0.018-0.1; VE=95.67%; Table 2). For the secondary outcome analysis, the incidence rate of symptomatic SARS-CoV-2 infection was 37.82 vs. 95.53 per 100 000 person-days in the partially vaccinated and unvaccinated cohorts, respectively, corresponding with an IRR of 0.3959 (95% CI, 0.224, 0.7; VE=60.41%; Table 2).
| Vaccination status | No. COVID-19 cases | Surveillance time, person-days | Incidence rate per 100,000 person days | Incidence relative ratio (IRR) (95% CI) | P-value |
|---|---|---|---|---|---|
| Fully vaccinatedb | 5 | 120,852 | 4.14 | 0.0433 (0.018, 0.1) | <0.001 |
| Partially vaccinatedc | 13 | 34371 | 37.82 | 0.3959 (0.224, 0.7) | <0.001 |
| Unvaccinated | 124 | 129799 | 95.53 |
Note: aWhen estimating incidence of symptomatic SARS-CoV-2 infections, follow-up was ceased on the day of COVID-19 case confirmation, the termination date of employment, or the end of follow-up on May 4, 2021. Participants with fewer than 14 days (<14 d) of follow-up or who contracted SARS-CoV-2 less than 14 days after the first vaccine dose were excluded in this analysis.
bIncluded those with data equal or longer than 14 days (≥ 14 d) after the second vaccine dose to the end of follow-up.
cIncluded those with data equal or longer than 14 days (≥ 14 d) from the first vaccine dose and fewer than 14 days after the second dose if it happened, otherwise to the end of the follow-up.
The incidence rates were defined for each of the two groups as the number of positive SARS-CoV-2 cases divided by the cumulative follow-up time of the HCWs in the group within the examined time frame.
The incidence rate ratio (IRR) was computed directly from the incidence rates of the two groups as simply the ratios of incidence rates between the vaccinated (fully and partially) group and unvaccinated group.
Table 2: Observed Incidence Rate of Symptomatic SARS-CoV-2 Infection in the vaccinated groupa.
Discussion
The COVID-19 has placed a massive physical and mental health burden on HCWs. Several studies provided strong short-term evidence that vaccinating HCWs can substantially reduce symptomatic and asymptomatic SARS-CoV-2 infection and, therefore, might help to reduce transmission of infection in the healthcare settings [3-11]. However, most studies among HCWs involved high vaccinated study population. In this retrospective cohort study, vaccination with two doses of the authorized available mRNA vaccines was associated with a significantly lower incidence rate for symptomatic SARS-CoV-2 infection in HCWs in the absence of highly vaccination rates (52.1%) and for a median follow-up of 139 days. The IRR for the fully vaccinated participants (91% received the BNT162b2) was 0.043 for symptomatic infection, corresponding to the estimated vaccine effectiveness of 95.67%. This study, while is mostly supporting what has already been published on mRNA vaccine effectiveness, has the added strength of a large unvaccinated population which has not been the case for other published studies. To achieve optimal immunity, completing the series of two doses of the mRNA vaccines is required in individuals without prior exposure to SARS-CoV-2 infection. Low COVID-19 vaccination rate was present in our study. In these cases, HCWs used appropriate personal protective equipment, social distancing, and frequent hand hygiene to maintain a safe medical practice. This practice should continue until the prevalence of SARSCoV- 2 has fallen to much lower levels to safeguard the healthcare settings.
Strengths
The data on study participants were collected in real time with a relatively longer follow-up time than prior studies. Furthermore, the vaccinated and unvaccinated cohorts were similar in size, and this is unique feature of our research as most studies among HCWs involved a highly vaccinated population.
Limitations
This study has several limitations. First, the inherent characteristics of a single-center, retrospective observational study might limit the generalizability of the findings. Second, HCWs were screened daily with an electronic "Fast Pass," and there may be a bias concerning the decision of "feeling ill" dependent on the vaccination status. Vaccinated recipients may be less likely to seek testing for minor symptoms, which may falsely increase vaccination effectiveness. Furthermore, there may also be a bias concerning overall behavior for mitigation measures between vaccinated and unvaccinated HCWs. Third, the study did not look at a regular collection of respiratory specimens in the participants irrespective of symptoms to see the impact on silent transmission, not just illness prevention. Furthermore, information on Variants of Concern (VOC) or on disease severity and viral load or Cycle Threshold (CT) value of PCR tests between vaccinated and unvaccinated HCWs was not available. Finally, we used the sample date of a positive PCR result as the event date, which might have introduced some misclassification of vaccination status relative to infection.
Conclusion
Among HCWs at a single-center in Pikeville, Kentucky, the receipt of mainly the BNT162b2 vaccine compared with no vaccine was associated with a significantly lower incidence of symptomatic SARSCoV- 2 infection in the absence of highly vaccination rates and for twenty weeks following vaccination. Direct patient contact or care will require the continuation of mitigation measures for HCWs in a healthcare setting until the rate of SARS-CoV-2 infection transmission drops significantly. Rigorous genetic surveillance is crucial to determine if breakthrough cases will increase with time and with which VOC, to evaluate vaccines that are most effective against future variants. Further studies are required to assess the long-term COVID-19 vaccine efficacy and the possible use of booster vaccinations to sustain protection.
Authors Contribution
Concept and design
Fadi Al Akhrass.
Acquisition, analysis, or interpretation of data
Gregory Green and Fadi Al Akhrass.
Drafting of manuscript
All authors contributed.
Administrative, technical, or material support
Fadi Al Akhrass.
Supervision
Fadi Al Akhrass.
Conflicts of Interest
No conflict of interest.
Disclaimer
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Pikeville Medical Center, the University of Pikeville, or the New England College of Optometry.
References
- Polack FP, Thomas SJ, Kitchin N (2020) C4591001 Clinical trial group. safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383: 2603-2615.
- Baden LR, El Sahly HM, Essink B (2021) Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med 384: 403-416.
- Thompson MG, Burgess JL, Naleway AL (2021) Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers-Eight U.S. Locations, December 2020-March 2021. CDC 70: 495-500.
- Angel Y, Spitzer A, Henig O (2021) Association between vaccination with bnt162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA 325: 2457-2465.
- Benenson S, Oster Y, Cohen MJ, Nir-Paz R (2021) BNT162b2 mRNA COVID-19 vaccine effectiveness among health care workers. N Engl J Med 384: 1775-1777.
- Keehner J, Horton LE, Pfeffer MA (2021) SARS-CoV-2 infection after vaccination in health care workers in california. N Engl J Med 384: 1774-1775.
- Daniel W, Nivet M, Warner J, Podolsky DK (2021) Early evidence of the effect of sars-cov-2 vaccine at one medical center. N Engl J Med 384: 1962-1963.
- Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E (2021) Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 397: 875-877.
- Hall VJ, Foulkes S, Saei A (2021) COVID-19 vaccine coverage in healthcare workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 397: 1725-1735.
- Swift MD, Breeher LE, Tande AJ (2021) Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis 4: ciab361.
- Thompson MG, Burgess JL, Naleway AL (2021) Prevention and Attenuation of COVID-19 with BNT162b2 and mRNA-1273. N Engl J Med 385: 320-329.
- Centers for Disease Control and Prevention (2021) Coronavirus disease 2019 (COVID19).
- Centers for Disease Control and Prevention (2021) COVID Data Tracker Weekly Review.
- Interim Guidance on Ending Isolation and Precautions for Adults with COVID-19 (2019) CDC.
- Chu H, Halloran ME (2004) Bayesian estimation of vaccine efficacy. Clin Trials 1: 306-314.
Citation: Akhrass FA, Reynolds N, Akhrass CA, Dawahare J, Raj R et al. (2021) The Association of Vaccination and the Incidence of New Cases of COVID-19 among Health Care Workers, December 16, 2020, through May 4, 2021. J Clin Exp Pathol 11: 394. DOI: 10.4172/2161-0681.1000394
Copyright: © 2021 Akhrass FA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2278
- [From(publication date): 0-2021 - Nov 28, 2025]
- Breakdown by view type
- HTML page views: 1609
- PDF downloads: 669