The Anesthetic Management of a Patient on VA ECMO Undergoing a Hemicolectomy
Received: 17-Oct-2016 / Accepted Date: 04-Nov-2016 / Published Date: 14-Nov-2016
Abstract
Veno-Arterial extracorporeal mechanical oxygenation (VA ECMO) provides temporary mechanical support to the patient with cardiac and/or respiratory failure in cases of cardiogenic shock or heart failure. VA ECMO requires systemic anticoagulation to prevent thrombosis in the oxygenator and circuit. We describe the unique case of a previously healthy young woman who suffered an acute coronary dissection and subsequent cardiogenic shock. Emergent placement on VA ECMO and systemic anticoagulation unmasked a bleeding colonic cancer which required urgent resection. Unfortunately, the ultimate prognosis in ECMO patients requiring noncardiac surgery is poor and there is a high risk of perioperative complications and even death.
Keywords: Heart failure; Anticoagulation; Isoflurane; Fibrinogen; Heparin; Anesthetics
78727Veno-Arterial extracorporeal (VA ECMO) provides temporary mechanical support to the patient with cardiac and/or respiratory failure in cases of cardiogenic shock or heart failure [1-3]. VA ECMO essentially decreases cardiac work and reduces oxygen consumption while providing sufficient organ perfusion. ECMO is a bridge to recovery, left ventricular assist device (LVAD) implantation or eventual cardiac transplantation. ECMO requires continuous systemic heparin anticoagulation otherwise thrombosis can occur in the oxygenator and circuit leading to devastating complications including embolism and death.
We present the case of a previously healthy 44 year old patient who suffered a ST elevation myocardial infarction (STEMI) complicated by left main coronary artery (LMCA) dissection and cardiogenic shock. This dissection was unique because it was not the result of iatrogenic injury. Spontaneous coronary artery dissection (SCAD) is an infrequent cause of acute coronary syndrome that is accompanied by a high morbidity and mortality rate, and can require emergent placement on VA ECMO [4]. While on systemic anticoagulation, the patient was found to have a bleeding colonic adenocarcinoma which required urgent resection. An anesthetic plan was formulated which would maintain hemodynamic stability, while balancing large fluid shifts in the perioperative period. Successful anesthetic management of this complex patient facilitated an uneventful intraoperative course.
Case Description
A 44 year-old female with no significant past medical history or family history presented with substernal chest pain and tachycardia with heart rate of 110-120 beats per minute. An electrocardiogram (EKG) showed ST elevations in leads I, aVL, V2-V5. The patient was diagnosed with an acute STEMI and underwent a cardiac catheterization at an outside hospital. During the procedure, there was difficulty advancing wire past the left anterior descending (LAD) lesion. However, the blockage was eventually able to be ballooned and a drug-eluting stent (DES) was placed. The patient experienced decreased blood pressure (SBP 60’s millimeters Hg), tachycardia (110 beats per minute), and decline in oxygen saturation to 80%. She became unresponsive and was intubated. A significant dissection occurred to the ostial circumflex while intervention on the LAD was performed. A repeat angiography showed LMCA/ left circumflex artery dissection. The patient subsequently went into cardiogenic shock with systemic blood pressures in the 40’s mmHg and became unresponsive. Advanced cardiac life support (ACLS) was initiated. She was intubated, and received intravenous epinephrine with an improved mental status. Given her low blood pressures, an intra-aortic balloon pump (IABP) was placed and a dopamine infusion was started.
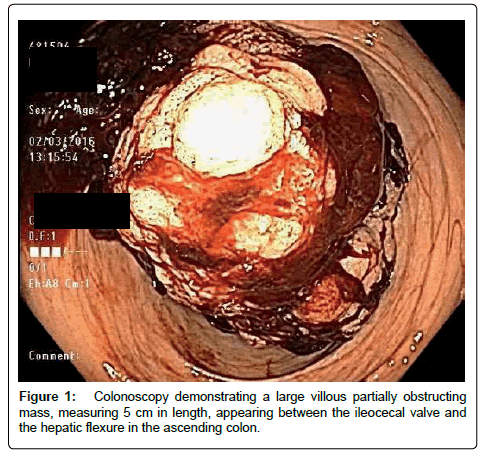
She was then emergently transferred to University hospital and placed on VA ECMO. A bedside echo was significant for left ventricular dilatation and dysfunction. Her ejection fraction was noted to be 15%, with anterolateral wall akinesis. There was no ventricular septal defect and no valvular abnormalities. For her continued hypotension, she was placed on a levophed infusion. Her lactate level was elevated to 11 millimoles per liter. The patient suffered significant blood loss from her cardiac catheterization and bilateral groin catheter sites, and was transfused for a hemoglobin of 7.1 grams per deciliter. She then developed loss of motor function ad loss of temperature sensation in her lower extremities bilaterally. A computed tomography angiogram (CTA) of her abdomen was obtained to rule out a spinal cord infarction, but incidentally showed a portion of intussusception of the ascending colon involving the hepatic flexure and proximal transverse colon. A colonoscopy demonstrated a partially obstructing bleeding mass in the ascending colon (Figure 1). Due to the increased risk for bleeding during the hemicolectomy, her systemic anticoagulation was discontinued.
The patient was scheduled for an open right hemicolectomy. On arrival to the operating room, she was receiving 0.02 mcg/kg/ min norepinephrine, 0.03 mcg/kg/min epinephrine, and 40 mcg/ kg/min propofol to maintain blood pressure of 106/70 mmHg. Her temperature was 35.6 degrees Celsius. Her access consisted of a multilumen access catheter (9 French MAC) which was placed in the left internal jugular vein. Activated Clotting Time (ACT) was 157 seconds at the beginning of the case. She was already intubated and her general anesthesia proceeded with 3 mg midazolam and 100 mg rocuronium for muscle relaxation. Maintenance of anesthesia was achieved with isoflurane and propofol infusions with re-dosing of rocuronium and boluses of fentanyl as needed. No additional pressors were started during the case. Central venous pressure and and pulmonary artery pressure were measured continuously to monitor volume status. Serial arterial blood gases were obtained to determine fluid status and need for blood transfusion. Intraoperatively, the patient was resuscitated with 1.3 liters of crystalloid, 500 milliliters of colloid (5% albumin) and 1 unit of packed red blood cells to treat a hemoglobin of 7.3 grams per deciliter. Her urine output was 900 milliliters for the case. Blood loss was estimated to be less than 100 milliliters with excellent surgical hemostasis. The patient remained intubated at the end of the case. Her hemoglobin at the end of the case was determined to be 10.6 grams per deciliter. She was taken to the Cardiac Surgical Intensive Care unit intubated and in stable condition.
She was successfully weaned off ECMO on post operative day (POD) 4. Nevertheless, her complicated hospital course necessitated placement of a temporary LVAD on POD 29 due to failure to wean off continuous high dose inotropes and pressors. Two months postoperatively, she suffered a cardiac arrest and expired despite resuscitative attempts.
Discussion
Spontaneous coronary artery dissection (SCAD) is a rare cause of acute coronary syndrome that occurs in young otherwise healthy female patients [4-7]. The underlying cause of SCAD remains uncertain, but some patients report extreme physical exertion or high levels of emotional stress shortly before the occurrence of SCAD [8]. In this case, the cardiologists believed that SCAD was not due to mechanical trauma since the injury occurred in the ostial circumflex, away from the region in which they were working. Usually, in hospital mortality rate is low, but when SCAD occurs in association with percutaneous coronary intervention there is a much higher complication rate [5,9].
There is no specific guideline in the management of SCAD. Current therapies are directed at medical therapy in the form of blood pressure control and anticoagulation, percutaneous coronary intervention to seal the dissection, or coronary artery bypass graft surgery. In patients with acute coronary syndrome, initial treatment should include antiplatelet and anti-ischemic agents, along with anticoagulation with heparin. Cardiogenic shock can be a devastating consequence of an acute coronary artery dissection, which can require ECMO placement for adequate resuscitation. There is also a very high rate of reoccurrence [5].
Since ECMO requires systemic anticoagulation, bleeding can result and lead to catastrophic consequences. However, there is a high mortality rate in patients who require noncardiac surgery while on ECMO [1]. Steps should be taken for patients that require emergency surgery to prevent massive bleeding including the cessation of systemic anticoagulation, transfusion of blood products, use of antifibrinolytics as well as surgical techniques for hemostasis [10]. It is recommended to hold heparin six hours prior to surgery and to restart heparin six hours after surgery, which we did for this patient [3]. However, there are no clear guidelines regarding the perioperative management of anticoagulation in the ECMO patient undergoing non-cardiac surgery [11]. It is important to maintain a platelet level greater than or equal to 100 × 109/L, fibrinogen greater than 150 mg/dL, international normalized ratio (INR) between 1.5 and 2.5, and a hematocrit greater than 30% while on ECMO [12]. However, in the ECMO patient undergoing non-cardiac surgery, it is recommended to transfuse blood products only when necessary due to the negative consequences of blood transfusions [5]. It is far more important to obtain good surgical hemostasis.
Increased risk for bleeding in ECMO patients for noncardiac surgery also leads to increased transfusion requirements. However, in this case, only one unit of packed red blood cells was given. These patients are also more likely to require postoperative mechanical ventilation and also experience an increase in postoperative creatinine [3].
The goal of ECMO is to minimize and eliminate any excess fluid that has accumulated in the extracellular space in the setting of sepsis, inflammation, or cardiac failure. There are concerns regarding balancing fluid requirements of bowel resection and third spacing versus minimizing volume overload in a patient with poor ventricular function. Since hypovolemia can lead to decreased flows on the ECMO machine and decreased organ perfusion, maintaining a euvolemic fluid status is important in the ECMO patient. In this case, the central venous pressure (CVP) and pulmonary artery pressures were used to help guide the patient’s fluid management.
Further anesthetic management of the ECMO patient for noncardiac surgery involves the delivery of medications. Studies have shown that there is significant sequestration of propofol, opioids and benzodiazepines within the polymeric components of the ECMO circuit with >50% reductions in the concentrations of these medications [13]. Thus, the ECMO patient may require a higher amount of anesthetic agent. Due to the pharmacokinetic changes while on ECMO, there is a need to constantly reassess the depth of sedation and analgesia, which can be monitored with the BIS-pectral index monitor. In this case, the patient was adequately anesthetized and there were no changes in her hemodynamic status throughout the case.
With the greater prevalence of heart failure and increasing number of patients placed on ECMO, there have been more of these complex patients that require urgent or emergent noncardiac surgery [14]. It is important to stress anesthetic factors that will optimize patient outcomes. Hypotension is possible during anesthetic induction and needs to be promptly treated by increasing ECMO flows, administering volume, or adding vasopressor agents. Achieving a mean arterial pressure greater than 65 millimeters Hg should be adequate to maintain perfusion pressure. Anesthetic maintenance is achieved with total intravenous agents, including sedatives, hypnotics, analgesics, and muscle relaxants. Central venous pressure (CVP) monitoring can be used as a trend monitor since ECMO flow rates can affect exact measurements. In addition, information gained from the pulmonary artery catheter is alson not reliable since there is minimal blood flow through the lungs while on ECMO. Newer flow-based hemodynamic monitoring devices may be more beneficial and are currently being investigated in the ECMO patient.
Conclusion
With the increasing number of patients requiring ECMO, more patients on ECMO will require noncardiac surgery. Managing a patient on ECMO requiring noncardiac surgery can be a difficult task. This patient population is usually acutely ill, with an overall dismal prognosis. Nevertheless, noncardiac surgery can be accomplished successfully if there is a better understanding of the effects the ECMO circuit has on the physiology of the body and pharmacology of the anesthetics.
References
- Chestovich PJ, Kwon MH, Cryer HG, Areti T, Jonathan HR (2011) Surgical procedures for patients receiving mechanical cardiac support. American Surg 77: 1314-1317.
- Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, et al. (2009) Extracorporeal membrane oxygenation for treating severe cardiac and respiratory disease in adults: Part I-overview of extracorporeal membrane oxygenation. J Cardiothor Vasc Anesth 23: 886-892.
- Taghavi S, Beyer C, Vora H, Senthil JN, Jay D, et al. (2014) Noncardiac surgery in patients on mechanical circulatory support. ASAIO Journal 60: 670-674.
- Tanis W, Stella PR, Pijlman AH, Kirkels JH, Peters RHJ, et al. (2008) Spontaneous coronary artery dissection: current insights and therapy Netherlands heart journa 16: 344-349.
- Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, et al. (2012) Clinical features, management and prognosis of spontaneous coronary artery dissection. Circulation 126: 579-588.
- Adlam D, Cuculi F, Lim C, Banning A (2010) Management of spontaneous coronary artery dissection in the primary percutaneous coronary intervention era. J Invasive Card 22: 549-553.
- Shamloo BK, Chintala RS, Nasur A (2010) Spontaneous coronary artery dissection: aggressive vs. conservative therapy. J Invasive Card 22: 222-228.
- Tweet M, Gulati R, Aase L, Hayes S (2011) Spontaneous coronary artery dissection: a disease-specific, social networking community-initiated study. May Clinic Proc 86: 845-850.
- Mortensen KH, Thuesen L, Kristensen IB (2009) Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv 74: 710-717.
- Murphy D, Hockings LE, Andrews RK, Aubron C, Gardiner EE, et al. (2015) Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 29: 90-101.
- Taghavi S, Jayaraian SN, Mangi AA, Kathryn H, Elizabeth D, et al. (2015) Examining Noncardiac Surgical Procedures in Patients on Extracorporeal Membrane Oxygenation. ASAIO Journal 61: 520-525.
- Oliver WC (2009) Anticoagulation and coagulation management for ECMO. Seminars in  Cardiothoracic Vascular  Anesthesia 13: 154-175.
- Chauhan A, Subin S (2011) Extracorporeal membrane oxygenation, an anesthesiologist's perspective: physiology and principles. Part 1 Ann Card anaesth 14: 218-229.
- Roberts SM, Hovord DG, Kodavatiganti R, Sathishkumar S (2015) Ventricular assist devices and non-cardiac surgery. BMC Anesthesiol 15: 185.
Citation: Jeanne KY, Bernstein WK (2016) The Anesthetic Management of a Patient on VA ECMO Undergoing a Hemicolectomy. Cardiovasc Ther 1: 114.
Copyright: © 2016 Jeanne KY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4669
- [From(publication date): 0-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 3761
- PDF downloads: 908