Terinary Epidemiology and Disease Control Role
Received: 26-May-2022 / Manuscript No. ECR-22-67228 / Editor assigned: 30-May-2022 / PreQC No. ECR-22-67228 / Reviewed: 13-Jun-2022 / QC No. ECR-22-67228 / Revised: 17-Jun-2022 / Manuscript No. ECR-22-67228 / Published Date: 24-Jun-2022 DOI: 10.4172/2161-1165.1000447
Abstract
Animals, both domesticated and non-domesticated, provide considerable financial and non-financial benefits to humans. Livestock provides food, fibre, hides, manure for fuel and fertiliser, and draught power to communities and families, as well as having cultural significance and playing a part in the status of individuals in certain societies. Non-domesticated animals provide a range of benefits to humans, including economic, health, recreational, scientific, and ecological values. Pet animals, particularly dogs and cats, are important companions in many households and contribute to the physical, social, and emotional development of children and the well-being of their owners, whereas non-domesticated animals provide a range of benefits to humans, including economic, health, recreational, scientific, and ecological values. Disease, on the other hand, can have a substantial impact on livestock output and product quality, as well as the lifetime and quality of the animals.
Keywords
Recreational; Infectious diseases; Veterinarians
Introduction
Given the potential for numerous disease viruses to be transmitted to humans, of the life of pets and the variety of wildlife Furthermore, it is predicted that 60 percent of developing infectious diseases in people are zoonotic, with over 70% of these beginning in wildlife. With the recent focus on enhancing food safety, food security, biodiversity, and animal and public health, steps to decrease the danger of disease introduction or dissemination within animal populations, as well as from animal to human populations, are increasingly being adopted. Control, prevention, and/or eradication of illnesses in animal and human populations necessitate a solid understanding of epidemiology. Veterinary epidemiology gives veterinarians the tools they need to research disease outbreaks, identify disease risk factors, investigate diseases with unknown causes, conduct disease surveillance and monitoring, administer herd health programmes, and develop and execute biosecurity measures.
As a result, this discipline is critical for disease control, eradication, and prevention. The role and importance of veterinary epidemiology in the control, prevention, and elimination of illnesses in domesticated and non-domesticated animals, as well as the execution of biosecurity programmes in farmed livestock populations, are the subject of this publication. Disease does not occur randomly in a population, but is more likely to occur in specified members/groups of a population, at specific times, and in specific locations; in other words, disease follows definite patterns, according to veterinary epidemiology. The identification of these patterns, as well as the risk factors that increase the likelihood of disease, as well as the risk factors that reduce the likelihood of disease, is critical to disease control because it allows measures to be implemented to reduce the frequency, severity, and impact of disease. Diseases are complex, with both direct and indirect causes, elements, or qualities interacting to create disease; for clarity, this interrelationship is sometimes depicted graphically as a causal web. Several illness causation models have been proposed. There have been suggestions for both infectious and non-infectious diseases. The epidemiologic trio, which is the standard model for infectious disease, is one of the simplest of these. The trio is made up of an external agent (bacteria, virus, parasite, fungus, or prion), a susceptible host, and an environment, which includes management and husbandry techniques, that brings the host and agent together [1].
Disease is caused by an interaction between the agent and the vulnerable host in an environment that allows the agent to be transmitted from a source to that host. We can reduce disease by modifying the environment, such as minimising faecal pollution, reducing overcrowding, or eliminating pathogen carriers or vectors. Similarly, we can lessen the severity of disease and hence the disease's impact by choosing disease-resistant animals or raising population resistance through natural or artificial mechanisms. In contrast, while some host factors, such as age and gender, are strongly connected to many diseases, these factors cannot be changed within a population. Females and younger animals were shown to be more susceptible to sheep and goat pox than males and older animals, respectively, while Zeng found that the prevalence of brucellosis in yaks increased with age. This type of data is useful for forecasting infection in a community. In a disease-control programme, however, it is difficult or impossible to act on these animal traits because they cannot be altered or completely eradicated from a population. It is critical to identify factors that raise the risk of disease and those that reduce the risk of disease (protective factors) so that potential strategies to lessen disease or prevent its introduction can be undertaken. Demographic, husbandry/ management, environmental, or socioeconomic factors can all be risk or protective factors for a disease, and they can all be studied using descriptive epidemiological research. The estimated odds ratio (OR) and its 95 percent confidence interval (CI) are often used to assess the strength of the link between a potential risk factor and a disease [2].
Literature Review
Key concepts of epidemiology
A fundamental tenet of veterinary epidemiology is that diseases follow patterns, not random distributions, and are more likely to strike certain individuals or groups of individuals, at particular times, and in particular places. Identification of these patterns, risk factors that increase the chance of disease, and risk variables that decrease the likelihood of disease is essential to disease control so that measures may be taken to limit the frequency, severity, and effect of disease.
For clarity's sake, this inter-relationship is sometimes shown graphically as a causal web. Diseases are complex, with direct and indirect causes, fac- tors, or characteristics acting together to generate illness. There are several disease causation models that may be used for both infectious and non-infectious disorders. The epidemio-logic triad, which is the conventional paradigm for infectious illness, is one of the most straightforward of these. The components of the triad include an external agent (a bacterium, virus, parasite, fungus, or prion), a vulnerable host, and an environment, such as management and husbandry techniques, which combine the host and agent. According to this theory, sickness develops as a result of the interaction between the agent and the vulnerable host in a transmission-friendly environment [3].
We can lessen sickness by modifying the environment, such as by lowering faecal pollution, overcrowding, or removing the carriers or vectors of infections.
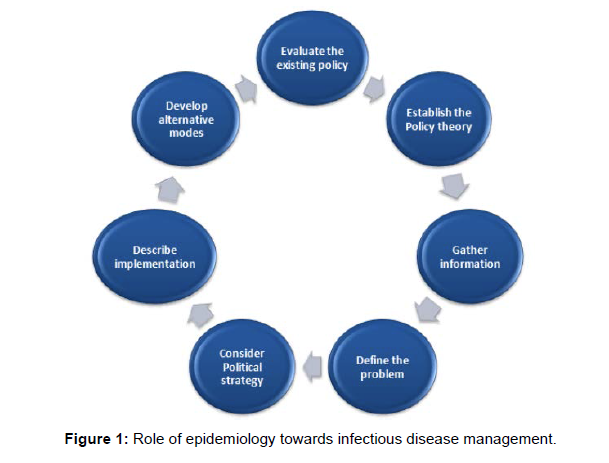
Similar to this, we may lessen the severity of sickness and subsequently its impact by choosing animals that are disease-resistant or by raising the population's resistance through natural or artificial ways. Contrarily, although while age and gender in particular are intimately connected with many illnesses, we are unable to control these aspects within a population. For instance, Zeng found that younger animals and females were more susceptible to sheep and goat pox than males and older animals, respectively (Figure 1).
Disease frequency
Prevalence and incidence are the two indicators of illness frequency used in epidemiology, and changes in these indicators are a crucial result of disease-control initiatives.
The proportion of people who are infected with the pathogen or who are seropositive on a serological test is often reported as the prevalence, which is a static measure of illness. In order to offer estimates of the illness frequency in the sampled total population and for comparing findings across research, this metric is often expressed as a percentage and should include 95% confidence intervals (CIs) [4].
The sensitivity and specificity of the tests performed, however, have an impact on test prevalence; as a result, many studies adjust test prevalence for these characteristics of a diagnostic test(s) using the formula true prevalence = (test prevalence + specificity 1) (sensitivity + specificity 1). For instance, found a genuine animal-level prevalence for FMD in cattle from Bhutan of 17.6 (95 percent CI: 15.6-19.5) after accounting for test uncertainty. When attempting to prove that a community is disease-free, estimation of a disease's genuine prevalence is essential since it enables comparison of control measures in various settings when various diagnostic procedures are used. In contrast to prevalence, which is a dynamic measure of the current frequency of a disease or an antibody, incidence is a dynamic measure of the spread of illness. Typically, incidence is expressed as an incidence rate (incidence density) or an incidence risk (cumulative incidence). For instance, a research found that during a four-month period, the cumulative incidence (incidence risk—new instances of illness within a specified time period) of lameness in dairy cattle was 29.6 percent, meaning that nearly one-third of the study's dairy cattle experienced lameness [5].
Identification of risk factors for disease
In order to execute potential steps to either minimise illness or prevent its introduction, it is vital to identify variables that either raise the risk of disease or those that decrease the risk of disease (protective factors). Demographic, husbandry/management, environmental, socioeconomic, and other risk or protective variables for a disease are evaluated by performing descriptive epidemiological studies (crosssectional, case-control, or cohort studies). The odds ratio (OR) and its 95% confidence interval (CI) are most frequently used to determine the strength of the link between a potential risk factor and a disease.
Discussion
The emphasis on biosecurity implementation has increased as the focus has shifted in the 21st and late 20th centuries from treating persons to preventing illness. Biosecurity is essential to keeping a farm, area, or nation disease-free. The management of the danger of pests and illnesses entering, emerging, establishing, or spreading and harming animals, plants, human health, the economy, the environment, or the community has been referred to as biosecurity. The majority of veterinarians are involved in assessing and preventing the spread of illness on specific farming operations that are under their supervision, even if this notion functions on a national and international scale. Farmlevel biosecurity's primary element is In order to limit the transmission of infectious pathogens among animal populations on a farm or to stop the infectious agent from leaving the farm, a set of management methods known as bio containment or internal biosecurity have been developed [6,7].
A number of acronyms, such as isolation, resistance, and sanitation (IRS) and sanitation, traffic control, assessment, isolation, resistance, and security (STAIRS), have been established to help organizations embrace and stress the fundamental ideas of biosecurity and bio containment. The owners, managers, and employees of livestock businesses, industry organizations, as well as rural and urban communities, are among the stakeholders who must be educated, trained, and involved for biosecurity to be successful at the enterprise, regional, and national levels. On both a national and international level, there are a tonne of publications and websites that discuss biosecurity. The use of biosecurity at the farm or company level is the main topic of this section [8-10].
Conclusion
Only necessary vehicles should be allowed access to cattle entrances,and the entry of these vehicles can be reduced by installing perimeter fence. The risk of disease entry from potentially contaminated feed trucks and livestock transport vehicles can be reduced by creating infrastructure that enables feed to be delivered externally to an enterprise and then moved via augers into storage bins/silos and laneways that are used to direct animals from buildings to outside the perimeter fencing [11]. Vehicles that must enter a building should only be allowed to enter and depart through one location that has facilities for cleaning and disinfecting the wheels and, preferably, the entire vehicle. The risk of introducing diseases, such as Newcastle disease, through the entry of contaminated equipment and fomites has been highlighted in numerous studies. This is because the introduction of equipment contaminated with faeces and other animal products (such as hair, feathers, and saliva) to a farm is also a potential disease introduction risk.
Acknowledgement
None
Conflict of Interest
None
References
- Hussein AA, Wilkoff BL (2019) Cardiac Implantable Electronic Device Therapy in Heart Failure. Circ Res 124(11):1584-1597.
- Sato K (2007) Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 1; 109(1):228-234.
- Kang K (2015) Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway following Myocardial Infarction. Stem Cells Int 659890.
- Schmidt C (2021) COVID-19 long haulers. Nat Biotechnol 39(8):908-913.
- Yilmaz R (2020) Mesenchymal stem cells treatment in COVID-19 patient with multi-organ involvement. Bratisl Lek Listy 121: 847-852.
- Aalsma M, Lapsley DK, Flannery D (2016) Narcissism, personal fables, adolescent adjustment. Psychol Sch PSYCHOL SCHOOLS 43(4):481 - 491.
- Edens JF, Marcus DK, Lilienfeld SO, Poythress Jr (2006) Psychopathic, not psychopath: Taxometric evidence for the dimensional structure of psychopathy. J Abnorm Psychol 115(1):131-144.
- Aluede O (2011) Managing bullying problems in Nigerian secondary schools: Some counselling interventions for implementation. The African Symposium: An Online Journal of the African Educational Research Network 11(1): 138-145.
- Edens JF, Marcus DK, Lilienfeld SO, Poythress Jr (2006) Psychopathic, not psychopath: Taxometric evidence for the dimensional structure of psychopathy. J Abnorm Psychol 115(1):131-144.
- Wang XR, Burkhardt NY, Kurtti TJ, Oliver JD, et al (2021) Mitochondrion-Dependent Apoptosis Is Essential for Rickettsia parkeri Infection and Replication in Vector Cells. mSystems 6(2):e01209-1220.
- Aung AK, Spelman DW, Murray RJ, Graves S (2014) Rickettsial infections in Southeast Asia: implications for local populace and febrile returned travelers. Am J Trop Med Hyg 91(3):451-460.
Indexed at, Google Scolar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scolar, Crossref
Citation: David W (2022) Terinary Epidemiology and Disease Control Role. Epidemiol Sci, 12: 447. DOI: 10.4172/2161-1165.1000447
Copyright: © 2022 David W. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1462
- [From(publication date): 0-2022 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 1126
- PDF downloads: 336