TERT Promoter Mutations and Tert Expression in Early-Stage (T1N0M0) Non-Small Cell Lung Cancer (NSCLC)
Received: 30-Jul-2015 / Accepted Date: 27-Aug-2015 / Published Date: 30-Aug-2015 DOI: 10.4172/2161-0681.1000248
Abstract
Background: The mutations detected in the promoter of the telomerase reverse transcriptase (TERT) gene,namely C228T and C250T, were first identified in melanoma and subsequently in several cancer models, notably glioma, thyroid cancer and bladder cancer. Recent findings demonstrate that mutation of the TERT promoter may be one of the most common genetic mechanisms contributing to telomerase activation in malignant cells. Mutation of the TERT promoter appears to be an indicator of worse outcome and is correlated with tumor aggressiveness and patient survival; however, to date the only two published studies reported a very low TERT mutation frequency in non-small cell lung cancer (NSCLC). Additionally, the relationship among the presence of these mutations, Tert expression and clinical outcome is unknown.
Materials and methods: In this study, the polymerase chain reaction and direct sequencing analysis of a TERT promoter region using formalin-fixed and paraffin-embedded tissues were performed to investigate the presence of TERT promoter mutations in a series of 95 early-stage (T1N0M0) NSCLC samples, including 48 adenocarcinomas (ADCs) and 47 squamous cell carcinomas (SCCs).Then, immunohistochemical (IHC) staining was performed to detect the expression of Tert.
Results and conclusions: We report that TERT promoter mutations are present in NSCLC with a frequency of 10.52%. TERT promoter mutations were identified in 7 (14.58%) ADCs and 3 (6.38%) SCCs. Tert was expressed in the cytoplasm of 55 samples out of 93 (59.1%) in 33 ADCs and 22 SCCs. The ADCs expressed Tert in a higher percentage of cases (71.7% vs. 46.8%). Although no correlations between the TERT mutations, Tert immunohistochemical expression, clinico-pathological parameters and survival were found in our series, this study represents a starting point for further research to better understand the molecular mechanisms involved in the transcriptional regulation of TERT and its role in lung cancer progression.
Keywords: TERT promoter mutations; Telomerase; Non-small cell lung cancer
323163Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, and non-small cell lung cancer (NSCLC) accounts for approximately 80% of these cases [1,2]. Surgical resection represents the treatment of choice for NSCLC during early stages of the disease; however, the survival rates remain low, and the study of tumors and patient genetic profiles represents a priority for improving the knowledge of the molecular mechanisms involved in human tumorigenesis.
Telomerase reverse transcriptase encodes the catalytic subunit of the enzyme telomerase, which is a multimeric ribonucleoprotein complex composed of a reverse transcriptase protein component (TERT) that catalyzes the addition of non-coding DNA elements to the ends of chromosomes and a functional RNA that contains the template region complementary to the telomeric sequence [3,4]. Critical shortening of the telomeres leads to unprotected chromosomal ends, with chromosomal fusion and breakage during mitosis. In the presence of telomerase, telomere length is maintained and allows the evasion of replicative senescence [5].
Telomerase activation is known to be a hallmark of cancer and is detected in up to 80% of malignant tumors. Furthermore, the transcriptional regulation of the catalytic component of the telomerase complex is a decisive factor for controlling telomerase activity [6]. Therefore, elucidating how the TERT gene is transcriptionally activated in cancer cells has been one of the key focuses of research. More recently, two novel mutations in the promoter region of TERT, both C>T transitions, were identified in human melanoma [7]. Subsequently, these substitutions were shown to be present in several malignant human tumors, including thyroid cancer [8,9], hepatocellular cancer [10], bladder cancer [11], gliomas [12] and malignant pleural mesothelioma [13]. These mutations, hereafter referred to as C228T and C250T, were mutually exclusive and were located within a region from -124bp and -146bp upstream of the ATG start site. Both C228T and C250T generated an identical 11-bp nucleotide stretch (5'-CCCCTTCCGGG-3') containing a consensus binding site for E-twenty-six (ETS) transcription factors [GGA(A/T), reverse complement] within the TERT promoter region. These two mutations have been demonstrated to confer increased transcriptional activity from the TERT promoter [14], suggesting a novel mechanism by which telomerase contributes to human tumorigenesis.
Although numerous data exist regarding TERT promoter mutations in various types of human malignancies, little is known regarding these mutations in lung cancer. The only two published studies reported a very low mutation frequency in NSCLCs. While Ma et al demonstrated that TERT promoter mutations are present in 2.57% of NSCLCs [15], neither C228T nor C250T mutations were validated in the 174 NSCLC samples analyzed by Li et al. [16].
The objective of our study was to investigate the relationship between TERT promoter mutations, Tert expression and clinical parameters in a series of 95 early-stage (T1N0M0) non-small cell lung cancer (NSCLC) patients.
Materials and Methods
Patient and tumor characteristics
A total of 95 patients with stage I (T1N0M0) NSCLC who underwent surgical resection at the Unit of Thoracic Surgery in the Department of Surgical, Medical, Molecular Pathology and Critical Area at Pisa University between 2000 and 2010 were retrospectively selected. All of the samples were formalin fixed and paraffin embedded (FFPE) for microscopic examination, and the histological diagnoses were formulated by two pathologists (G.F. and A.S.) according to the World Health Organization classifications [17-19]. The most representative paraffin blocks of the tumor tissues were selected for the molecular analysis for each case. The clinicopathological characteristics and survival data were collected for all of the patients. Informed consent was obtained from each patient for the tissue collection and molecular analysis.
DNA isolation
Ten-micron sections were prepared from the FFPE tissues. After standard deparaffinization, the tumor tissue was manually macro-dissected from one to two sections, and the samples were lysed and digested with proteinase K. DNA purification was performed using the spin column procedure (QIAmp DNA Mini Kit; QIAGEN) according to the manufacturer’s instructions. The DNA quality and quantity were evaluated using a NanoDrop ND-1000 spectrophotometer.
PCR and direct sequencing
A 163-bp TERT promoter region covering the two mutation hotspots was amplified by PCR using the following primers: TERT_F, 5’-CAGCGCTGCCTGAAACTC-3’ and TERT_R, 5’-GTCCTGCCCCTTCACCTT-3’ [7]. The quality and efficiency of the PCR amplification were confirmed by running the PCR products on a 1.5% agarose gel, and the PCR products were subjected to a purification procedure. Cycle sequencing analysis of the purified amplicons was performed with the ABI big dye terminator version 3.1 cycle sequencing kit (Applied biosystems) according to the manufacturer’s instructions using the PCR amplification primers for bidirectional sequencing. The fragments were processed on an ABI PRISM 3130 genetic analyzer (Applied biosystems) and the data were analyzed using Sequencing Analysis 5.0 Software (Applied biosystems).
Tert immunohistochemistry
We tested the samples with two different Tert antibodies to compare the two results. Three-micron thick tumor sections from all of the 95 samples were stained with anti-telomerase reverse transcriptase rabbit monoclonal [Y182] antibody (Abcam - cod. ab32020) at a 1:150 dilution. Ten samples were also stained with anti-telomerase catalytic subunit rabbit polyclonal antibody at a 1:300 dilution. The staining was performed on an automated stainer system using the UltraView DAB Detection Kit.
A pathologist (A.S.) examined the immunohistochemical slides without any prior information on the clinico-pathological features of the patient samples. The tumor samples were considered positive if a cytoplasmic staining in at least 5% of all tumor cells was present. The percentages of Tert-positive tumor cells and staining intensity were evaluated for each positive sample. A proportion score (PS) was assigned representing the estimated proportion of positive staining cells and was graded according to the following 5 classes: less than 5% (score 0); between 5% and 20% (score 1); between 21% and 50% (score 2); between 51% and 70% (score 3); over 71% (score 4). An intensity score (IS) was also assigned and graded according to the following 4 classes: none (score 0); weak (score 1); intermediate (score 2); strong (score 3). Then, a final total score was obtained from the sum of PS and IS, ranging from 0 to 7.
Statistical analysis
All of the statistical analyses were carried out using JMP10 software (SAS). The chi2 test was used to determine the correlation between the TERT promoter mutations and the different parameters. To evaluate the usefulness of the TERT promoter mutations as a prognostic factor in this cohort of patients, we performed a survival analysis using the Kaplan-Meier method followed by a log-rank test to determine the significance. P-values less than 0.05 were considered statistically significant.
Results
Patient and tumor characteristics
Ninety-five patients participated in this study, including 67 male and 28 female patients with an age at diagnosis ranging from 48-81 years (mean 66.8 years, median 67). The patient features and the tumor characteristics are shown in Table 1.
| A | n (%) |
|---|---|
| Gender | |
| Male | 67 (70.52%) |
| Female | 28 (29.48%) |
| Histology | |
| ADCa | 48 (50.52%) |
| SCCb | 47 (49.48%) |
| Tumor grading | |
| G1 | 4 (4.21%) |
| G2 | 66 (69.47%) |
| G3 | 25 (26.32%) |
| Recurrence | |
| Yes | 26 (27.36%) |
| No | 69 (72.64%) |
| Smoking habits | |
| current | 42 (44.21%) |
| former | 38 (40%) |
| never | 15 (15.79%) |
Table 1: Clinicopathological features of the patients.
Of the 95 patients, 75 (78.95%) had a lobectomy and the remaining 20 patients (21.05%) had a wedge resection. According to the histology, 48 (50.53%) of the samples were adenocarcinomas (ADCs) and 47 (49.47%) were squamous cell carcinomas (SCCs). All of the patients were classified as T1N0 according to tumor size, pleural infiltration and nodal involvement. According to the degree of differentiation, 4 tumors were graded as well differentiated (G1), 66 as moderately differentiated (G2), and 25 as poorly differentiated (G3). The histologic patterns of the 48 adenocarcinomas were characterized as follows: 16 acinar (33.33%, 75% predominantly acinar and 25% pure acinar), 15 lepidic (31.25%, 86.66% predominantly lepidic and 13.34% pure lepidic), 7 mucinous (14.58%, 14.28% predominantly mucinous and 85.72% pure mucinous), 7 papillary (14.58%, 100% predominantly papillary) and 3 solid (6.26%, 33.33% predominantly solid and 66.67% pure solid).
The clinico-pathological features and survival data were available for all of the patients, with follow-up through 30 July 2013. The median disease-free interval (DFI) and overall survival (OS) were 54.5 months (range, 6-161 months) and 70 months (range, 11-161 months), respectively. A total of 26 (27.36%) patients presented relapses during the follow-up period. The location of metastasis was known for only 22 cases; 5 patients developed local recurrence, 7 developed relapses in the contralateral lung, 3 developed relapses in the homo-lateral lung, and 7 patients developed distant metastases.
The smoking habits of all of the patients were known. There were 42 current smokers (44.21%), 38 former smokers (40%) and 15 never-smokers (15.79%). At the end of the follow-up 78 patients (82.10%) were alive and 17 (17.90%) had died.
TERT Promoter Mutation Analysis
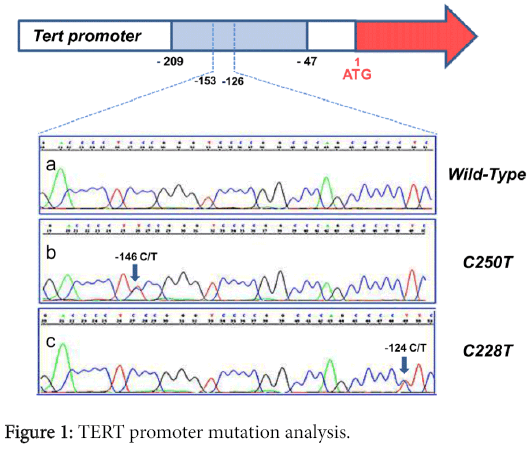
Sequencing analysis was performed in the TERT promoter region containing the two previously reported mutation hot spots. Representative electropherograms are shown in Figure 1.
Among the 95 tumor specimens, 10 tumors were found to have TERT promoter mutations (10.52%). In 5 cases, the C228T substitution was present, and 5 cases showed the C250T point mutation. According to histology, 7 out of the 48 (14.58%) adenocarcinomas analyzed were altered with the following distribution regarding the histological subtypes: 1 C228T and 1 C250T in 2 out of the 16 acinar tumors; 1 C228T and 1 C250T in 2 out of the 15 lepidic tumors; and 1 C228T and 1 C250T in 2 out of the 7 mucinous ADCs and 1 C250T in 1 out of the 3 solid tumors. None of the 7 analyzed ADCs with a papillary pattern contained a mutation in the promoter region of TERT. Regarding squamous cell carcinomas, we found two instances of C228T and one of C250T in 3 out of 47 cases (6.38%).
Sequence analysis revealed that these mutations affected only one of the alleles of the TERT gene locus; their somatic nature was confirmed by their absence in normal tissues collected at a distance from the tumor in the 10 patients harboring TERT mutations.
In addition, 7 NSCLCs carried mutations at other positions within the TERT promoter core in an 88-bp region starting 165bp upstream of the ATG start site. The C184T mutation was detected in one case of squamous cell carcinoma, C240T was detected in two cases of squamous cell carcinoma, C241T was detected in two cases of adenocarcinoma, C243T was detected in one case of squamous cell carcinoma, and C269T was detected in one case of squamous cell carcinoma.
Tert Immunohistochemistry
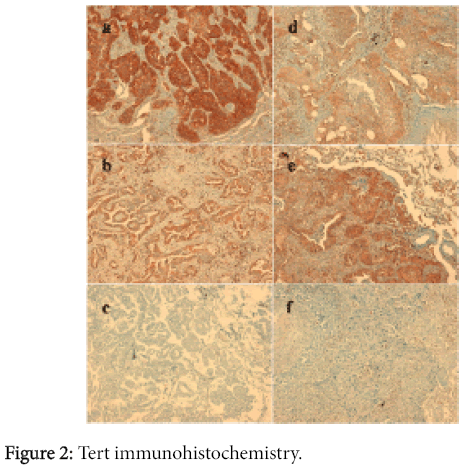
During the immunohistochemistry evaluation, 93 out of the 95 analyzed samples were suitable. Tert was expressed in cytoplasm of 55 samples out of 93 (59.1%) including 33 ADCs and 22 SCCs (Figure 2). The ten samples we analyzed with both antibodies have been selected in term of either immunostaining percentage or intensity as follows: three cases with negative staining, two cases with 2+IS and 20% of tumour cells, one case with 3+IS and 60% of tumour cells, one case with 2+IS and 70% of tumour cells, one case with 3+IS and 70% of tumour cells, one case with 2+IS and 80% of tumour cells, one case with 2+IS and 90% of tumour cells. Similar results were obtained when comparing the two antibodies, both in term of percentages of positive tumor cells as well as staining intensity, so we decided to analyze the whole series using anti-telomerase reverse transcriptase rabbit monoclonal [Y182] antibody (Abcam-cod. ab32020).
There was no statistically significant association between the expression of Tert and the histological type, though ADCs appear to express the protein in a higher percentage of cases (71.7% vs. 46.8%).
Considering all of the samples, the proportion of positive staining cells ranged from 0 to 100%, with a mean value of 29% and a median value of 50%. Thirty-eight cases out of 93 (40.9%) showed a PS (proportional score) of 0; 9 out of 93 (9.7%) showed a score of 1; 10 out of 93 (10.7%) showed a score of 2; 6 out of 93 (6.5%) showed a score of 4; 0 cases showed a score of 3; 17 out of 93 (18.3%) showed a score of 5; 12 out of 93 (12.9%) showed a score of 6 and 1 out of 93 (1%) showed a score of 7.
Correlation between TERT Mutational Status and Immunohistochemistry
We tried to compare the TERT mutational status with the immunohistochemical expression of Tert using the following four different grouping systems: positive vs. negative, high vs. low percentage; high vs. low intensity; and the eight classes of the final total score. No statistically significant association was found between the immunohistochemical expression of Tert and the TERT mutational status using any of the four grouping systems.
No significant association was found between mutational status and immunoistochemical staining when the seven samples harbouring other mutations located within an 88-bp region were included.
Relationship between TERT Mutations and Clinicopathological Features
No statistically significant association was found between the mutational status of the tumors and the clinico-pathological features (Table 2).
| Mutational status of TERT promoter region | p-value | ||
|---|---|---|---|
| Mutant | Wild type | ||
| All cases (n=95) | 10 (10.52%) | 85 (89.48%) | |
| Age | |||
| ≤68 years (n=50) | 5(10%) | 45 (90%) | 0.86 |
| >68 years (n=45) | 5 (11.11%) | 40 (88.89%) | |
| Gender | |||
| Males (n=67) | 5 (7.46%) | 62 (92.54%) | 0.14 |
| Females (n=28) | 5 (17.86%) | 23 (82.14%) | |
| Histology | |||
| ADCa (n=48) | 7 (14.58 %) | 41 (85.42 %) | 0.18 |
| SCCb(n=47) | 3 (6.38%) | 44 (93.62%) | |
| Smoking habits | |||
| Smoker (n=42) | 3 (3.15%) | 39 (41.05%) | 0.43 |
| Former smoker (n=38) | 4 (4.22%) | 34 (35.79%) | |
| Never smoker (n=15) | 3 (3.15%) | 12 (12.64%) | |
Table 2: Correlation between the clinico-pathological features of the patients and TERT-promoter genotype.
The frequency of the mutational status of the promoter region of TERT did not vary significantly according to histology (ADC and SCC, p=0.18), sex (p=0.14), age (p=0.86), or smoking habits (p=0.43).
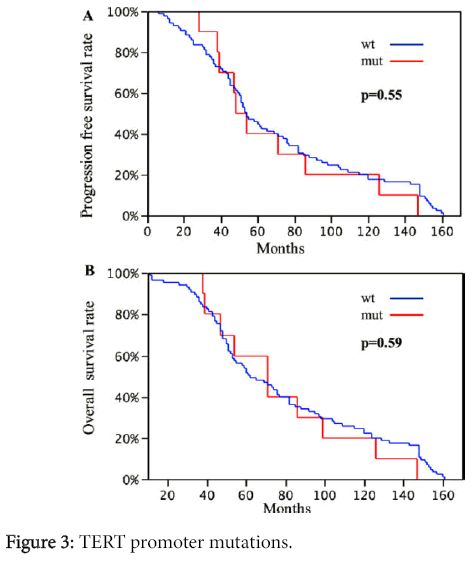
No significant difference in disease-free-interval and overall survival was found between the patients harboring TERT promoter mutations and patients without a mutation (OS p=0.55 and DFI p=0.59, respectively) (Figure 3).
Discussion
Whereas telomerase is inactive in the majority of non-neoplastic cells, its activation is identified in up to 80% of malignant tumors and it is known to be a hallmark of cancer [6]. Telomerase activation in cancer has been attributed to several mechanisms, including epigenetic deregulation, as well as genetic amplification of the locus containing the TERT gene [20]. Recently, mutations within the TERT gene promoter, particularly at two positions located at -124 and -146 relative to the ATG start site, were found in melanoma [7,14,21] and in several other human cancers [8-13], suggesting a putative role in telomerase activation in malignant cells.
The frequency of TERT mutations appears to be very different among cancer types. High frequencies of TERT promoter mutations have been reported in bladder cancer [11,22,23], melanoma [7,24], pleomorphic dermal sarcoma [25], myxoid liposarcoma [12], glioma [12], basal and squamous cell carcinoma of the skin [26], and in liver cancer [27]. Conversely, a very low frequency of these mutations has been found in other types of cancers, particularly in esophageal squamous cell [28] and gastric carcinomas [29].
Furthermore, in melanoma, Heidenreich et al. [24] reported that mutations were mostly associated with the presence of regional and distant metastasis. These findings raise the possibility of the eventual use of TERT promoter mutations as prognostic factors in disease management and as putative therapeutic targets.
Our results revealed that TERT promoter mutations occurred in 10.52% of early-stage (T1N0M0) NSCLC patients, which is in contrast to the two previously published reports. Specifically, Li et al screened the TERT promoter region in 174 NSCLC FFPE samples using PCR and direct sequencing. They identified only three mutations but reverse sequencing validated none of these; the authors concluded that was maybe caused by DNA damage in the process of formalin fixing and paraffin embedding. Although the sensitivity of sequencing results is affected by the low quality of FFPE samples in comparison with unfixed fresh-frozen specimens, we demonstrated that the DNA purified from FFPE specimens is suitable to detect TERT promoter mutations in NSCLCs and they occur in NSCLC samples.
Regarding the second one, Ma et al. analyzed TERT promoter region in 467 lung cancer patients at different TNM stage. Their results showed that these mutations were presented in 2.57% of NSCLCs, with five hot spot alterations (C228T and C250T) and six point mutation located at other position. The cases examined by Ma et al belong to a heterogeneous series of patients in terms of clinical stage, so the TERT promoter mutation rate found in their study is different from our results in only early-stage specimens. In conclusion, the low number of former publications makes it impossible to reach a definitive conclusion on the effective frequency of TERT promoter mutations, therefore requiring further analysis of these alterations [15,16].
Interestingly, we observed a higher frequency of mutations in ADCs (14.58%) compared with SCCs (6.38%). Although these data do not reach statistical significance, the two previous studies on NSCLCs did not concentrate on histology. Additionally, a recent work by Cheng et al. [30], which is focused on SCC of different anatomical sites, did not locate any TERT promoter mutations in SCC of the lung.
Regarding the ADC subtypes, among the 7 cases with a papillary pattern, no mutation was identified, while mutations were found among the other patterns analyzed (12.5% in acinar ADCs, 13.33% in lepidic ADCs, 28.57% in mucinous ADCs, 33.3% in solid ADCs). The small number of patients in each histological category makes it impossible to reach a definitive conclusion on this issue. Our results are the first demonstrating this distribution of TERT promoter mutations among NSCLC histotypes and prevalent patterns of the ADC subtypes. Because ADCs of the lung are mostly characterized by multiple patterns in the same tumor, whether TERT mutations can map to different histological patterns in the same tumor would be interesting to test. Therefore, a larger population will be required to confirm our results regarding the putative absence of mutations in papillary adenocarcinoma compared with other subtypes and to better understand the molecular aspects of the ADC subtypes.
All of the detected mutations that consisted of a cytosine-to-thymine transition at positions -124 and -146 from the TERT translation start site were mutually exclusive. In contrast to melanoma, we did not find any tandem CC>TT mutations. In addition, we found seven other mutations located within an 88bp region starting from -165bp upstream of the ATG start site. None of these mutations generate de novo consensus binding sites for transcription factors [31].
Many studies have demonstrated that TERT promoter mutations are associated with increased telomerase expression in several cancer types, such as melanoma [7,14] and malignant pleural mesothelioma [13], suggesting that TERT promoter mutations may activate telomerase in some tumors types. In our series, no differences in Tert IHC staining were observed between TERT-mutated and TERT-wild type tumors, in accordance with a recent study [32]. This finding suggests that molecular mechanisms distinct from promoter mutations may contribute to telomerase activation in lung cancer.
Moreover, high telomerase expression and activity have been found to be associated with both high-grade tumors and a more advanced clinical stage in lung cancers [33,34]. Our series is mainly composed of moderately differentiated tumors and a low number both of well differentiated cases and poorly differentiated ones, which inhibited our ability to correlate telomerase expression and tumor grade.
Considering the immunohistochemical results in terms of the PS (proportional score), 61.28% of the samples analyzed showed a score lower than 4, suggesting that our samples globally exhibit a low Tert expression level. Because our series is composed of early-stage NSCLCs, this finding is in line with a previously cited report demonstrating that in stage I NSCLC, telomerase expression was lower than in the other stages [34]. An interesting aspect concerns the presence of TERT promoter alterations also in association with other gene mutations. Recent studies, in fact, reported a statistical relationship between BRAF and TERT promoter mutations in melanomas [7,24,35]. In particular, in our previous study [21] we found that the coexistence of TERT promoter and BRAF mutations correlated more strongly with most important adverse clinicopathological parameters, including thickness, sentinel node metastases, and absence of regression than either mutation alone. Similar results, notably a significant association between the coexistence of TERT promoter and BRAF mutations and worse outcome in terms of disease-free survival, were reported by Xing et al. in papillary thyroid carcinoma [36].
In conclusion, the data in the literature support the hypothesis that mutations in the TERT promoter could represent an additional mechanism of genetic alteration in various human cancers. Moreover, our study revealed that TERT promoter mutations are present even in non-small cell lung cancer, with a higher incidence in adenocarcinomas. We found that TERT promoter mutations had no correlation with either Tert expression or clinicopathological features, but future studies will clarify the possible coexistence of TERT promoter mutations with EGFR or KRas or BRAF mutation and their association with clinical features. Our findings represent a starting point for further research to better understand the molecular mechanisms involved in the transcriptional regulation of TERT in lung cancer and its role in tumor progression.
Acknowledgments
This work was supported by Grant PRIN 2009LMEEEH_004 from the Italian Ministry for University and Scientific Research and by Grant 10316_2010 from Italian Association for Cancer Research.
References
- Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, et al. (2003) Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control.J Natl Cancer Inst 95: 1276-1299.
- Scagliotti GV, Fossati R, Torri V, Crinò L, Giaccone G, et al. (2003) Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer.J Natl Cancer Inst 95: 1453-1461.
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, et al. (1994) Specific association of human telomerase activity with immortal cells and cancer.Science 266: 2011-2015.
- Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond.Nat Rev Genet 6: 611-622.
- Daniel M, Peek GW, Tollefsbol TO (2012) Regulation of the human catalytic subunit of telomerase (hTERT).Gene 498: 135-146.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation.Cell 144: 646-674.
- Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, et al. (2013) TERT promoter mutations in familial and sporadic melanoma.Science 339: 959-961.
- Liu X, Bishop J, Shan Y, Pai S, Liu D, et al. (2013) Highly prevalent TERT promoter mutations in aggressive thyroid cancers.EndocrRelat Cancer 20: 603-610.
- Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, et al. (2013) Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease.J ClinEndocrinolMetab 98: E1562-1566.
- Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, et al. (2013) High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions.Nat Commun 4: 2218.
- Liu X, Wu G, Shan Y, Hartmann C, von Deimling A, et al. (2013) Highly prevalent TERT promoter mutations in bladder cancer and glioblastoma.Cell Cycle 12: 1637-1638.
- Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, et al. (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal.ProcNatlAcadSci USA 110: 6021-6026.
- Tallet A, Nault JC, Renier A, Hysi I, Galateau-Sallé F, et al. (2014) Overexpression and promoter mutation of the TERT gene in malignant pleural mesothelioma.Oncogene 33: 3748-3752.
- Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, et al. (2013) Highly recurrent TERT promoter mutations in human melanoma.Science 339: 957-959.
- Ma X, Gong R, Wang R, Pan Y, Cai D, et al. (2014) Recurrent TERT promoter mutations in non-small cell lung cancers.Lung Cancer 86: 369-373.
- Li C, Hao L, Li Y, Wang S, Chen H, et al. (2014) Prognostic value analysis of mutational and clinicopathological factors in non-small cell lung cancer.PLoS One 9: e107276.
- Colby TV, Noguchi M, Henschke C, Vazquez MF, Geisinger K, et al. (2004) Adenocarcinoma. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds.). World Health Organization classification of tumours: pathology and genetics of tumours of the lung, pleura, thymus, and heart. IARC Press, Lyon, France, pp. 35-44.
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, et al. (2011) International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J ThoracOncol 6: 244-285.
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, et al. (2013) Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 137: 668-684.
- Zhang A1, Zheng C, Lindvall C, Hou M, Ekedahl J, et al. (2000) Frequent amplification of the telomerase reverse transcriptase gene in human tumors.Cancer Res 60: 6230-6235.
- Macerola E, Loggini B, Giannini R, Garavello G, Giordano M, et al. (2015) Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness.Virchows Arch 467: 177-184.
- Hurst CD, Platt FM, Knowles MA (2014) Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine.EurUrol 65: 367-369.
- Allory Y, Beukers W, Sagrera A, Flandez M, Marques M, et al. (2014) Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. EurUrol 65: 360-366.
- Heidenreich B, Nagore E, Rachakonda PS, Garcia-Casado Z, Requena C, et al. (2014) Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma.Nat Commun 5: 3401.
- Griewank KG, Schilling B, Murali R, Bielefeld N, Schwamborn M, et al. (2014) TERT promoter mutations are frequent in atypical fibroxanthomas and pleomorphic dermal sarcomas.Mod Pathol 27: 502-508.
- Scott GA, Laughlin TS, Rothberg PG (2014) Mutations of the TERT promoter are common in basal cell carcinoma and squamous cell carcinoma.Mod Pathol 27: 516-523.
- Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, et al. (2013) High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions.Nat Commun 4: 2218.
- Zhao Y, Gao Y, Chen Z, Hu X, Zhou F, et al. (2014) Low frequency of TERT promoter somatic mutation in 313 sporadic esophageal squamous cell carcinomas.Int J Cancer 134: 493-494.
- Qu Y, Shi L, Wang D, Zhang B, Yang Q, et al. (2014) Low frequency of TERT promoter mutations in a large cohort of gallbladder and gastric cancers.Int J Cancer 134: 2993-2994.
- Cheng KA, Kurtis B, Babayeva S, Zhuge J, Tantchou I, et al. (2015) Heterogeneity of TERT promoter mutations status in squamous cell carcinomas of different anatomical sites.Ann DiagnPathol 19: 146-148.
- Akiyama Y (1995) TF Search: Searching Transcription Factor Binding Sites.
- Muzza M, Colombo C, Rossi S, Tosi D, Cirello V, et al. (2015) Telomerase in differentiated thyroid cancer: promoter mutations, expression and localization.Mol Cell Endocrinol 399: 288-295.
- Marchetti A, Bertacca G, Buttitta F, Chella A, Quattrocolo G, et al. (1999) Telomerase activity as a prognostic indicator in stage I non-small cell lung cancer.Clin Cancer Res 5: 2077-2081.
- Lantuejoul S, Soria JC, Moro-Sibilot D, Morat L, Veyrenc S, et al. (2004) Differential expression of telomerase reverse transcriptase (hTERT) in lung tumours.Br J Cancer 90: 1222-1229.
- Populo H, Boaventura P, Vinagre J, Batista R, Mendes A, et al. (2014) TERT promoter mutations in skin cancer: the effects of sun exposure and X-irradiation. J Invest Dermatol 134: 2251-2257.
- Xing M, Liu R, Liu X, Murugan AK, Zhu G, et al. (2014) BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J ClinOncol 32: 2718-2726.
Citation: Giordano M, Macerola E, Boldrini L, Giannini R, Servadio A, et al. (2015) TERT Promoter Mutations and Tert Expression in Early-Stage (T1N0M0) Non-Small Cell Lung Cancer (NSCLC). J Clin Exp Pathol 5:248. DOI: 10.4172/2161-0681.1000248
Copyright: © 2015, Giordano M, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 17244
- [From(publication date): 10-2015 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 12540
- PDF downloads: 4704