The Association of Silymarin, Vitamin C, Vitamin E, Coenzyme Q10 and Selenomethionine for the Treatment of Non Alcoholic Fatty Liver Disease
Received: 11-Nov-2018 / Accepted Date: 05-Jan-2019 / Published Date: 15-Jan-2019 DOI: 10.4172/2161-069X.1000589
Abstract
Objective: Non-Alcoholic Fatty Liver Disease (NAFLD) is an emerging disease of metabolic origin characterized by the accumulation of fat in the liver. There are currently no specific drugs for the treatment of NAFLD, even if some pharmacological molecules have showed beneficial effects. Many studies reported that silymarin, vitamin E, vitamin C, coenzyme Q10 and selenium can be effective in NAFLD. The present study aims to evaluate the efficacy and safety of a food supplement composed by silymarin, vitamin C, vitamin E, coenzyme Q10 and selenomethionine (Medronys epato®) in patients with NAFLD.
Methods: We enrolled 151 patients with mild/moderate/severe NAFLD. The patients were divided into 2 groups. A group of patients (n=80) received 1 capsule a day of Medronys epato® for 90 days. The second group (n=71) received placebo. The patients were evaluated at 3 times: baseline (T0), after 45 days of treatment (T1) and after 90 days of treatment (T2). At each step an evaluation of the following criteria was carried out: a) Blood parameters (ALT, AST, ALP, GGT and ferritin); b) Liver ultrasound scan; c) Side effects; and d) Patient’s judgment.
Results: After 45 days and after 90 days of treatment, the food supplement composed by silymarin, vitamin C, vitamin E, coenzyme Q10 and selenomethionine (Medronys epato®) showed greater efficacy than placebo by decreasing liver enzymes (ALT, AST, ALP, GGT) and ferritin. The patients of Medronys epato® group improved hepatic steatosis on ultrasound examination, while the patients of placebo group did not improve their ultrasound examination. No adverse effects were reported in either group.
Conclusion: The complex silymarin, vitamin C, vitamin E, coenzyme Q10 and selenomethionine (Medronysepato®) improves blood parameters (ALT, AST, ALP, GGT and ferritin) and symptoms in patients with NAFLD, with high tolerability and satisfaction of patients.
Keywords: Non-alcoholic fatty liver disease; Alanine aminotransferase; Aspartate aminotransferase; Alkaline phosphatase; Gamma-glutamyl transpeptidase; Silymarin; Coenzyme Q10; Vitamin C; Vitamin E; Selenomethionine
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD), considered a benign condition until a few years ago, is an emerging disease of metabolic origin [1,2]. In some cases NAFLD can have an evolutionary course, in particular when inflammation and hepatocellular damage are associated with steatosis [1,2].
This particular condition, called “Non-Alcoholic Steato-Hepatitis” (NASH) is associated with activation of fibrogenesis and may evolve into cirrhosis or hepatocellular carcinoma [3]. NAFLD is currently the most frequent cause of chronic hepatopathy in outpatient clinical practice [3]. The diagnosis is based on the use of image techniques and/or on histological examination after exclusion of secondary causes of steatosis [4].
NAFLD is defined by an excessive accumulation of triglycerides in the liver (>5%), in the presence of an alcohol consumption (accurately determined) <30/20 g per day respectively for M/F (male/female) [5].
The NAFLD can be suspected when there is an enlarged liver associated with the alteration of some blood parameters [4]. In details, an increase in transaminases (ALT and AST), an increase in other liver enzymes, such as Gamma-Glutamyl Transpeptidase (GGT), an increase in alkaline phosphatase (ALP) and/or ferritin, are shown [3,4]. The diagnosis can be ascertained by ultrasound scan [3,4].
The diagnosis of NAFLD is also strengthened by the simultaneous presence of alterations typical of the metabolic syndrome due to a state of insulin resistance: in particular hyperglycemia, dyslipidemia, arterial hypertension, abdominal obesity [1,2].
Obese and diabetic patients are more likely to develop NAFLD and therefore, the increased incidence of NAFLD in Western countries over the last 15-20 years is potentially related to the recent increase in obesity and diabetes in different age groups [2]. However, some patients with NAFLD are normal weight, not diabetic and have a normal lipid profile as well as having normal parameters related to liver function [2].
The management of patients with NAFLD basically consists of treating hepatic disease and associated comorbidities (obesity, dyslipidemia, diabetes) [2-5]. Many studies indicate that lifestyle modifications may reduce transaminase values and degree of steatosis [2].
There are currently no specific drugs for the treatment of NAFLD, even if some pharmacological molecules, commonly used and registered for other indications, have shown beneficial effects in patients with NAFLD/NASH [5,6]. In any case, the pharmacological approach should be reserved for patients with greater severity of hepatic injury (advanced fibrosis) by coordinating with the specialist [6].
Some well-designed studies have reported that silymarin, vitamin E, vitamin C, coenzyme Q10 and selenium can exert beneficial effects in NAFLD [7-16].The data indicate that these compounds are effective in the reduction of the biochemical, inflammatory and ultrasonic indices of liver steatosis [7-16].
Methodology
Our study aims to evaluate the complex silymarin, vitamin C, vitamin E, coenzyme Q10 and selenomethionine (Medronys epato®) in patients with Non-Alcoholic Fatty Liver Disease (NAFLD), through observation of the improvement of blood parameters, the improvement of the ultrasound results and the improvement of the associated symptomatology, together with the tolerability profile. The composition of 1 capsule of Medronys epato® is:
• Silybum marianum 175 mg
• E.s.t. 80% in silymarin 140 mg
• Coenzyme Q 10: 10 mg
• Vitamin C: 60 mg
• Vitamin E: 20 mg
• Selenium: 41,5 mcg
We enrolled 151 patients with mild/moderate/severe NAFLD. In details: mild (60 patients), moderate (61 patients) and severe (30 patients). The patients were divided into 2 groups. A group of patients (n=80) received 1 capsule a day of Medronys epato®. The second group (n=71) received placebo. The patients were evaluated at 3 times: baseline (T0), after 45 days of treatment (T1) and after 90 days of treatment (T2). At each step an evaluation of the following criteria was carried out:
• Blood parameters: ALT (Alanine Amino Transferase), AST (Aspartate Amino Transferase), ALP (Alkaline Phosphatase), GGT (Gamma-Glutamyl Transpeptidase) and ferritin;
• Liver Ultrasound scan;
• Any symptoms reported by patient: dyspepsia, nausea, abdominal swelling;
• Side effects;
• Patient’s judgment: classified as “no benefit”, “real benefit” or “great benefit”. Data collection was carried out by individual physicians as follows:
• Blood parameters: T0, T1, T2;
• Patient’s symptoms and benefit: T0, T2;
• Side effects: T2.
Baseline characteristics of the study population
The study enrolled 151 patients, 71 women and 80 men. Their baseline characteristics are reported in the Table 1. All patients were affected by Non-Alcoholic Fatty Liver Disease of different degree diagnosed through the evaluation of the following blood parameters: ALT, AST, ALP, GGT and ferritin. Liver ultrasound was carried out by physicians in order to confirm the diagnosis of liver steatosis and to determine steatosis degree. In the group of treatment, the degrees of steatosis were divided as follows: 30% mild steatosis, 46% moderate steatosis, 24% severe steatosis (Table 1).
| Variable | Medronys epato group® (n = 80) |
Placebo group (n =71) |
p value |
|---|---|---|---|
| Sex M/F | 50/30 | 30/41 | - |
| Age (Y) - mean (± SD) | 62 (± 13.8) | 44 (± 9.2) | 0.713 |
| Steatosis degree % | 30% mild | 52% mild | - |
| 46% moderate | 33% moderate | 0.388 | |
| 24% severe | 15% severe | - | |
| M/F: Male/Female; Y: Years; Data are expressed as mean (± SD) or number % | |||
Table 1: Baseline characteristics of Medronys epato® and placebo group.
In the group of placebo, the degrees of steatosis were divided as follows: 52% mild steatosis, 33% moderate steatosis and 15% severe steatosis (Table 1).
The mean value of markers of liver damage in the two groups at baseline are reported in Table 2. 65% of patients was affected by other diseases beyond liver steatosis. Among the other common diseases were calculi, heart failure, hypertension, diabetes and dyslipidemia.
| Medronys epato group® (n=80) | Placebo group (n=71) | |||||
|---|---|---|---|---|---|---|
| T0 (Baseline) | T1 (45days) | T2(90 days) | T0 (Baseline) | T1 (45 days) | T2 (90 days) P value |
|
| Markers of liver damage - mean (± SD) | ||||||
| ALT, U/L | 71.6 (±31.8) | 52 (± 24.4) | 39.4 (±14.6) | 82.4 (±18.2) | 83.6 (±18.1) | 78.7 (±17.8) |
| AST, U/L | 64 (± 30.4) | 45.7 (±21.2) | 32.7 (±11.4) | 60.4 (±10.5) | 61.1 (±10.2) | 56.55 (±10.3) |
| ALP, U/L | 104.7 (±13.2) | 87.6 (±53) | 81.3 (±54.8) | 87.2 (±12.9) | 85.9 (±3.8) | 83.6 (±13.6) |
| GGT, U/L | 116.0 (±17) | 85.7 (± 24) | 71.0 (±29) | 49.7 (±9.2) | 51.4 (±9.2) | 46.08 (±8.7) |
| Ferritin, µg/L | 116.0 (±13) | 105.4 (±11) | 93.6 (±10) | 234.5 (±22) | 346 (±11.2) | 340 (±13.1) |
| Data are expressed as mean (± SD); ALT: Alanine Amino Transferase; AST: Aspartate Amino Transferase; ALP: Alkaline Phosphatase; GGT: Gamma-Glutamyl Transpeptidase; p value < 0.001, Medronys epato at T1 and T2 vs T0 (baseline); p value > 0.05, placebo at T1 and T2 vs T0 (baseline); a p value less than 0.05 is considered statistically significant. | ||||||
Table 2: Baseline, T1 (45 days) and T2 (90 days) data for Medronys epato® and placebo group.
Clinical parameters and blood sample collection
Laboratory test results obtained as part of screening included tests for serum ferritin, serum Alanine Amino Transferase (ALT), Aspartate Amino Transaminase (AST), Alkaline Phosphatase (ALP) and Gamma-Glutamyl Transpeptidase (GGT).
Blood samples were collected after 12–14 h from fasting, by an antecubital venous puncture. Serum samples were obtained by centrifugation. Serum levels of Aspartate Amino Transferase (AST), Alanine Amino Transferase (ALT), Gamma-Glutamyl Transpeptidase (GGT), Alkaline Phosphatase (ALP) and ferritin were directly measured using standard automated laboratory methods on Cobas 6000 (Roche, Rotkreuz, Switzerland), by using the relative kits, according to the manufacturer’s instructions.
Lifestyle instructions for study participants
With regard to lifestyle changes, patients were given standardized, 5 minute oral information on the hepatic and general benefits of healthy eating, weight loss, and exercise. Patients were asked to follow these advices if engaging in weight loss, and to refrain from very hypocaloric and other alternative diets.
Statistical analysis
Calculations were performed as paired data, by comparing the biochemical (serum liver- indices) values recorded at baseline, after 45 days and after 90 days. Continuous variables are presented as mean ± SD. p<0.05 was considered statistically significant for analysis. The degrees of steatosis through ultrasound examination recorded in basal conditions and at the end of treatment (90 days) were also compared. All calculations were made using SPSS Version 25.0 for Microsoft Windows.
Results And Discussion
Since there are currently no specific drugs for the treatment of NAFLD we decided to analyze the effects of a food supplement composed by silymarin, vitamin E, vitamin C, coenzyme Q10 and selenium (Medronys epato®) in patients with mild/moderate/severe NAFLD.
Several studies have reported that silymarin, vitamin E, vitamin C, coenzyme Q10 and selenium, administered singly or in combination can exert beneficial effects in NAFLD reducing biochemical, inflammatory and ultrasonic indices of liver steatosis [7-16].
In this placebo-controlled trial, 1 daily dose of Medronys epato® for 90 days showed efficacy in reduction of ALT, AST, ALP, GGT and ferritin both at T1 (45 days of treatment) and T2 (90 days of treatment) in patients with NAFLD. As evident from baseline clinical characteristics, the participants comprised a large spectrum NAFLD cohort and several comorbidities. This makes our findings easily generalizable to the common clinical ambulatory setting.
Markers of liver injury-ALT or alanine amino transferase
The study enrolled 151 patients with mild/moderate/severe Non- Alcoholic Fatty Liver Disease (NAFLD) as detailed above. The patients were divided into 2 groups. A group of patients (n=80) received 1 capsule a day of Medronys epato® for 90 days. The second group (n=71) received placebo for 90 days.
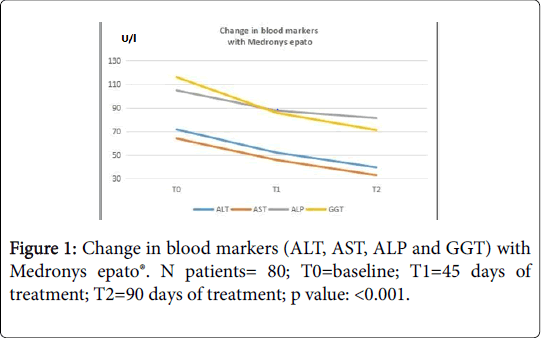
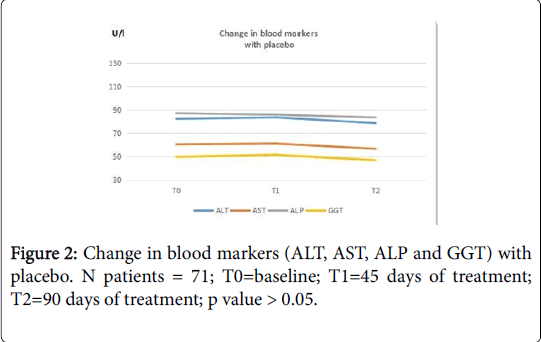
Blood evaluation of ALT values was performed at baseline (T0), after 45 days of treatment (T1) and after 90 days (T2) of treatment. The data show that Medronys epato® significantly decreases the value of ALT in patients with NAFLD (Table 3)[T0= 71.6 (± 31.8) U/L; T1=52.0 (± 24.4) U/L; T2=39.4 (± 14.6) U/L; p value: <0.001; see Figure 1)] compared with placebo [T0=82.4 (± 18.2) U/L; T1=83.6 (± 18.1) U/L; T2: =78.7 (± 17.8) U/L; p value >0.05; see Figure 2)].
Markers of liver injury-AST or aspartate amino transferase
A blood evaluation of AST (Aspartate Amino Transferase) values was carried out during the study, at baseline (T0), after 45 days of treatment (T1) and after 90 days of treatment (T2). The data show that Medronys epato®significantly decreases the values of AST in patients with NAFLD [T0 =64 (± 30.4) U/L; T1=45.7 (± 21.2) U/L; T2=32.7 (± 11.4) U/L; p value= <0.001; see Figure 1)] compared with placebo [T0=60.4 (± 10.5); T1=61.1 (± 10.2); T2=56.55 (± 10.3); p value >0.05; see Figure 2)].
Markers of liver injury-ALP
A blood evaluation of ALP (Alkaline Phosphatase) values was carried out during the study, at baseline (T0), after 45 days of treatment (T1) and after 90 days of treatment (T2).
The mean values of ALP in the Medronys epato® group and in the placebo group were within the reference range values at baseline (T0). The reference range of ALP was 40-129 U/L for males and 35-104 U/L for females. In the group of Medronys epato® a reduction of ALP values was observed both at T1 and T2 from baseline [(T0=104.7 (± 13.2) U/L; T1=87.6 (±53) U/L; T2=81.3 (± 54.8) U/L; p value= <0.001; see Figure 1] and the final values were in any case within reference range values. The reduction of ALP measured in the group of treatment was greater than placebo [T0=87.2 (± 12.9) U/L; T1 =85.9 (± 13.8) U/L; T2=83.6 (± 13.6) U/L; p value >0.05; see Figure 2].
Markers of liver injury-GGT
A blood evaluation of GGT (Gamma Glutamyl Transpeptidase) values was carried out during the study, at baseline (T0), after 45 days of treatment (T1) and after 90 days of treatment (T2).
The data show that Medronys epato® significantly decreases GGT values in patients with NAFLD [T0=116.0 (± 17) U/L; T1=85.7 (± 24) U/L; T2=71.0 (±29) U/L; p value= < 0.001; see Figure 1] compared with placebo [T0=49.7 (± 9.2) U/L; T1 = 51.4 (± 9.2) U/L; T2=46.08 (± 8.7) U/L; p value >0.05; see Figure 2].
Markers of liver injury-Ferritin
A blood evaluation of ferritin values was carried out during the study, at baseline (T0), after 45 days of treatment (T1) and after 90 days of treatment (T2).
The data show a reduction in the ferritin values at T1 and T2 for Medronys epato® [T0=116.0 (±13) μg/L; T1=105.4 (±11) μg/L; T2=93.6 (± 10) μg/L; p value= <0.001] while in the placebo group ferririn values increased both at T1 and T2 [T0 = 234.5 (± 22) μg/L; T1=346 (± 11.2) μg/L; T2=340 (± 13.1) μg/L; p value> 0.05; see Table 2].
Liver ultrasound examination
The patients underwent liver ultrasound examination at baseline (T0), after 45 days (T1) and after 90 days (T2) to assess the degree of liver steatosis. In the group of Medronys epato®, the ultrasound results at T1 were recorded in 54 patients (68%). Considering the data recorded for the 54 patients, at T1, 17 patients (31.5%) did not show improvement of the ultrasound scan, 17 patients (31.5%) showed a little improvement in the ultrasound scan, while 20 patients (37.0%) showed a significant improvement in the ultrasound scan.
The ultrasound results at T2 were recorded in 58 patients (72.5%). Considering the data recorded for 58 patients, at T2, 13 patients (22.4%) showed no improvement in the ultrasound scan, 9 patients (15.5%) showed a little improvement in the ultrasound scan, while 36 patients (62.1%) showed a significant improvement in the ultrasound scan. The results obtained were satisfactory. The placebo group did not improve hepatic steatosis on ultrasound examination.
Patient judgment
At the end of the treatment (90 days) with Medronys epato®, we asked to the patients if they had obtained benefit from the treatment and of which entity. The parameters used were: a) no benefit; b) real benefit; and c) great benefit.
The data were collected for all the patients (151). The 96% of patients in Medronys epato® group reported an improvement of symptoms after treatment with Medronys epato®.
In details, in Medronys epato® group, 3 patients (4%) reported that they had no benefit by treatment, 52 patients (65%) reported a real benefit and 25 patients (31%) reported a great benefit by treatment.
In placebo group benefit for patients has not been recorded. No adverse events were reported either in Medronys epato® group or in placebo group.
Conclusion
In conclusion, the present study has demonstrated that patients with NAFLD assigned to Medronys epato® (silymarin, vitamin C, vitamin E, coenzyme Q10 and selenomethionine) significantly benefited in terms of improvement in liver enzymes (ALT, AST, ALP, GGT), ferritin, liver ultrasound and symptoms compared with placebo during the 3- months period. Furthermore, this nutritional supplementation was generally safe and well tolerated, and no adverse events were reported.
Conflicts of Interest
Conflict of interest disclosed was none.
References
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, et al. (2016) Global epidemiology of nonalcoholic fatty liver disease - meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64: 73-84.
- Marzocchi R, Zannoni C, Moscatiello S, Marchesini G (2004) The non-alcoholic epathic steatosis: an emerging pathology of metabolic interest. Italian J Diabetol Metabol 24: 107-115.
- Benedict M, Zhang X (2017) Non-alcoholic fatty liver disease: An expanded review. World J Hepatol 9: 715-732.
- Dowman JK, Tomlinson JW, Newsome PN (2011) Systematic review: The diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther 33: 525-540.
- Abd El-Kader SM, El-Den Ashmawy EM (2015) Non-alcoholic fatty liver disease: The diagnosis and Management. World J Hepatol 7: 846-858.
- Sumida Y, Yoneda M (2018) Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol 53:362–376.
- Zhong S, Fan Y, Yan Q, Fan X, Wu B, et al. (2017) The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease A meta-analysis (PRISMA) of randomized control trials. Medicine (Baltimore) 96: e9061.
- Aller R, Izaola O, GÏŒmez S, Tafur C, Gonzalez G, et al. (2015) Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci 19: 3118-3124.
- Loguercio C, Federico A, Trappoliere M, Tuccillo C, De Sio I, et al (2007) The effect of a silybin- vitamin e-phospholipid complex on nonalcoholic fatty liver disease: a pilot study. Dig Dise Sci 52: 2387-2395.
- Loguercio C, Andreone P, Brisc C, Bugianesi E, Chiaramonte M, et al. (2012) Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med 52: 1658–1665.
- Wellington K, Jarvis B (2001) Silymarin: A review of its clinical properties in the management of hepatic disorders. BioDrugs 15: 465-489.
- Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, et al. (2006) A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non- alcoholic fatty liver disease: preliminary observations. Gut 55: 901-902.
- Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F (2013) Silymarin in non alcoholic fatty liver disease. World J Hepatol 5: 109-113.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S (2003) Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 98: 2485-90.
- Vidlar A, Vostalova J, Ulrichova J, Student V, Krajicek M, et al. (2010) The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy-a six month placebo-controlled double-blind clinical trial. Biomed Pap Med Fac Univ Palacky 154: 239-44.
- Farsi F, Mohammadshahi M, Alavinejad P, Rezazadeh A, Zarei M, et al. (2015) Functions of Coenzyme Q10 Supplementation on Liver Enzymes, Markers of Systemic Inflammation, and Adipokines in Patients Affected by Nonalcoholic Fatty Liver Disease: A Double-Blind, Placebo- Controlled, Randomized Clinical Trial. J Am Coll Nutri 35: 346- 53.
Citation: Adriana R, Annalisa C, Antonio DN, Orazio G, Donatella S, et al. (2019) The Association of Silymarin, Vitamin C, Vitamin E, Coenzyme Q10 and Selenomethionine for the Treatment of Non Alcoholic Fatty Liver Disease. J Gastrointest Dig Syst 9: 589. DOI: 10.4172/2161-069X.1000589
Copyright: © 2019 Adriana R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4696
- [From(publication date): 0-2019 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 3886
- PDF downloads: 810