Technique of Phosphorus Recovery from Dehydrated Sludge by Incineration
Received: 07-Feb-2022 / Manuscript No. JBRBD-22-53437 / Editor assigned: 09-Feb-2022 / PreQC No. JBRBD-22-53437 (PQ) / Reviewed: 23-Feb-2022 / QC No. JBRBD-22-53437 / Revised: 25-Feb-2022 / Manuscript No. JBRBD-22-53437 (R) / Published Date: 28-Feb-2022 DOI: 10.4172/2155-6199.1000496
Abstract
Water sludge contains significant amounts of phosphorus, and in order to recover the phosphorus, some kinds of methods are investigated. One of the methods, phosphorus recovery from the dehydrated sludge by incineration is considered to be useful. The dehydrated sludge was mixed with the reagent of NaOH, KOH or Na2 CO3 , and incinerated at 750°C or 900°C. Phosphorus in the generated ash of the sludge was dissolved by the addition of the hot water, and recovered by the evaporation of the extract. The recovered phosphorus was confirmed to be an alkali metal phosphate, and the recovery rate reached about 75%.
Keywords: Dehydrated sludge; Phosphorus recovery; Incineration; Alkali
Keywords
Dehydrated sludge; Phosphorus recovery; Incineration; Alkali
Introduction
Phosphorus is a very important element, and regarded to be one of the indispensable elements of life. However it distributes in a limited area of the world, and Japan is importing all of it from other nations, and finding the domestic sources are needed. A large amount of wastewater sludge is discharged through the constructing a sewage treatment facility [1]. The water sludge contains a significant amount of phosphorus, and the phosphorus concentration in the sludge is increased by the introduction of the advanced phosphorus removal technique [2]. However, a useful recycling technique is not established, and some of them are used as compost for fertilizer, but this usage is not become popular because of the hygienic factor [3]. In Japan, most of the sludge is now incinerated through heat energy recovery to reduce the amount of the waste. Some of the generated ash of the sludge is used as cement raw material [4] or construction ingredients [5]; however, the phosphorus in the sludge is discharged into the environment without utilization. In order to recover the phosphorus from the sludge, some kinds of the methods using alkali [6] or acid [7, 8] are investigated. The acid treatment can recover the phosphorus with high performance [9]. However, the recovered phosphorus mainly contained a form of the aluminum phosphate [10] which is used as sintering material or encapsulation reagent of the heavy metals [11], but the usage is limited. On the other hand, with the alkali treatment, the recovered phosphorus is mainly composed of calcium phosphate with small amounts of aluminum which is widely used for fertilizer. However, the recovery rate is low compared with the acid treatment [12], and introduction of the technique is not practically going on. We found that the phosphorus recovery rate became high by the treatment at high temperature [13, 14]. However, how to use the heat source of the treatment is an important matter. In Japan, almost all dehydrated sludge is already incinerated as mentioned above, and the techniques about the incineration are already established. Therefore, incineration of the water sludge with alkali compounds is considered to be a effective method for phosphorus recovery. Based on this concept, the investigation of the phosphorus recovery was carried out, and phosphorus was successively recovered [15].
Method
Raw material
The dehydrated sludge (referred to as raw sludge) which was discharged at the Yokkaichi-City Waste Water Treatment facility was used. The aq. solution of the NaOH and KOH or powder of Na2CO3 was mixed with the raw sludge (water content 82%), and dried at 105˚C for 24 hours.
Incineration method of the sludge
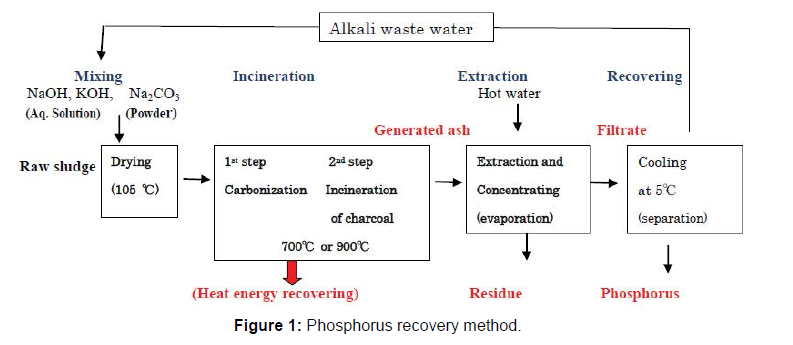
A basic matter is to find out the suitable incineration method of the sludge. The raw sludge was mixed with the alkali compounds with a finite ratio followed by incineration. A suitable instrument for the incineration is not provided, so in order to make easy incineration of the sludge, the incineration was carried out in 2 steps. In the first step, the dried sludge mentioned above was put into the evaporating dish (made of porcelain), and heated to about 500˚C using an oven. The volatile organic components in the sludge were evaporated, and the sludge was changed to charcoal. In the second step, the charcoal was incinerated at 750˚C (NaOH, KOH) or 900˚C (Na2CO3) using an electric furnace. These temperatures are considered best from former research [16]. In the practical treatment, the incineration will be carried out using the furnace at once. The ash (referred to as generated ash) was generated by this treatment. As a comparison, raw sludge was incinerated the same way without the addition of the alkali.
Extraction of the phosphorus
The generated ash was mixed with hot water (about 90˚C) to dissolve the phosphorus component in the ash, and was filtrated using filter paper (Toyo Roshi Kaisha, LTD). The filtrate was concentrated by evaporation, and cooled at 5˚C for one day. The phosphorus was recovered as a form of crystal from the cooled filtrate by separation using filter paper. On this occasion, significant amounts of the alkali waste water remained. This waste water will possibly be reused as the alkali for the phosphorus recovery [17], but in this experiment reuse of the alkali waste water was not examined. The insoluble components in the generated ash were separated as the residue using filter paper. The outline of the method is shown in Figure 1.
Alkali mixing rate
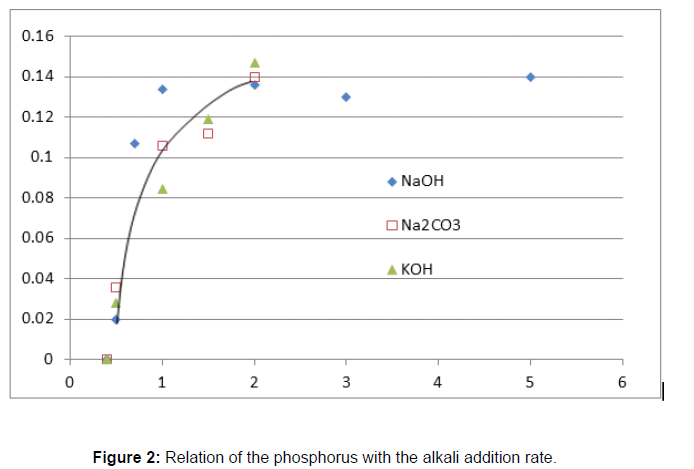
In order to find out the appropriate mixing rate, 50g of raw sludge was mixed with of 0.5g to 5g of NaOH (reagent, which was dissolved with 10mL of water), 0.5g to 2g of KOH (reagent, which was dissolved with 10mL of water), and also mixed with 0.5g to 2g of Na2CO3 (solid powder) respectively. These mixtures were dried as described above, and were incinerated. The generated ash was mixed with 200mL of water, and filtrated using the filter paper. Phosphorus concentration of the filtrate was analyzed using the molybdenum color metric method. The result shows that the phosphorus concentrations of the filtrate are increased with higher addition rate of the alkali (NaOH, KOH or Na2CO3) toward 2g, later, trend of the phosphorus concentration plateau shown in the Figure 2. From the result, the appropriate addition rate of the alkali was determined (Table 1) [18].
| Reagent | Amount of the raw sludge | Amount of the alkali | Incineration temperature (˚C) |
|---|---|---|---|
| NaOH | 50g | 1g | 750 |
| KOH | 50g | 2g | 750 |
| Na2CO3 | 50g | 3 2g | 900 |
Table 1: Appropriate addition ratio.
State of the recovered materials
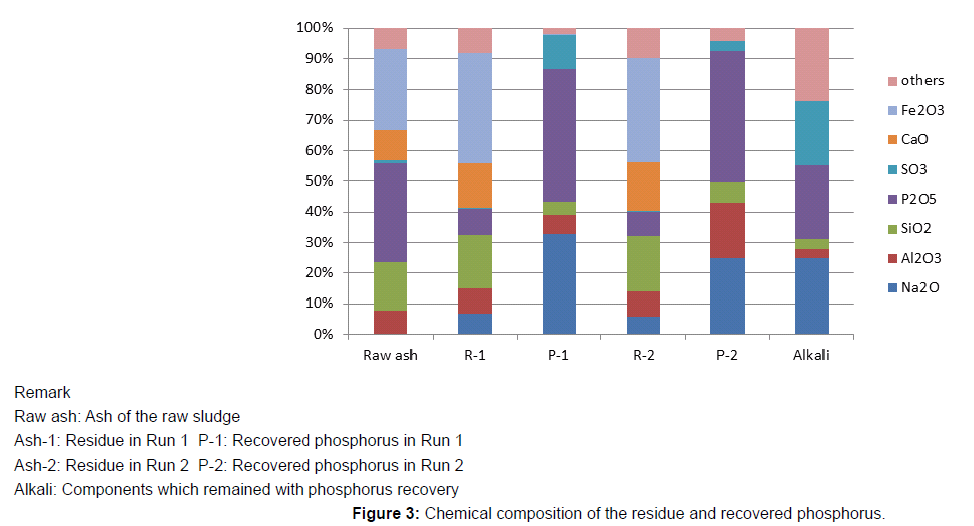
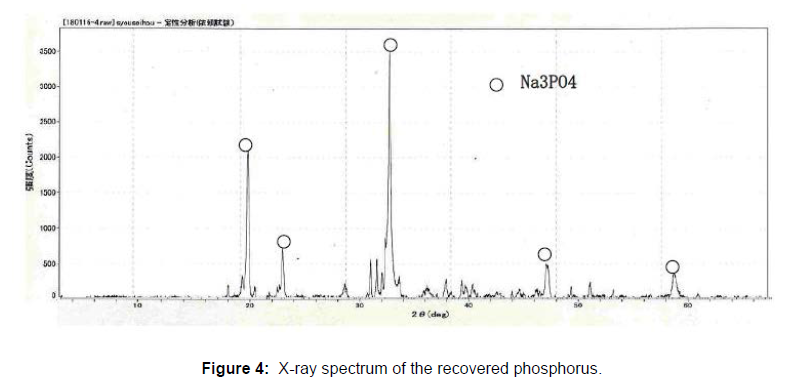
In order to confirm the chemical state of the recovered phosphorus, as the 1st run, 500g of the sludge was mixed with 10g of NaOH (dissolved by 20mL of water), and incinerated as mentioned above. The phosphorus containing filtrate (500mL) was concentrated to 100 mL, and the phosphorus was recovered as a form of the crystal, and the alkali waste water (about 50mL) was left. In the 2nd run, 1100g of the sludge was also mixed with 22g of NaOH, and incinerated as described above. The filtrate (1000mL) was concentrated to 200 mL, and the phosphorus was recovered with the alkali waste water (about 100mL). These alkali waste water were dried for the analysis. The amounts of the recovered phosphorus and residues are shown in the Table 2. The phosphorus recovery rate was estimated by comparing the phosphorus concentrations in the ashes (ash of the raw sludge and residues), and the recovery rates were estimated 75% to 75%. The recovered phosphorus was analyzed using X-ray analyzer (Rigaku Cooperation SPECTRO XEPOS). The recovered phosphorus are mainly composed of Na2O and P2O5 (Figure 3) and confirmed to be made of Na3PO4 by the X-ray diffraction Rigaku Cooperation (Figure 4). The alkali waste water contained a significant amounts of the alkali (Na2O) and phosphorus, and these components are considered to be reusable as the raw material.
| Item | Raw sludge | NaOH | Generated ash | Filtrate | Residue | Phosphorus | P recovery rate |
|---|---|---|---|---|---|---|---|
| Run 1 | 500g | 10g | 11.6g | 500mL | 8.5 | 7.7 | 0.74 |
| Run 2 | 1100g | 22g | 43 | 1000mL | 19 | 26 | 0.75 |
Table 2: The amounts of the recovered materials and recovery rate.
Chemical change of the ash components of the sludge
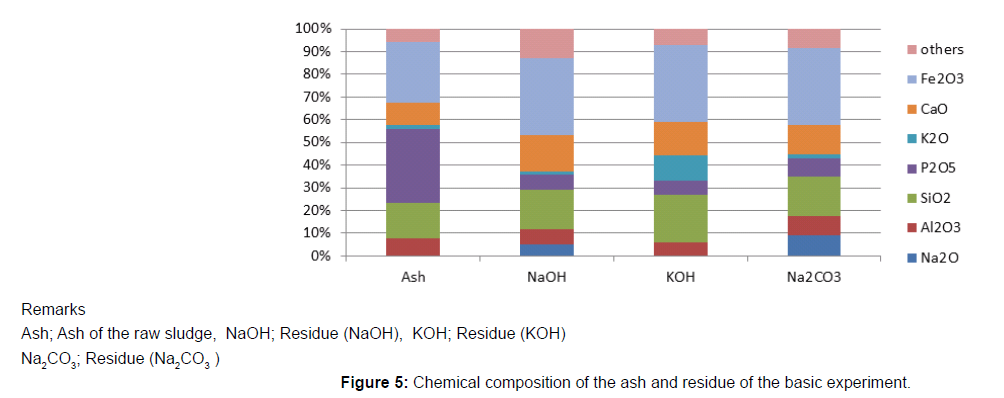
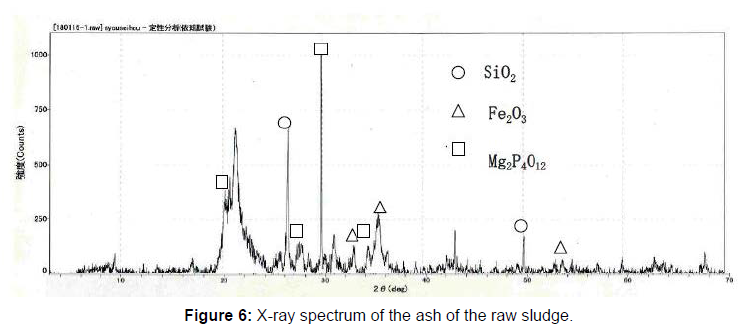
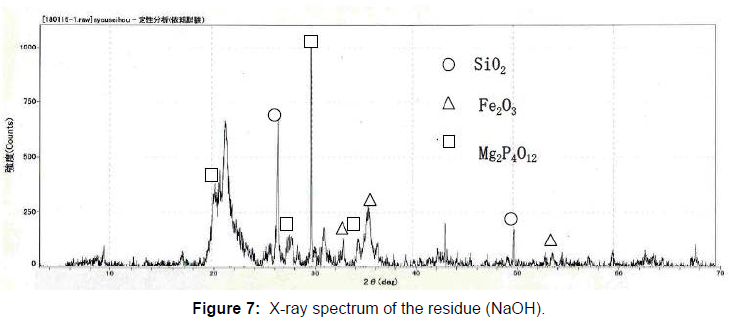
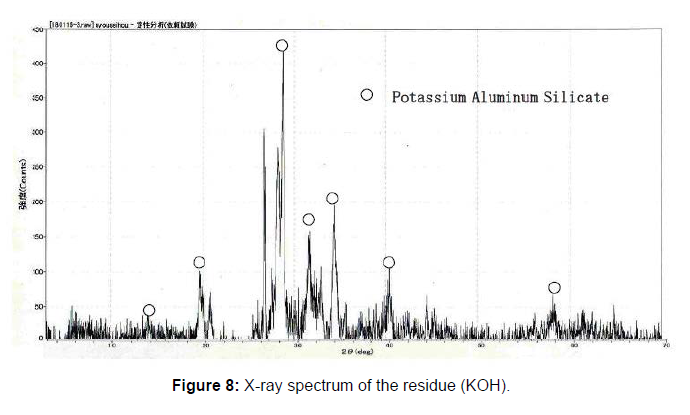
50g of the raw sludge was mixed with each alkali metal compound, and treated as described above, and the chemical compositions of the ash of raw sludge and the residue were analyzed using an X-ray analyzer, and the results are shown in Figure 5. The phosphorus component in the residue was decreased, compared to the ash of raw sludge because of the phosphorus extraction. On the contrary, other components like Fe3O3, SiO2, Al2O3 CaO were almost same. The component of Na2O increased in the case of the NaOH or Na2CO3, and K2O also increased in the case of the KOH. In order to identify the reaction through the phosphorus recovery, X-ray diffraction analysis was carried out on the ash of the raw sludge and the residue using X-ray diffraction analyzer. The X-ray diffraction spectrums are shown in Figure 6-8. The ash of the raw sludge, SiO2 and Fe2O3 component was dominant, on the contrary compounds made of Na,Al,Si or K,Al,Si were found in the residues. Some ash components like SiO2, Al2O3 react with alkali metal compounds and form Zeolite, which is widely known. It is considered that some kinds of chemical reactions happened like the one through the phosphorus recovery processes, and the reactions are enacting an important role.
Future prospect of this method
In order to find out a practical way of phosphorus recovery from the dehydrated sewage sludge, the incineration method of the sludge was investigated. The sludge was mixed with alkali metal compounds, and incinerated at the 750˚C or 900˚C. Phosphorus was recovered by the extraction from the generated ash with the addition of water followed by concentration. The recovery rate of the phosphorus reached about 75%, and the recovered phosphorus was considered to be mainly made of alkali metal phosphate (considered to be sodium phosphate or potassium phosphate) which has many usages, and which can be refined using crystallization techniques. Various kinds of chemical changes were found in the ash components through the phosphorus recovery, which is considered to be related with the phosphorus recovery. Phosphorus recovery from dehydrated sludge is a simple and energy effective way, and considered to be useful. However, there are many matters which will need to be considered before the method can be a practical technique.
Adaptability against various kinds of the raw sludge
The chemical composition of the sludge differ depending of the discharging source, and also chemical state of the phosphorus. The reaction of the phosphorus recovery is considered to be related with the ash components as mentioned above, especially the ratio of the sodium, aluminum and silicon are important matters. Therefore, the recovery rate is considered to differ from where the raw sludge originated, and some adjustment of the method will be needed on accepting the raw sludge.
Incineration facility
In order to the make a practical technology, mass treatment is needed. The sludge has high viscosity, and mixing with large amounts of the alkali reagents is needed. The mixture of the sludge and alkali is incinerated by the furnace, but the furnace should be made of alkali durable materials.
Usage of the ash components
Significant amounts of the residue (generated ash) are habitually discharged. Large amounts of the ash are discharged in Japan, and finding the dumping sites are very difficult, therefore finding out the usage of them is an important matter. Some of them are now used for cement raw material as mentioned above, however, high content of the phosphorus in the cement is considered to lower the cement quality, and the usage will be limited. In order to expand further utilizations, wide utilization of the ash is an important matter. The residue of this method is considered to be compounds of aluminum silicate what chemical and physical properties are not yet investigated, and finding out the usage of the ash is important, so further investigation is needed.
Acknowledgements
The authors express their appreciation to Dr. Shigeo Hayashi (Mie Prefectural Institute of Industrial Technology) for his assistance with the X-ray analysis, and also appreciation to Dr. Eric Bray (Professor of Yokkaichi University) for his advice on making the article and also express their appreciation to Mr. Kenji Takahashi for his assist on the experiment. The authors also express their appreciation to the Hinaga Wastewater Treatment Center (Yokkaichi City Government, Waterworks and Sewage Bureau) offering us the ash.
References
- https://www.npobin.net/research/data/176thTsuchiya.pdf#search=%27%E4%B8%8B%E6%B0%B4%E6%B1%9A%E6%B3%A5%E7%99%BA%E7%94%9F%E9%87%8F%27.
- https://www.jstage.jst.go.jp/article/jriet1972/11/11/11_11_826/_pdf
- http://soil.en.a.u-tokyo.ac.jp/jsidre/search/PDFs/04/0409-18.pdf
- https://www.taiheiyo-cement.co.jp/english/service_product/recycle_mw/index.html
- https://www.eng.nipponsteel.com/english/whoweare/r_and_d/reports/pdf/vol03_14.pdf
- Yosida K, Takahashi Y, Hatano M (2001) The Experiment on the Recovering Sodium Phosphate from Incinerated Ash of Sewage Sludge. In Proceedings of 12th Annual Conferenc
Google Scholar Crossref Indexed at
- Shima H, Takahashi M (1997) Gekkan Water.Gekkan [Mizu] 552: 36-40.
- Nakamura Y, Otsuka M, Haruta S, Omori D (2019) Practical Enrichment of Strains of Sulfur-Oxidizing Bacteria and Economical Medium for Bacteria Leaching. J JSWE42 (4): 145-154.
Google Scholar Crossref Indexed at
- Takahashi M, Kato S, Shima H, Sarai E, IchiokaT, et al. (2001) Technology for Recovering Phosphorus from Incinerated Wastewater Treatment Sludge. Chemosphere 44: 23-9.
Google Scholar Crossref Indexed at
- Ingham J, Ryan J, Keyakida E, Ri J (1996) Phosphorus and Metal Recovery from Sewage Treatment Sludge. In Proceedings of the 7th Annual Conference of the Japan Society of Waste Management Expert 280-282.
Google Scholar Crossref Indexed at
- Naeem A, Mustafa S, Rehana N, Dilara B, Murtaza S (2003) Selective removal of Pb2+ by AlPO4. Environ Technol 24: 779-785.
- Sasaki T, Kudo H, Sato Y, Abe T, Sugawara R (2015) Synthesis of phosphorus fertilizer from sewage sludge ash and alkaline wastewater, assessment of contamination by heavy metals, and evaluation of the characteristics of the fertilizer. Japan J Soil Sci Plant Nutr 86: 290-298.
Google Scholar Crossref Indexed at
- Sato K, Takahashi M, Onari Y, Kato S, Enjyoji H (2004) A Technique for Recovering Sodium Phosphate from Incinerated Ash of Sewage Treatment Sludge by Hydrothermal Synthesis. Transaction of the Material Research Society of Japan 29 (5): 2021-2024.
Google Scholar Crossref Indexed at
- Takahashi M, Takemoto Y, Yuuki E (2020) Phosphorus Transfer in the Ash through Acid or Alkali Extraction Processes. Mater Sci Eng 10 (1-2): 24-29.
- Takahashi M, Talemoto Y, Iwasaki S, Kamemoto K, Iida K, et al. 2020 Phosphorus Recovery from Dehydrated Waste Water Sludge by Incineration Using Alkali Metal Compounds. In Proceedings of the 31st Annual Conference of the Japan Society of Waste Management Expert 199-200.
- Takahashi M, Takemoto Y, Yuuki E (2019) Phosphorus Recovery from Incinerated Ash of Sewage Sludge by Heat Treatment. Mater Sci Eng9 (1-2): 13-6.
- Takahashi M, Takemoto Y, Iwasaki S, Yuuki E (2020) Phosphorus Recovery from the Ash of Sewage Sludge Using NaOH by Heat Treatment-2-Reuse of the Alkali Water Generated by Phosphorus Recovery. Mater Sci Eng 10(4):158-162.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2115
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1606
- PDF downloads: 509