Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Review Article Open Access
The Association between Maternal Vitamin D Status in Gestation and Pre-Eclampsia
| Arsala L, Silva Costa FD and Murthi P* | |
| Department of Perinatal Medicine, Pregnancy Research Centre and Department of Obstetrics and Gynaecology, Royal Women’s Hospital Parkville VIC 3052, Australia | |
| Corresponding Author : | Padma Murthi NorthWest Academic Centre The University of Melbourne St Albans, Victoria 3021, Australia Tel: 61383958075 E-mail: padma@unimelb.edu.au |
| Received July 14, 2014; Accepted August 14, 2014; Published August 14, 2014 | |
| Citation: Arsala L, Silva Costa FD, Murthi P, (2014) The Association between Maternal Vitamin D Status in Gestation and Pre-Eclampsia. J Preg Child Health 1:107. doi: 10.4172/2376-127X.1000107 | |
| Copyright: © 2014 Arsala L et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pregnancy and Child Health
Abstract
Preeclampsia is a complex and life threatening pregnancy disorder is mojor cause of maternal and neonatal morbidity and mortality. A growing body of evidence has demonstrated that low vitamin D is associated with the pathophysiology of preeclampsia. As such, identification of the mechanisms behind this relationship and placental vascular endothelial dysfunction presents itself as a significant modifiable disease risk factor, which if identified and managed appropriately, may make a significant impact in reducing the burden of disease.
| Keywords |
| Vitamin D; Preeclampsia; Gestation; Pregnancy; Endothelial dysfunction |
| Introduction |
| Preeclampsia is a life threatening pregnancy disorder, which is classically characterised by hypertension and proteinuria and complicates 2-8% of all pregnancies [1]. The pathophysiology of preeclampsia is poorly understood, however, it is often described as a 2-stage process whereby Stage I is characterised by abnormal placental invasion and formation resulting in impaired placental perfusion. It is thought that reactive oxygen species and pro-inflammatory cytokines released from the ischaemic placenta result in oxidative stress and placental endothelial cell dysfunction [2,3]. This creates a pathophysiological state resulting in Stage II of preeclampsia with the clinical detection of hypertension, proteinuria and eventual organ damage [2]. |
| In recent times, it has become increasingly evident that preeclampsia is no longer an isolated disease of pregnancy, but rather has a significant impact on the risk of subsequent maternal and paediatric cardiovascular disease [4,5]. Preeclampsia has been shown to be an independent risk factor for maternal cardiovascular disease 10 to 15 years after the affected pregnancy, with an increase in the risk of cardiovascular disease of similar magnitude to that of dyslipidaemia [4,6]. Furthermore, children of preeclamptic pregnancies have been found to have elevated blood pressure and increased cardiovascular risk later in life [7]. |
| Current research highlights low levels of the steroid hormone vitamin D as a potentially significant independent risk factor for the development of preeclampsia and cardiovascular disease [3,8]. Vitamin D plays a critical role in vascular endothelial function, and deficiency leads to dysfunction in the preeclamptic placenta [3]. This review discusses vitamin D function in the placenta, deficiency of which increases the risk of preeclampsia, a significant cause of maternal and neonatal morbidity, and as such presents a potentially significant modifiable risk factor [9]. |
| Vitamin D Physiology and Metabolism |
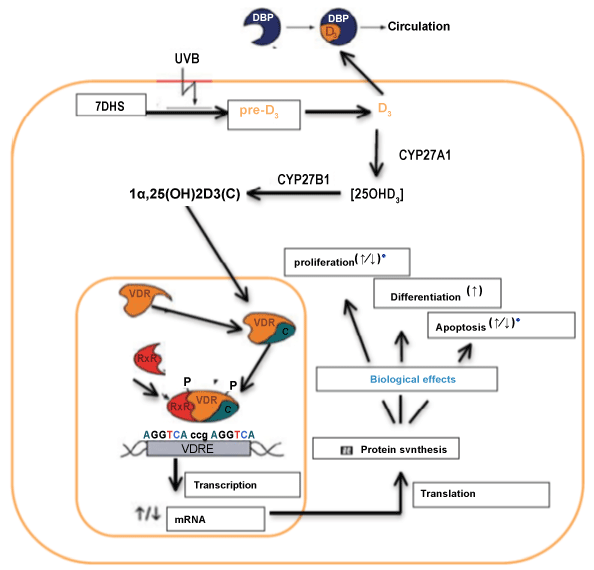
| Vitamin D is a steroid hormone classically involved in calcium homeostasis. The two main forms are vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) (Table 1) [10,11]. The main source of vitamin D in humans (~90%) is in the form of vitamin D3, which is derived from synthesis in the skin via exposure of 7-dehydrocholesterol, concentrated in the stratum basale and stratum spinosum, to ultraviolet B (UV-B) radiation. Vitamin D2 is obtained from the diet, and is derived from ultraviolet irradiation of ergosterol, found in fungi [11]. Both metabolites are transported in the blood bound to vitamin D binding protein (DBP) (Figure 1) [11,12]. These inactive vitamin D metabolites must undergo a two-step hydroxylation process to become biologically active [11]. Initially, vitamin D2 and D3 undergo hydroxylation in the maternal liver, via the action of vitamin D 25-hydroxylase enzyme (CYP27A1), to form the inactive steroid precursor 25-hydroxy-vitamin D (25[OH]D). 25[OH]D is the major circulating and stored form of vitamin D [10,11]. To enable biological activation, further hydroxylation occurs via the action of 25-hydroxyvitamin D-1-α-hydroxylase, found in the maternal kidneys and placenta, to form the active metabolite 1,25 hydroxy-vitamin D [1,25 [OH]2D [10,11]. This process in the kidneys is tightly regulated by the parathyroid hormone, serum calcium and phosphorous levels in a physiological regulatory loop [11]. Measurement of serum vitamin D levels involve detection of 25-hydroxy-vitamin D (25[OH]D). We measure 25[OH]D in preference to the active 1,25[OH]D due to the latter having a half-life of several minutes, as compared to 3 weeks for the former [12]. 1,25[OH]2D concentrations are much higher once pregnancy begins [13]. |
| Mechanism of Vitamin D Action in the Placenta |
| In recent times it has been highlighted that vitamin D has many other important non-classic roles including placental and immunomodulatory functions [14]. Furthermore, vitamin D deficiency is becoming increasingly prevalent and has been linked to adverse pregnancy outcomes, including preeclampsia, insulin resistance, gestational diabetes mellitus, low birth weight (<2500 g) and high rate of Caesarean section [14-16]. The actions of the active metabolite, 1,25[OH]2D, are mediated via binding to the vitamin D receptor (VDR), which is present in a diverse range of tissues. Sites of VDR expression include endothelial cells, cells of the innate and adaptive immune systems, pancreatic β-cells, the brain and reproductive tissues including placental trophoblast cells [17,18]. The VDR is a nuclear receptor for steroid hormones and mediates its action via two main methods, one of which is at the gene expression level and the other a more rapid non-genomic response involving signalling cascades [9]. Identified roles of vitamin D include induction of decidualisation in early pregnancy to enable implantation, angiogenesis, modulation of immune function and regulation of hormone function in pregnancy [19,20]. Human placental trophoblast cells both produce and respond to 1,25[OH]2D, and the P450 cytochrome enzymes encoded by CYP27B1 (1α-hydroxylase) and CYP24A1 (25-hydroxylase) genes are local regulators of 1,25[OH]2D in these cells [3]. CYP24A1 encodes 25-hydroxylase, the vitamin D catabolyzing enzyme, converting 25[OH]D and 1,25[OH]2D to their inactive metabolites. |
| Recent studies have demonstrated that in preeclamptic placentas there is increased expression of this catabolyzing enzyme, suggesting elevated degradation of active vitamin D in these placentas as compared to healthy placentas [3]. Furthermore, researchers have found reduced expressions of VDR and DBP in preeclamptic placentas as compared to normal placentas, providing direct evidence of disrupted vitamin D metabolism in the preeclamptic placenta [3]. |
| Although the exact molecular mechanisms by which vitamin D deficiency affects the risk of developing preeclampsia is yet to be determined, there are a number of potential avenues by which they are hypothesised to occur. Reduced placental perfusion during Stage I may result in the placenta producing substances, including proinflammatory cytokines that initiate the ensuing multi-system sequelae characterising Stage II of preeclampsia [17,19]. Pro inflammatory cytokines such as tumour necrosis factor-α, (TNF-α) interleukin-6 (IL-6) and interferon-γ (IFN- γ), as well as the pro-inflammatory nuclear transcription factor nuclear factor-κB (NF-κB), are increased in pregnant women with vitamin D deficiency [17,19]. Furthermore, vitamin D has been found to inhibit proliferation of T helper 1 (Th1) cells and reduce their production of pro-inflammatory cytokines IFN- γ, IL-2, and TNF-α [17]. Thus, hypothesising that maternal vitamin D deficiency predisposes to a pro-inflammatory state with increased levels of oxidative stress, leading to endothelial cell dysfunction, may have sound premise. |
| Vitamin D Deficiency and Vascular Endothelial Dysfunction |
| The diagnosis and management of vitamin D deficiency is a contentious issue with varying local practices [10]. The adequate level of serum 25(OH)D levels remains contested, with levels ranging from 80-250 nmol/L being considered vitamin D replete [11]; the majority of the literature supports the consensus of <50 nmol/L being classified as vitamin D deficient [10,11]. This can be further subclassified into three categories differentiated by severity; marginal (25-50 nmol/L), moderate (12.5-25 nmol/L), and severe (<12.5 nmol/L) [20]. |
| In recent times, there have been a number of studies that have demonstrated a link between vitamin D deficiency and placental dysfunction in preeclampsia (Table 2), with particular emphasis on Stage I of preeclampsia as the key period during which vitamin D deficiency would have a significant impact. Bodnar et al demonstrated via a nested case control study that maternal vitamin D deficiency at less than 22 weeks’ gestation was a strong independent risk factor for preeclampsia [9]. The authors hypothesise that potential mechanisms may include abnormal implantation in the absence of sufficient vitamin D leading to subsequent impaired placental perfusion and oxidative stress which results in the preeclamptic physiological sequlae [9]. Furthermore, similar results were reported by Robinson et al in another case control study where women with early onset severe preeclampsia were found to be significantly vitamin D deficient at the time of diagnosis [2]. Another observational study from Norway on 23,423 nulliparous pregnant women demonstrated that vitamin D supplementation during pregnancy reduced the risk of preeclampsia by 27% [20]. These observational studies demonstrate a probable link between vitamin D deficiency during pregnancy and prevalence of preeclampsia, although the exact mechanism by which this occurs is yet to be determined. |
| However, there have been contradictory results from some studies with a prospective cohort study by Shand et al (n=221) finding no association between vitamin D deficiency and the incidence of preeclampsia in early pregnancy in a group of women identified as being at high risk of developing preeclampsia [21,22]. This conflicting result may have been due to the small sample size and selective patient subset. Furthermore, the quantification of vitamin D status during different gestational ages may have also contributed to the incongruent findings. In particular the authors looked at vitamin D concentrations between 10 to 20th week of pregnancy [22], whereas most studies show a significant association between lower concentrations of vitamin D and preeclampsia at the third trimester [23]. Therefore, studies assessing vitamin D levels at the first trimester and the subsequent risk of developing preeclampsia may conceal the true risk of such deficiency on development of preeclamptic disease later in the pregnancy. As such, studies may not be directly comparable at such varying gestation times. |
| Subsequently, in a prospective cohort study (n=697) assessing longitudinal vitamin D status in pregnancy has demonstrated a positive association between vitamin D deficiency at 24-26 weeks’ gestation and preeclampsia [19]. Furthermore, Tabesh et al found that there was a significant association between vitamin D deficiency and the risk of developing preeclampsia on systematic review and meta-analyses of published observational studies [23]. |
| It must be noted that these cross sectional studies have innate limitations, in that causal relationships are unable to be established [23]. Further research and stronger study design in the form of randomised clinical trials is required to elucidate the exact relationship between vitamin D and preeclampsia, although the feasibility of such studies in a patient cohort of pregnant women may be debatable. |
| Conclusion |
| It is clear that we can no longer consider vitamin D as a humble steroid hormone involved solely in mineral homeostasis - it has more complex actions than previously accredited with. In particular, we cannot ignore the multitude of evidence from observational studies linking vitamin D deficiency to preeclampsia. Further research is required to determine the exact mechanisms by which vitamin D influences placental formation, and in particular the role that vitamin D plays in Stage I of the development of preeclampsia, and the specific downstream molecular mechanisms involved in endothelial dysfunction. |
| The limitations of observational studies conducted to date have been discussed, highlighting the beneficial role a randomised clinical trial would play in providing us with definitive evidence based answers. Although, this may be technically difficult due to the nature of pregnancy and ethical obligations of patient safety, a careful study design can make this a reality. Other clinical questions that may be answered include the correct vitamin D analogue to test, the timing of vitamin D testing and ideal window of vitamin D supplementation in order to correctly diagnose deficiency and to effectively treat this deficiency in order to provide the greatest benefit for preventing the development of maternal preeclampsia. |
| If we were able to answer these clinical questions, and further identify the specific molecular and genetic mechanisms involved in the feto-placental unit, we could better understand the intricate relationship between vitamin D deficiency and placental endothelial vascular dysfunction, thus allowing us to modify the development of long term maternal and paediatric adverse health outcome. |
References
- Behrouz GF, Farzaneh GS, Leila J, Jaleh Z, Eskander KS (2013) Presence of auto-antibody against two placental proteins , annexin A1 and vitamin D binding protein, in sera of women with pre-eclampsia. J ReprodImmunol 99: 10-16
- Robinson CJ, Alanis MC, Wagner CL, Hollis BW, Johnson DD (2010) Plasma 25-hydroxyvitamin D levels in early-onset severe preeclampsia. Am J ObstetGynecol 203: 366.
- Ma R, Gu Y, Zhao S, Sun J, Groome LJ, et al. (2012) Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J PhysiolEndocrinolMetab 303: E928-935.
- McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ (2008) Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 156: 918-930.
- Lazdam M, Davis EF, Lewandowski AJ, Worton SA, Kenworthy Y, et al. (2012) Prevention of vascular dysfunction after preeclampsia: a potential long-term outcome measure and an emerging goal for treatment. J Pregnancy 2012: 704146.
- Magee LA, von Dadelszen P (2007) Pre-eclampsia and increased cardiovascular risk. BMJ 335: 945-946.
- Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, et al. (2010) Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension 56: 159-165.
- Hermes W, Franx A, van Pampus MG, Bloemenkamp KW, van der Post JA, et al. (2010) 10-Year cardiovascular event risks for women who experienced hypertensive disorders in late pregnancy: the HyRAS study. BMC Pregnancy Childbirth 10: 28.
- Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, et al. (2007) Maternal vitamin D deficiency increases the risk of preeclampsia. J ClinEndocrinolMetab 92: 3517-3522.
- De Laine KM, Matthews G, Grivell RM (2013) Prospective audit of vitamin D levels of women presenting for their first antenatal visit at a tertiary centre. Aust N Z J ObstetGynaecol 53: 353-357.
- Gezmish O, Black MJ (2013) Vitamin D deficiency in early life and the potential programming of cardiovascular disease in adulthood. J CardiovascTransl Res 6: 588-603.
- Barrett H, McElduff A (2010) Vitamin D and pregnancy: An old problem revisited. Best Pract Res ClinEndocrinolMetab 24: 527-539.
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL (2011) Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 26: 2341-2357.
- Bener A, Al-Hamaq AO, Saleh NM (2013) Association between vitamin D insufficiency and adverse pregnancy outcome: global comparisons. Int J Womens Health 5: 523-531.
- Huang JY, Qiu C, Miller RS, Siscovick DS, Williams MA, et al. (2013) Maternal birthweight is associated with subsequent risk of vitamin D deficiency in early pregnancy. PaediatrPerinatEpidemiol 27: 472-480.
- Scholl TO, Chen X, Stein P (2012) Maternal vitamin D status and delivery by cesarean. Nutrients 4: 319-330.
- Ota K, Dambaeva S, Han AR, Beaman K, Gilman-Sachs A, et al. (2014) Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum Reprod 29: 208-219.
- Mizwicki MT, Norman AW (2009) The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal 2: re4.
- Wei SQ, Audibert F, Hidiroglou N, Sarafin K, Julien P, et al. (2012) Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG 119: 832-839.
- Haugen M, Brantsaeter AL, Trogstad L, Alexander J, Roth C, et al. (2009) Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women. Epidemiology 20: 720-726.
- Stroud ML, Stilgoe S, Stott VE, Alhabian O, Salman K (2008) Vitamin D - a review. AustFam Physician 37: 1002-1005.
- Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ (2010) Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG 117: 1593-1598.
- Tabesh M, Salehi-Abargouei A, Tabesh M, Esmaillzadeh A (2013) Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis. J ClinEndocrinolMetab 98: 3165-3173.
- Seely EW, Wood RJ, Brown EM, Graves SW (1992) Lower serum ionized calcium and abnormal calciotropic hormone levels in preeclampsia. J ClinEndocrinolMetab 74: 1436-1440.
- Oken E, Ning Y, Rifas-Shiman SL, Rich-Edwards JW, Olsen SF, et al. (2007) Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann Epidemiol 17: 663-668.
- Azar M, Basu A, Jenkins AJ, Nankervis AJ, Hanssen KF, et al. (2011) Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: a longitudinal study.Diabetes Care 34: 1258-1264.
- Baker AM, Haeri S, Camargo CA Jr, Espinola JA, Stuebe AM (2010) A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J ClinEndocrinolMetab 95: 5105-5109.
- Powe CE, Seely EW, Rana S, Bhan I, Ecker J, et al. (2010) First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension 56: 758-763.
- Hossain N, Khanani R, Hussain-Kanani F, Shah T, Arif S, et al. (2011) High prevalence of vitamin D deficiency in Pakistani mothers and their newborns. Int J GynaecolObstet 112: 229-233.
- Fernández-Alonso AM, Dionis-Sánchez EC, Chedraui P, González-Salmerón MD, Pérez-López FR; Spanish Vitamin D and Women's Health Research Group (2012) First-trimester maternal serum 25-hydroxyvitamin D₃ status and pregnancy outcome. Int J GynaecolObstet 116: 6-9.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
| Figure 1 |
Post your comment
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 13654
- [From(publication date):
October-2014 - Dec 18, 2024] - Breakdown by view type
- HTML page views : 9259
- PDF downloads : 4395
