Tetracyclines as Masterful Regulators of Mitochondrial Function in Viral and Infectious Disease States
Received: 20-Dec-2022 / Manuscript No. JIDT-23-85376 / Editor assigned: 23-Dec-2022 / PreQC No. JIDT-23-85376 (PQ) / Reviewed: 06-Jan-2023 / QC No. JIDT-23-85376 / Revised: 13-Jan-2023 / Published Date: 20-Jan-2023 DOI: 10.4172/2332-0877.1000530
Abstract
Of all the antibiotic classes used in medicine, the Tetracycline’s (Tcs) stand out as extraordinary compounds and as bacteriostatic agents inhibiting protein synthesis in bacteria, the genesis of their success as medicinal and commercially valuable life-saving drugs. But they also possess unique anti-inflammatory activity in humans, where their activity is multifold having a long and rich history of affecting such pathways. Historically, the 1st generation fermentation Tcs affected the growth of α-proteobacteria rickettsias, known to cause infectious diseases such as spotted fevers and typhus, where chlortetracycline 1 was so effective against rickettsial disease it was approved for clinical use in 1947 even before the chemical structure was solved in the early 1950s.
Keywords: Infectious Diseases; Tetracycline’s; Bacteriostatic agents; Inflammation
Introduction
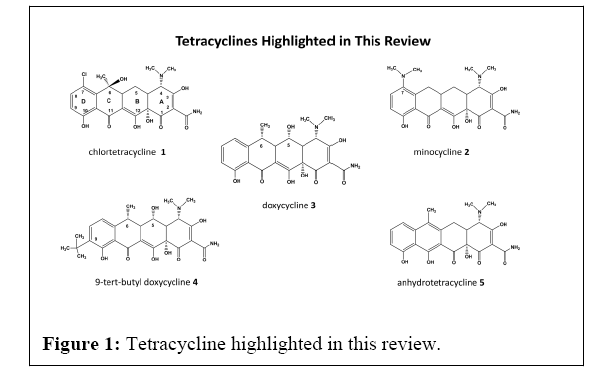
Sequential generations of tetracycline’s soon emerged, with improved antibacterial activity, pharmacokinetics, and safety, generating the semi-synthetic 2nd generation Tcs Minocycline 2 (Mino) and Doxycycline 3 (Doxy), both more potent, stable, and bioactive across a broad spectrum of bacterial strains (Figure 1).
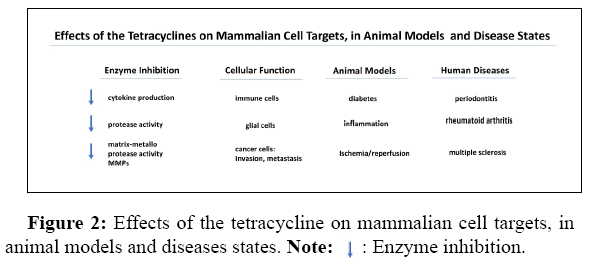
At the same time reports appeared that the Tcs also had bioactivity in mammals, modifying bone growth, metabolism dynamics, and chemotaxis in immune cells, but these properties were largely ignored until 1983, when Golub described the inhibitory activity of Mino and Doxy against mammalian collagenase. This was the start of a consolidated scientific front highlighting the activity of the Tcs, where reports of the bioactivity of the Tcs keep expanding and evolving to this day. While many non-antimicrobial and mammalian activities have been reported they include the following activities but not limited to them [1,2] (Figure 2).
The study of Tcs in humans continues in the clinic, as www.clinicaltrials.gov shows Mino and Doxy have 292 and 422 clinical studies currently registered, with only about 10% or less of these studies looking at their antibacterial activity. The majority are directed at human diseases related to inflammation, neuroinflammation and neurodegeneration, and diseases of the cardiovascular system, unrelated to infections. In comparison, comparison with other antibiotic classes, such as the -lactams, cephalosporins, or azithromycin, which have 215, 259, and 682 trials on-going, respectively, and directed to their uses primarily as antibiotics. This signals that the tetracycline’s possess unique activities as anti-inflammatories and potential use human disease therapeutics as Mino and Doxy are readily available to repurpose in human diseases.
We started synthesizing new tetracycline derivatives in 1987, working with Stuart B. Levy, MD, at Tufts University School of Medicine, and by 1996 we formed Paratek Pharmaceuticals, in Boston, MA, to synthesize new Tcs against resistant common bacterial ESKAPE pathogens. At Paratek, we synthesized 1000’s of novel derivatives and studied them in bacterial, mammalian cell and animal models, mainly, successfully bringing our inventions to the clinic with the FDA approval of NuzyraTM and SeysaraTM in 2018 [3,4].
We also followed the non-antibiotic uses of the Tcs, and launched projects to understand the Structure-Activity-Relationships (SAR) of the compounds we generated against mammalian disease states, their effect on parasites such as malaria, then developed in vitro and in vivo assays studying multiple sclerosis, arthritis, stroke and spinal muscular atrophy, among the many reported uses of the Tcs. We found many of our compounds were active across the array of tests, and that certain structural changes decreased antibacterial activity, and were extremely potent in inflammation-based pathologies in vivo. We also knew the Tcs were dynamic and pleiotropic, possessing antioxidant, metal chelation as well as RNA binding characteristics, but effects on mitochondria were not considered, a detriment to our understanding.
Concurrently, another scientific front was developing on the activity of the Tcs, where Houtkooper and Auwerx were studying longevity in Caenorhabditis elegans worms, simple nematodes with wellcharacterized biology and anatomy and used in drug discovery [5]. They found Doxy, which was being used extensively in the Tet-On- Off expression system, also induced the Mitochondrial Unfolded Protein Response (mtUPR) in mitochondria, clearing dysfunctional proteins, a major cause cellular stress. They also found that Tcs reduced oxygen consumption and attenuated respiration which suggested that the mitonuclear protein imbalance is a common mechanism linking mitochondrial function to cell fitness, and subsequently lifespan regulation. Doxy resulted in worm longevity, mitochondrial fitness, and the process of mitohormesis a positive fitness response to mitochondrial stress.
Literature Review
Since In 2018, the Auwerx laboratory at the Swiss Federal Institute of Technology, Lausanne, (EPFL), experts in mitochondrial dynamics and physiology, and ours began a collaboration, where over 50 structurally different tetracycline’s representing changes at all the positions along the ring system, were screened for mtUPR activation activity using the C. elegans hsp-6::gfp reporter strain, a measure of mtUPR mitohormesis and mitochondrial signalling. The results of this collaboration serves as the basis for our current manuscript reviewed here “Tetracycline-induced mitohormesis mediates disease tolerance against influenza” [6,7].
The mtUPR assay in the Auwerx lab revealed the initial SAR of mtUPR activation by the structurally varied tetracyclines, where the initial SAR shows that Tcs harbor significant activity in activating the mtUPR, particularly at the C4 to C9 positions, and that nonantibacterial agents were more potent in this regard. In mitochondria, Dox showed induction of the ATF-4 Integrated Stress Response (ISR), a stress factor and it also induced the type I IFN response, as confirmed by the increased expression of inflammasome markers CGAS, CXCL10 and TANK-Binding Kinase 1 (TBK1) [6,7]. The ISR and related Mitochondrial Stress Response (MSR) pathways exist in the immune response to pathogens, sensing bacterial and viral DNAs, causing TBK1 signalling, and increasing the secretion of type I IFNs, IFNα, and IFNβ, a form of chemical stimulation of the mitochondria, causing fitness and mitohormetic response during infectious disease progression [8].
Discussion
From these studies, representing clinically relevant and C2-C10 position modified compounds we found numerous potent Tcs, while two compounds, a C9 derivative of Doxy, 9-Tert-Butyl doxycycline 4 (9-TB), and Anhydrotetracycline (Anh) 5, showed the most activity in the mtUPR assay. Both compounds have little or no antimicrobial activities against ESKAPE pathogens, were more efficacious, with 10- fold activity compared to Doxy in activating the mtUPR response. Other derivatives also activated the mtUPR, while the clinical antibiotics Nuzyra™ , Tygacil™, Mino, or Tcs based on the minocycline scaffold, were inactive as mtUPR activators and downstream mitohormesis.
The pharmacology of 9-TB and Anh were then characterized in a Human Embryonic Kidney (HEK) 293T cell line, showing that they affected the MSR mitochondrial versus nuclear coded OXPHOS protein expression, and both reduced basal mitochondrial oxygen consumption rates. Additionally, both compounds were superior in inducing the ISR, MSR, and increased subsequent IFN β secretion. These compounds were more potent than Doxy in triggering the MSR, ISR and type I IFN responses in C. elegans, mouse marrow derived macrophages, HEK cells, in a dose-dependent and controllable way.
Resistance to viruses by innate immunity is mediated by mtDNA instability and the MSR, during the immune response against Influenza A (IFV). We then the IFV infection model to determine if the Tc-induced MSR enable mice to survive a viral infection. Using BLAB/c N mice, we found 9-TB increased their survival rate in an infectious challenge model, and in a higher viral titer model of therapeutic intervention, where the survival rates were 50% and 20%, respectively. Mice infected with even higher viral loads showed that both 9-TB and Doxy delayed mortality and health decline, while the Tet-induced MSR did not cause adverse effects itself, and resulted in lower levels of Interleukin 6, a pro-inflammatory cytokine and an antiinflammatory myokine. The compounds were acting to increase the survival of mice to IFV infection, and doing so regardless of their viral load. The 20% survival upon the dosing of low-doses of 9-TB (0.25 and 0.05 mpkd) show that such modified Tcs can increase tolerance to IFV in a clinical setting.
We also studied the effect of the compounds on the micro biome, collecting faecal samples from Doxy and 9-TB treated animals and using DNA and metagenomic analysis, and while Doxy changed the gut microflora significantly, decreasing strain diversity, 9-TB was devoid of such changes and resembled that of control mice not dosed with any compounds. This suggests 9-TB does not affect mice gut microbiota in vivo and corroborates with our minimum inhibitory concentration values generated in vitro with ESKAPE pathogens.
The mechanisms governing the Tc activity was studied by transciptome analysis of the lung, liver and kidney, all targets of failure syndromes after infection by respiratory viruses such as IFV and SARS-CoV2. Gene-set enrichment analysis showed that 9-TB down-regulated multiple inflammatory and “immune response” related terms in lung tissue, along with “t-cell” and “b-cell” activation. Similar gene set analysis revealed that terms related to inflammatory, immune, and apoptosis responses were enriched when induced by IFV, and significantly reduced by 9-TB (see the Revigo representation). Surprisingly, genes set expression related to lung development, cell function and cilia structure were decreased by IFV, and their expression increased by 9-TB, showing that inflammation and cell loss of lung epithelial and cilia structures occurred by IFV, and 9-TB counteracted the viral infection severity and corrected ciliopathies. While such activity has been eluded to by the use of Doxy in COVID-19 infections, the exact mechanisms have not been elucidated but may be related to this new data. Using RNA-Seq profiles we then found that 9-TB stopped the loss of club, ciliated and alveolar cells, crucial to lung function, and decreased neutrophil, natural killer cell, and monocyte populations, all operative in an IFV or SARS-CoV2 infection. Doxy showed lesser activity in comparison and induced gene sets involved in the cytopathology of liver and kidney cells. 9-TB showed a reduced response suggesting that an improved safety profile may be designed into future Tc derivatives against liver and kidney cells an intriguing medicinal chemistry possibility. Altogether, we show that Tet-induced mitohormesis causes a response to IFV by preventing lung damage by discreet mechanisms and by lowering inflammation responses in vivo.
Conclusion
The Tcs can induce a mitohormetic response, activating the ATF4- ISR pathway, leading to type I IFN signaling in vitro and in animals. This response is beneficial in lethal infections where Tcs can increase the survival and disease tolerance, without affecting the pathogen itself. More remarkable, is that the most potent compounds were not the clinically-used Tcs, man-made antibacterial versions of the tetracycline scaffold, and that Tcs activating the mtUPR, ISR and MSR, are all mitochondrial-based. Tc activators that possess nonantibiotic properties against clinical pathogens is also desirable, selectively structurally targeting the mitobiome over the microbiome, as shown in our cell studies and their lack of activity against ESKAPE pathogens, gut flora or commensal bacteria. The low stress induced by the Tcs are variable and adaptive, generating a type I IFN response, followed by cellular and tissue protection from activated immune cells. While the mechanisms of this response are currently unknown, it could be the that the pleiotropic nature of the Tcs and their multiple roles in enzyme inhibition, type I IFN activation, and/or calcium dynamics may be desirable as well and the key to new therapeutics that will facilitate host-tolerance to immune responses to infections and tissue damage.
References
- Nelson MH, Wolfgang H, Greenwald RobertA (2001) Tetracyclines in biology, chemistry and medicine.
- Garrido-Mesa N, Zarzuelo A, Gálvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169: 337-352.
[Crossref] [Google Scholar] [PubMed]
- Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, et al. (2015) Structure activity relationship of the aminomethycyclines and the discovery of omadacycline. Antimicrob Agents Chemother 59: 7044-7053.
[Crossref] [Google Scholar] [PubMed]
- Tanaka SK, Steenbergen J, Villano S (2016) Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem 24: 6409-6419.
[Crossref] [Google Scholar] [PubMed]
- Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, et al. (2013) Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 23: 451-457.
[Crossref] [Google Scholar] [PubMed]
- Brüning A, Brem GJ, Vogel M, Mylonas I (2014) Tetracyclines cause cell stress-dependent ATF4 activation and mTOR inhibition. Exp Cell Res 320: 281-289.
[Crossref] [Google Scholar] [PubMed]
- Mottis A, Li TY, El Alam G, Rapin A, Katsyuba E, et al. (2022) Tetracycline-induced mitohormesis mediates disease tolerance against influenza. J Clin Invest 132: e151540.
[Crossref] [Google Scholar] [PubMed]
- Münch C (2018) The different axes of the mammalian mitochondrial unfolded protein response. BMC Biol 16: 81.
[Crossref] [Google Scholar] [PubMed]
Citation: Nelson ML (2023) Tetracycline’s as Masterful Regulators of Mitochondrial Function in Viral and Infectious Disease States. J Infect Dis Ther 11:530. DOI: 10.4172/2332-0877.1000530
Copyright: © 2023 Nelson ML. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1424
- [From(publication date): 0-2023 - Nov 23, 2024]
- Breakdown by view type
- HTML page views: 1288
- PDF downloads: 136