Case Report Open Access
The Antimicrobial Therapy of the Future: Combating Resistances
| Beatriz Suay-García and María Teresa Pérez-Gracia* | ||
| Instituto Ciencias Biomédicas, Universidad CEU Cardenal Herrera, Moncada, Valencia, Spain | ||
| Corresponding Author : | María Teresa Pérez-Gracia Area Microbiología. Departamento Farmacia Instituto Ciencias Biomédicas Universidad CEU Cardenal Herrera Moncada, Valencia, Spain Tel: +34-00-96139 5272 Fax: +34-00-96 136 9000 E-mail: teresa@uch.ceu.es |
|
| Received April 25, 2014; Accepted May 29, 2014; Published June 06, 2014 | ||
| Citation: Suay-García B, Pérez-Gracia MT (2014) The Antimicrobial Therapy of the Future: Combating Resistances. J Infect Dis Ther 2:146. doi:10.4172/2332-0877.1000146 | ||
| Copyright: © 2014 Pérez-Gracia MT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
The appearance of antibiotic resistance is an increasing problem in our society, where the adaptation of microorganisms to conventional therapies has been favored due to their incorrect use. This has driven the scientific community to develop new therapeutic alternatives hoping to obtain treatments that are more effective against increasingly resistant bacteria. The purpose of this study is to review the existent knowledge on the therapeutic alternatives that are being developed to treat cases of infection with antibiotic resistant bacteria. To do so, scientific publications were consulted on the MEDLINE database using different search terms. The bibliography consulted indicates that a great variety of therapeutic alternatives are currently being developed among which the most relevant are probiotics, synthetic peptides, bacteriophages and nanoparticles. Some of these measures, such as probiotics, are already being introduced in some hospitals with positive results. Others, such as synthetic peptides, bacteriophages or nanoparticles are still in the early stages of development and clinical trials. Moreover, there is a clear tendency to go back to the more classical medicine turning to plant extracts and essential oils whose active ingredients have been proven to have therapeutic activity. This shift towards less conventional therapies is marking the beginning of a post-antibacterial era in which antibiotics will most probably be replaced for probiotics, bacteriophages, synthetic peptides or even inorganic nanoparticles.
| Key Words |
| Antibiotics; Resistance; Therapeutic alternatives |
| Introduction |
| The appearance of antibiotic resistances is a serious problem facing today’s society where the adaptation of microorganisms to conventional therapies is being favored due to the wrong use of these treatments. |
| Since antibiotics were first used, bacteria have evolved in order to survive these treatments by effectively developing resistance mechanisms. The four main resistance mechanisms are the modification of the antibiotic by means of the production of enzymes that inactivate the antibiotic, the alteration of bacterial proteins that act as therapeutic targets, changes in membrane permeability, preventing the chemical agent from entering the cell and the active pumping of the antibiotic [1]. |
| In the 60s, a group of renowned experts in infectious diseases and microbiologists [2] gathered to discuss the question: “Is there a real need for new antibiotics?” At the end of the meeting they came to the conclusion that it was imperative to accelerate the development of new antibiotics because many Gram-positive bacteria, including staphylococci and pneumococci, were already showing a high level of resistance to existing treatments. They also highlighted that Gram-negative bacteria were also starting to show signs of resistance. |
| Since that meeting, scientists have seen that the number of resistant bacteria has been growing exponentially in the last few decades. Therefore, the scientific community has started a series of alternatives to avoid certain infections from becoming mortal illnesses due to a lack of an effective antibiotic. Having infections that currently have a cure become mortal due to resistances would be a serious public health problem and a step backwards in the field of medicine. |
| The aim of this study was to review the current knowledge of the therapeutic alternatives being developed to treat cases of microbial infections with antibiotic resistance. |
| Materials and Methods |
| The bibliographic revision took place searching MEDLINE and EBSCO databases using the key words “antibiotic resistance”, “antibiotic alternatives”, “phages”, “peptide antibiotics” and “medicinal plants with antibiotic properties”. Both, abstracts and complete articles published between 1965 and 2014 were consulted. |
| Current State of Antibiotic Resistances |
| The discovery of antibiotics in 1940 represented one of the most remarkable events in the history of medicine seeing as their use has allowed for the treatment of infectious diseases up to now. However, as years have gone by, many factors, mainly the ability of adaptation of bacteria and the indiscriminate use of antimicrobial agents, have led to the development of resistances and the emergence of infectious diseases produced by microorganisms that no longer respond to common antibiotic therapies. |
| A resistant strain is that which has a wild phenotype that allows it to naturally “resist” a specific antibiotic. The base of said resistance is generally a structure of the bacteria that acts as a barrier [3]. The mechanisms biochemical of antibiotic resistance can be divided in four main categories (Table 1) [4]: |
| Antibiotic modification. |
| Changes in the therapeutic target. |
| Alteration of membrane permeability. |
| Active pumping of the antibiotic (Efflux effect). |
| According to the World Health Organization [5], the most common bacterial infections are those in which the problem of antibiotic resistance is more evident: diarrhoea’s, respiratory tract infections, meningitis, sexually transmitted diseases and nosocomial infections. The people infected with antibiotic-resistant microorganisms usually have longer hospitalization periods and require treatments that combine two or three antibiotics, which might be less effective, more toxic and more expensive. This fact constitutes a worldwide health issue, which also has great implications at an economic and social level. |
| According to data published by the Centers of Disease Control of the United States [6]: |
| Around two million patients in the United States become infected in the hospital every year. |
| Of these patients, about 90,000 die every year as a result of superinfection. |
| At least two million people become infected with bacteria that are resistant to antibiotics. |
| At least 23,000 people die each year as a direct result of these infections. |
| More than 70 percent of the diseases acquired in hospitals are resistant to at least one of the antibiotics commonly used for their treatment. |
| The people infected with antibiotic-resistant microorganisms usually have longer hospitalization periods and require treatments that combine two or three antibiotics, which might be less effective, more toxic and more expensive. |
| The resistant bacteria most commonly isolated in hospitals are: Staphyloccocus aureus, Enterococcus, Klebsiella, Enterobacter, Escherichia coli, Pseudomonas and more recently Acinetobacter. On the other hand, there are multi-resistant bacteria that produce infections in the community outside hospitals such as Streptococcus pneumoniae, Neisseria gonhorroeae, Mycobacterium tuberculosis and Streptococcus pyogenes [5,6]. |
| In the last decades of the 20th century, the appearance of antibiotics such as fluoroquinolones, oxyimino-cephalosporins and carbapenems seemed to be the ultimate solution against bacterial infections. However, in the last few years, bacteria have proved to have very effective mechanisms to generate resistances [7]. For example, Escherichia coli has become a high mortality causing pathogen because it has developed the ability to produce extended spectrum beta-lactamases (ESBL) [8,9]. Calbo et al. [10] performed a study in which they concluded that the prevalence of infections due to ESBL producing E. coli had increased from 0.47% to 1.7% in a three year period, which shows that the emergence of these strains is increasing. Moreover, some of the most severe nosocomial infections are caused by carbapenemase producing bacteria, such as certain strains of Klebsiella pneumonia [11] and Pseudomonas spp. [12]. This was the case of an outbreak of K. pneumoniae that took place in a Greek hospital in which, between the months of May and November 2012, 19 patients of the intensive care unit were infected [13]. |
| As for Gram-positive bacteria, Staphylococcus aureus must be noted seeing as it is a species that appears frequently both in the hospital and community environment. The methicillin resistant S. aureus strain (MRSA) has proved to be resistant against the vast majority of beta-lactam antibiotics and it appears to be spreading outside hospitals. A study [14] that took place in the United States showed that out of the 4131 samples taken, 2,093 (51%) were identified as MRSA. Specimen sources were wound or abscess (54%), blood (24%), lower respiratory tract (11%), and other sterile site (10%). One strain type (USA300/t008/IV) constituted almost half of all MRSA isolates (1,500 isolates; 48%) and was the most common at all body sites. It caused 38% of nosocomial MRSA infections and 37% of MRSA bloodstream infections. Multidrug-resistant phenotypes were found among 34 USA300 isolates (3%) from 18 states. |
| Seeing as the number of multi-resistant bacteria is increasing, especially within hospitals, many health systems have adopted antimicrobial stewardship protocols [15], considered a key factor in avoiding resistance appearance. These programs are established in order to select an appropriate treatment and optimize its dose and duration while trying to minimize toxicity and appearance of resistances considering that, in a study conducted in USA, up to 50% of antibiotic prescriptions were incorrect or unnecessary. The programs are designed to be directed by hospital staff and having in the team’s core a doctor with expertise on infectious diseases and a pharmacist specialized in infectious diseases [16]. |
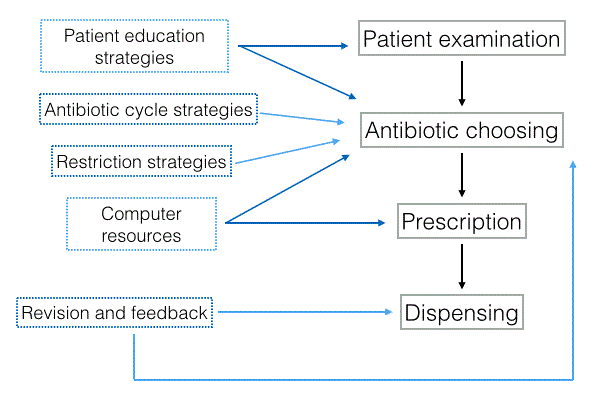
| The antibiotic stewardship programs are organized following an established protocol that has a scheme similar to the one described in Figure 1. It is based on strategies founded on empirical evidence with the idea of adapting each program to the needs and resources of the community. Thus, after a diagnosis based on laboratory evidence, the antibiotic that better adapts to the patient is prescribed, seeing as treatment adherence is essential in these cases. This is followed by a series of strategies [17] to educate the patients in order to make them understand the importance of their treatment. Finally, once the treatment is completed, there is a follow-up to confirm that the treatment has been effective. |
| A study conducted by Hecker et al. [18] suggests that this antimicrobial stewardship program not only reduces the appearance of resistances and adverse effects, but also improves the patient’s adherence to the treatment. |
| Therapeutic alternatives |
| Regardless of the efforts of the health community to make a correct use of antibiotics with programs such as the ones describe above, the appearance of resistances keeps increasing. For this reason, several lines of investigation are being developed with the purpose of finding alternatives to conventional antibiotics. |
| Probiotics |
| Probiotics are living microorganisms, identical or similar to the ones found naturally in the human body that can be beneficial. Among their functions, three mechanisms that take place in the intestine must be noted due to their antimicrobial activity. These are: their ability to modulate the intestine’s microbiome, being able to replace pathogens, the production of antimicrobial compounds and immune system enhancement [19]. |
| Many studies show the efficacy of oral probiotics as antimicrobial agents. Thus, Szajewska et al. [20] conducted a study in 2013 which showed the effectiveness of the DSM 17938 Lactobacillus reuteri strain in reducing the clinical phase of acute gastroenteritis in children less than 5 years of age. Besides the digestive system, which appears to be the most evident therapeutic target, many studies have focused on treating respiratory diseases based on the immunomodulative capacity of probiotics. Regarding this matter, positive results have been obtained from clinical studies [21] geared towards upper respiratory tract infections, nosocomial pneumonia and cystic fibrosis. |
| Once the therapeutic potential of probiotics was clear, different administration routes began to be evaluated amongst which, faecal transplant stands out. This technique [22] consists in collecting faecal samples from healthy donors and preparing suspensions that will be administered to the patients via enema or nasogastric tube. It is a very interesting alternative seeing as it has proved to be effective even when oral administration of probiotics failed. |
| The faecal transplant is turning out to be a key therapeutic tool in hospitals to combat Clostridium difficile infections (CDI) as this microorganism presents multiple resistances. This technique is already being used in prestigious hospitals such as the Mayo Clinic in the USA [23], which has already established an experimental program to treat CDI patients that do not respond to antibiotics. Moreover, they suggest the development of a standardized protocol in order to include it in the therapeutic arsenal of all hospitals [24]. |
| A study published in the New England Journal of Medicine [25] suggests that transplanting donor faeces to a patient suffering a recurrent infection with Clostridium difficile can turn out to be more effective than antibiotic treatment. The authors compared this treatment with the use of vancomycin. For this purpose, 43 patients were randomly divided in two groups; the first one received a faecal transplant from healthy donors in the small intestine while the second group was treated with a standard antibiotic regime during 2 weeks. The transplant was effective in 94% of the cases, while the infection was barely controlled with pharmacological treatment. It must be noted that, the faecal treatment was carried out in less than two hours, while antibiotic treatments take days. The authors of this study declared, “the faecal transplant is the most powerful probiotic we can imagine because it introduces healthy microbial flora in a pathologic environment”. They then went on to say that this therapy will most probably be used in the future to treat metabolic disorders such as obesity or the irritable bowel syndrome. |
| This therapy has shown to be so safe and effective that its application in children is already being studied [26]. The study took place at the Helen DeVos Children’s Hospital in Grand Rapids, Michigan. It included 10 patients between 7 and 20 years of age with ulcerative colitis. Each patient received 5 faecal transplants via enema in one week. 78% of the patients had a notable reduction of the symptoms in one week. After the study was concluded 33% of the patients no longer had any symptoms of ulcerative colitis. It must be noted that none of the patients suffered side effects. This is a very interesting treatment for children because ulcerative colitis is a very sever inflammatory disease that affects the bowels [26]. The symptoms include abdominal pain, bloody diarrhoea, fever, rectal pain, weight loss, nauseas, vomiting, joint pain, mouth sores and slow development in children. In fact, children are sometimes forced to stay at home due to this disease. |
| However, larger and longer studies are necessary before the process can be recommended for clinical practice. |
| Nanoparticles |
| Nanoparticles are microscopic particles of less than 100 nm in size, which locates them in an intermediate step between atoms and bulk materials. For this reason, these particles are becoming of high interest in a great range of industries, one of these being their use as antimicrobial agents [27]. It has been observed that this bactericidal activity depends on its size, its concentration and its stability at the temperature at which it is used. |
| Alzam et al. [28] synthesized nanoparticles of three metal oxides, ZnO, CuO and Fe2O3, via sol-gel combustion to test their antimicrobial activity against Gram-positive (Staphylococcus aureus and Bacillus subtilis) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria. The study concluded that ZnO was the most active nanoparticle, which happened to be the smallest (18 nm), while the least active was Fe2O3, being the largest in size (28 nm). In view of these results, other investigation groups have focused their attention in improving the synthesis of ZnO nanoparticles in order to optimize its activity. This is the case of Díez-Pascual et al. [29] who have developed a nanocompound of ZnO with a terminal hydroxide which they add to a poli (ether ether ketone) (PEEK) base, achieving higher antibacterial activity against E. coli and S. aureus than the ZnO particles without modification. |
| Another nanoparticle that is currently being studied for its antimicrobial activity is the silver nanoparticle [30]. Being an innovative therapy, there are very few studies focused on describing the mechanism of action of these nanoparticles. However, several mechanisms have been already proposed. Most of the existing studies have based their hypothesis in morphological and structural changes that have been observed after treating the bacteria with silver compounds. It has not yet been determined whether silver has one or several targets but it is believed that, due to its high reactivity with sulfate compounds, it reacts with enzymes containing this element that are found in the bacterial membrane. Consequently, the membrane loses permeability making it impossible for the bacteria to carry out respiration [31]. This collapses electron flow through the membrane, which obstructs ATP production and, therefore, causes bacterial death [32,33]. |
| Regardless of the little information available on its mechanism of action, many scientific publications have proven the efficiency of silver compounds against resistant strains of bacteria. In fact, these compounds are so promising that they are being introduced in textiles, plastic, synthetic paint, catheters, synthetic implants and many other products in order to provide them with antimicrobial properties. In Mexico, Mycronide has been producing and selling colloidal silver used for water purification and fruit and vegetable disinfection [34]. Samsung has also used silver nanoparticles, in this case, in their latest models of washing machines and dishwashers. Other examples of chemical companies that have started producing products with silver nanoparticles are Dow Chemical, BASF and Procter and Gamble [35]. |
| In conclusion, nanoparticles are antibacterial agents that can be effectively used against antibiotic resistant bacteria. Their mechanism of action is based in their ability to interact with proteins present in the microorganisms in order to inhibit a process or essential activity for the bacteria to be able to carry out the infection. |
| Bacteriophages |
| Bacteriophages, virus that infect bacteria, were discovered in the early 20th century by Frederick C. Twort and Félix d’Hérelle. Immediately after their discovery they were used to treat infections in humans [36] but, with the appearance of the first antibiotics, they fell into disuse. Currently, with the increasing problem of multi-resistant bacteria, they are being studied again as a possible therapeutic alternative [37]. |
| Being a treatment with a long history of studies and clinical trials, the advantages and disadvantages of this therapy have been widely described (Table 2). As advantages in favor over antibiotics, it has been observed that phages have the ability to evolve simultaneously with their corresponding bacteria which means that, in the case that the bacteria develops resistance towards the phage being used, it would be relatively simple to obtain a new phage compared to all the implications of developing a new antibiotic. Another advantage of phages is that they present a higher penetration capacity in the infected area seeing as they are not dependent on concentration and do not suffer metabolism and elimination processes. Another important factor in favor of bacteriophage therapy is that, with the information available at the moment, phages appear to produce less adverse effects than antibiotics, especially those related to allergies and gastrointestinal problems. |
| Like any innovative therapy, there are also a series of disadvantages derived from its application. The first and most important is that, due to its high specificity, each strain within a species requires the preparation of its own bacteriophage, which makes these therapies very restrictive. Furthermore, cases of immunogenicity have been observed due to the generation of antiphage antibodies by the immune system. There have also been cases in which the bacteria develop phage resistance [38], but the promptness with which a new effective phage can be obtained is such that this problem becomes irrelevant. |
| In some cases such as the one of Pseudomonas aeruginosa, the treatment with bacteriophages has been used for more than 50 years. In the early 90’s, positive results were obtained in a dog with otitis and in a human with an infected burn [39]. |
| Recent studies, such as that of Jun et al. [40], continue proving the therapeutic potential of bacteriophages to become antibiotic substitutes. In this case, a multi-resistant Vibrio parahaemolyticus strain was inoculated in mice to test a phage (pVp-1) that had proved to be capable of infecting several strains of this species. After monitoring, it was noted that the mice treated with bacteriophages showed protection against V. parahaemolyticus and even survived the inoculation of lethal doses of bacteria both, using intraperitoneal and oral route. |
| Antimicrobial peptides |
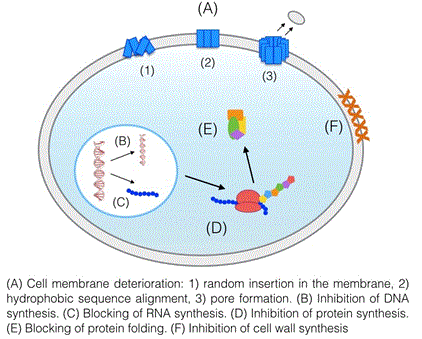
| Antimicrobial peptides are an abundant and varied group of molecules that can be found in nature or synthesized in the laboratory. Up to now, a large number of mechanisms for these peptides have been described (Figure 2). Most peptides appear to attack the cell membrane directly, damaging it and forming pores which result in an outward flow of molecules essential for the bacteria’s survival. In some cases, the interaction with the membrane only takes place in order for the peptides to penetrate inside the cell, where they have secondary mechanisms whose purpose is to inhibit the biosynthesis of the cell wall or the biosynthesis of DNA, RNA and proteins [41]. |
| Following, we will describe alpha-helical cationic peptides and phosphorodiamidate morpholino oligomers (PPMOs). |
| Alpha-helical cationic peptides |
| Alpha-helical cationic peptides are produced by practically all living organisms, being a part of their unspecific immediate defence against infections [42]. Its mechanism of action consist in the destabilization of the cellular membrane to form pores and open channels through with an outward flow of vital molecules for the bacteria’s survival is established [43]. This activity is due, mainly, to their amphipathic character, their cationic charge and their size. |
| Notwithstanding the fact that this alternative is in the early stages of its development, its advantages and disadvantages have already been described [44] (Table 2). In their favor, one must note their broad therapeutic spectrum, their fast antibacterial activity and the low probability of having bacteria develop resistances against this treatment. As for its disadvantages, the most relevant are its high cost, its low stability and the possible cytotoxicity that may take place in the host cells. |
| Antimicrobial peptides are one of the newest therapies which mean that they are still in development stages and there are no clinical trials available. In a 2013 study [45], a cationic peptide (Apep10) was isolated using chromatography with the aim of testing its antibacterial properties. After the pertinent studies it was observed that Apep10 inhibited growth of bacteria such as Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pneumoniae among others, with minimum inhibitory concentrations ranging between 8 and 64 µg. Another investigation group [46] focused on peptides containing tryptophan residues seeing as this amino acid is known for presenting a strong activity in modifying cell membranes. The activity of peptides modified with tryptophan was compared to that of its unmodified counterparts, having observed that the first presented a higher “in vitro” antibacterial activity. |
| Phosphorodiamidate morpholino oligomer |
| PPMOs are structural analogues of DNA that inhibit translation due to an antisense mechanism [47]. |
| Researchers in the University of Oregon in USA have conducted a study [48] to evaluate the antimicrobial activity of a series of PPMOs against Acinetobacter both, “in vitro” and “in vivo” using rodents. The results obtained were positive seeing as PPMOs proved to be bactericidal and showed minimum inhibitory concentrations within a clinically relevant range. |
| In an effort to optimize these new treatments, two studies [49,50] were conducted based on the idea that, if PPMOs are combined with the cationic peptides described previously, the penetration of the antimicrobial agent through the membranes of Gram-negative bacteria would be facilitated, and, therefore, there would be an increase in its activity. In both cases, conjugated PPMOs and non-conjugated PPMOs were tested against E. coli and both studies concluded that the antimicrobial activity increased when using conjugated PPMOs. |
| Medicinal plants |
| Practically every culture in the world has a tradition of natural medicine that includes a great variety of plant species. Some of this stand out for their antimicrobial activities, as it is the case of Bergenia ciliata or Sesamum indicum. For this reason, seeing the ongoing increase of resistances against antibiotics, there is a starting tendency to study these traditional medicines with the hope of developing new effective therapies. Table 2 describes the pros and cons of using medicinal plants as antimicrobial agents. |
| In this line of work, Awan et al. [51] studied a group of species typical of the Pakistani culture with a high content in tannins and phenolic compounds seeing as it is believed that they are responsible for the antimicrobial activity of their extracts. Extracts of several species were tested against a group of bacteria known for their high rate of resistances, such as Staphylococcus aureus, Pseudomonas aeruginosa and Serratia marcescens, among others. After performing antibiograms using the disk diffusion method, it was observed that the range of activity shown by the extracts was comparable to the reference antibiotics, using as a parameter the area of inhibition. |
| Skeup et al. [52] conducted a similar study but this time they focused on 7 plant species found in the Cameroonian diet and they tested their extracts against a selection of multi-resistant Gram-negative bacteria. Of the 7 species studied, the extract of Sesamum indicum proved to be the most active, being effective against 77.77% of the microorganisms tested. |
| A third group [53] studied the bactericidal activity of the methanol extracts of 21 timber-yielding plants from India against 9 species of uropathogenic multi-resistant bacteria. Among all the plant species studied, the extracts that showed higher antibacterial activity, and, therefore, higher therapeutic potential, were those of Cassia tora and Anogeissus acuminata. |
| In an article published in the European Journal of Microbiology and Immunology [54], the antimicrobial activity of the fluid extracts of Bergenia ciliata, Jasminium officinale and Santalum album against the 5 species that responsible for most nosocomial infections (Staphylococcus aureus, Bacillus subtilis, Proteus vulgaris, Pseudomonas aeruginosay Escherichia coli) was evaluated. The cold aqueous extract of B. ciliata showed a high activity against B. subtilis, having obtained an area of inhibition comparable to that of erythromycin. As for J. officinale and S. album, their aqueous extracts showed variable activity which did not allow the drawing of proper conclusions about its therapeutic use. |
| Conclusion |
| As it has been proven in this review, there is a great variety of therapeutic alternatives being developed to fight the increasing number of multi-resistant bacteria. These therapies go from the use of organisms such as probiotics and bacteriophages, to the synthesis of nanoparticles and peptides that could become the synthetic antibiotics of the future. Furthermore, medicinal plants should also be considered seeing as they have a high concentration of active substances, which could be an interesting source of new compounds with antibacterial activity. Some of these treatments, such as bacteriophages and synthetic peptides are still in the early stages of development so further studies are required in order to assess the feasibility of applying these treatments in clinical practice. Other alternatives such as probiotics and medicinal plants have already been extensively used to treat infections in humans with positive results, having displayed a high activity and practically no side effects at a relatively low cost. Finally, nanoparticles are in an intermediate stage of development since they are already being used as disinfecting agents at an industrial level (silver nanoparticles) but more studies are required when it comes to its application to treat bacterial infections in humans. What is clear is that, due to the exponentially increasing problem of resistant bacteria happening in developing countries, it is imperative to encourage and continue the development of therapeutic alternatives to fight these multi-resistant microorganisms. |
| Acknowledgement |
| Work cited in this review from the author´s laboratory was supported in part by grants from the Universidad CEU Cardenal Herrera (PRUCH 25/10, PRUCH 39/11 and Santander-PRUCH 19/12). |
References
- Dever LA, Dermody TS (1991) Mechanisms of bacterial resistance to antibiotics. Arch Intern Med 151: 886-895.
- Finland M, Kirby WM, Chabbert YA, Chain EB, Dowling HF, et al. (1965) Round table: are new antibiotics needed? Antimicrob Agents Chemother (Bethesda) 5: 1107-1114.
- Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10: S122-129.
- Martínez-Martínez L, Calvo J (2010) [Development of resistances to antibiotic drugs: causes, consequences and importance to the public health system]. EnfermInfeccMicrobiolClin 28 Suppl 4: 4-9.
- World Health Organization. Antimicrobial resistance: global report on surveillance 2014.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United Sates, 2013.
- Livermore DM (2012) Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med 27: 128-142.
- Park SH, Choi SM, Lee DG, Kim J, Choi JH, et al. (2011) Emergence of extended-spectrum β-lactamase-producing escherichia coli as a cause of community-onset bacteremia in South Korea: risk factors and clinical outcomes. Microb Drug Resist 17: 537-544.
- Pérez-Gracia MT, Suay B, Boix I, Ferrandis S, Galán F, et al. (2014) Detección de bacteriasproductoras de ß-lactamasas y sucaracterización molecular en animalesdomésticos. Proceedings of the XVIII Congreso de la Sociedad Española de EnfermedadesInfecciosas y MicrobiologíaClínica.
- Calbo E, Romaní V, Xercavins M, Gómez L, Vidal CG, et al. (2006) Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J AntimicrobChemother 57: 780-783.
- Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I et al. (2014) Carbapenemase-producing Klebsiellapneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 58: 2322-2328.
- Dortet L, Boulanger A, Poirel L, Nordmann P (2014) Bloodstream infections caused by Pseudomonas spp.: how to detect carbapenemase producers directly from blood cultures. J ClinMicrobiol 52: 1269-1273.
- Poulou A, Voulgari E, Vrioni G, Koumaki V, Xidopoulos G, et al. (2013) Outbreak caused by an ertapenem-resistant, CTX-M-15-producing Klebsiellapneumoniae sequence type 101 clone carrying an OmpK36 porin variant. J ClinMicrobiol 51: 3176-3182.
- Diekema DJ, Richter SS, Heilmann KP, Dohrn CL et al. (2014) Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from nationwide surveillance study. Infect Control HospEpidemiol 35: 285-292.
- Fishman N (2006) Antimicrobial stewardship. Am J Med 119(6 Suppl 1), 62-70.
- Ohl CA, Luther VP (2011) Antimicrobial stewardship for inpatient facilities. J Hosp Med 6 Suppl 1: S4-15.
- MacDougall C, Polk RE (2005) Antimicrobial stewardship programs in health care systems. ClinMicrobiol Rev 18: 638-656.
- Hecker MT, Fox CJ2, Son AH3, Cydulka RK4, Siff JE4, et al. (2014) Effect of a stewardship intervention on adherence to uncomplicated cystitis and pyelonephritis guidelines in an emergency department setting. PLoS One 9: e87899.
- National Center for Complementary and Alternative Medicine. Oral Probiotics: An Introduction [online]. Rockville Pike M.D. [ref. Februrary 24th, 2014].
- Szajewska H, Urbańska M1, Chmielewska A1, Weizman Z2, Shamir R3 (2014) Meta-analysis: Lactobacillus reuteri strain DSM 17938 (and the original strain ATCC 55730) for treating acute gastroenteritis in children. Benef Microbes .
- Alexandre Y, Le Blay G2, Boisramé-Gastrin S1, Le Gall F3, Héry-Arnaud G3, et al. (2014) Probiotics: a new way to fight bacterial pulmonary infections? Med Mal Infect 44: 9-17.
- Grehan MJ, Borody TJ, Leis SM, Campbell J, Mitchell H, et al. (2010) Durable alteration of the colonic microbiota by the administration of donor fecal flora. J ClinGastroenterol 44: 551-561.
- Patel NC, Griesbach CL, DiBaise JK, Orenstein R (2013) Fecalmicrobiota transplant for recurrent Clostridium difficile infection: Mayo Clinic in Arizona experience. Mayo ClinProc 88: 799-805.
- Kelly CR, Kunde SS2, Khoruts A3 (2014) Guidance on preparing an investigational new drug application for fecalmicrobiota transplantation studies. ClinGastroenterolHepatol 12: 283-288.
- van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, et al. (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368: 407-415.
- Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, et al. (2013) Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J PediatrGastroenterolNutr 56: 597-601.
- ScienceDaily. Nanoparticle [online]. [ref. February 24th, 2014].
- Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, et al. (2012) Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine 7: 6003-6009.
- Díez-Pascual AM, Díez-Vicente AL (2014) Development of nanocomposites reinforced with carboxylated poly(ether ether ketone) grafted to zinc oxide with superior antibacterial properties. ACS Appl Mater Interfaces 6: 3729-3741.
- Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. BiotechnolAdv 27: 76-83.
- Holt KB, Bard AJ (2005) Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry 44: 13214-13223.
- Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, et al. (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52: 662-668.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, et al. (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16: 2346-2353.
- Kumar A, Vemula PK, Ajayan PM, John G (2008) Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat Mater 7: 236-241.
- Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27: 341-353.
- Encyclopedia Britannica. Bacteriophage [en línea]. Edinburgh, Scottland. [ref. de 26 de febrero 2014]. Disponible en
- Debarbieux L, Saussereau E, Maura D (2013) [Phagotherapy: a nightmare for bacteria, a dream for physicians?]. BiolAujourdhui 207: 181-190.
- Summers WC (2001) Bacteriophage therapy. Annu Rev Microbiol 55: 437-451.
- Soothill J (2013) Use of bacteriophages in the treatment of Pseudomonas aeruginosa infections. Expert Rev Anti Infect Ther 11: 909-915.
- Jun JW1, Shin TH, Kim JH, Shin SP, Han JE, et al. (2014) Bacteriophage Therapy of a Vibrio parahaemolyticus Infection Caused by a Multiple-Antibiotic-Resistant O3:K6 Pandemic Clinical Strain. J Infect Dis .
- Peters BM, ShirtliffME, Jabra-Rizk MA (2010) Antimicrobial peptides: primeval molecules or future drugs? PLoSPathog 6: e1001067.
- Hancock RE, Lehrer R (1998) Cationic peptides: a new source of antibiotics. Trends Biotechnol 16: 82-88.
- Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3: 238-250.
- Marr AK, Gooderham WJ, Hancock RE (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. CurrOpinPharmacol 6: 468-472.
- Tang W, Zhang H, Wang L, Qian H (2014) New cationic antimicrobial peptide screened from boiled-dried anchovies by immobilized bacterial membrane liposome chromatography. J Agric Food Chem 62: 1564-1571.
- Bi X, Wang C, Dong W, Zhu W, Shang D (2014) Antimicrobial properties and interaction of two Trp-substituted cationic antimicrobial peptides with a lipid bilayer. J Antibiot (Tokyo) 67: 361-368.
- Geller BL, Deere J, Tilley L, Iversen PL (2005) Antisense phosphorodiamidatemorpholino oligomer inhibits viability of Escherichia coli in pure culture and in mouse peritonitis. J AntimicrobChemother 55: 983-988.
- Geller BL, Marshall-Batty K, Schnell FJ, McKnight MM, Iversen PL, et al. (2013) Gene-silencing antisense oligomers inhibit acinetobacter growth in vitro and in vivo. J Infect Dis 208: 1553-1560.
- Mellbye BL, Weller DD, Hassinger JN, Reeves MD et al. (2010) Cationic phosphorodiamidatemorpholino oligomers efficiently prevent growth of Escherichia coli in vitro an in vivo. J AntimicrobChemother 65: 98-106.
- Tilley LD, Mellbye BL, Puckett SE, Iversen PL, Geller BL (2007) Antisense peptide-phosphorodiamidatemorpholino oligomer conjugate: dose-response in mice infected with Escherichia coli. J AntimicrobChemother 59: 66-73.
- Awan UA, Andleeb S, Kiyani A, Zafar A, Shafique I, et al. (2013) Antibacterial screening of traditional herbal plants and standard antibiotics against some human bacterial pathogens. Pak J Pharm Sci 26: 1109-1116.
- Seukep JA, Fankam AG, Djeussi DE, Voukeng IK, Tankeo SB, et al. (2013) Antibacterial activities of the methanol extracts of seven Cameroonian dietary plants against bacteria expressing MDR phenotypes. Springerplus 2: 363.
- Mishra MP, Padhy RN (2013) In Vitro antibacterial efficacy of 21 Indian timber-yielding plants against multidrug-resistant bacteria causing urinary tract infection. Osong Public Health Res Perspect 4: 347-357.
- Khan UA, Rahman H, Niaz Z, Qasim M, Khan J, et al. (2013) Antibacterial activity of some medicinal plants against selected human path ogenic bacteria. Eur J MicrobiolImmunol (Bp) 3: 272-274.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 15790
- [From(publication date):
August-2014 - Apr 18, 2025] - Breakdown by view type
- HTML page views : 11166
- PDF downloads : 4624
