Research Article Open Access
Taxonomic Identification of Hallucinogenic Mushrooms Seized on the Illegal Market Using a DNA-Based Approach and LC/MS-MS Determination of Psilocybin and Psilocin
Veniero Gambaro1, Gabriella Roda1*, Giacomo Luca Visconti1, Sebastiano Arnoldi1, Eleonora Casagni1, Caterina Ceravolo1, Lucia Dell’Acqua1, Fiorenza Farè1, Chiara Rusconi1, Lucia Tamborini1, Stefania Arioli2* and Diego Mora2
1Department of Pharmaceutical Sciences, University of Milan, Via Mangiagalli 25, Milan, Italy
2Department of Food Science and Technology and Microbiology, Via Celoria 2, Milan, Italy
- *Corresponding Author:
- Gabriella Roda
Department of Pharmaceutical Sciences
University of Milan, Via Mangiagalli 25, 20133, Milan, Italy
Tel: 390250319065
E-mail: gabriella.roda@unimi.it
- Stefania Arioli
Department of Food Science and Technology and Microbiology
Via Celoria 2, 20133, Milan, Italy
E-mail: stefania.arioli@unimi.it
Received date: August 25, 2015; Accepted date: September 10, 2015; Published date: September 17, 2015
Citation: Gambaro V, Roda G, Visconti GL, Arnoldi S, Casagni E, et al. (2015) Taxonomic Identification of Hallucinogenic Mushrooms Seized on the Illegal Market Using a DNA-Based Approach and LC/MS-MS Determination of Psilocybin and Psilocin. J Anal Bioanal Tech 6:277. doi:10.4172/2155-9872.1000277
Copyright: © 2015 Gambaro V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The taxonomic identification of mushrooms suspected to contain hallucinogenic active principles was carried out using a DNA-based approach, thus highlighting the usefulness of this approach in the forensic identification of illegal samples also when they are difficult to identify because the morphologic identification is prevented, due to the bad conservation of the vegetable material. To confirm the presence of the illegal active principles, the optimization of a LC/MS-MS method for the qualitativequantitative analysis of psilocin and psilocybin in mushroom samples seized by the judicial authority is described. For the quantitative determination it was necessary to identify and synthesize a proper internal standard (IS, i.e., 5-hydroxy-N,N-diethyltryptamine), endowed with chromatographic features suitable for the analysis of the active principles. LC/MS-MS analysis evidenced that the amount of psilocybin ranged from 0.5 to 1.4% while that of psilocin from 1.3 to 2.5% (w/w), confirming literature data. The concentration of psilocin was higher in the cap and in the distal part of the stem (near to the soil) than in the part of the stem proximal to the cap. On the other hand the concentration of psilocybin was higher in the cap and in the proximal part, being lower in the distal part of the stem.
Keywords
Psilocybin; Psilocin; Hallucinogenic mushrooms; DNAbased identification; LC/MS-MS
Introduction
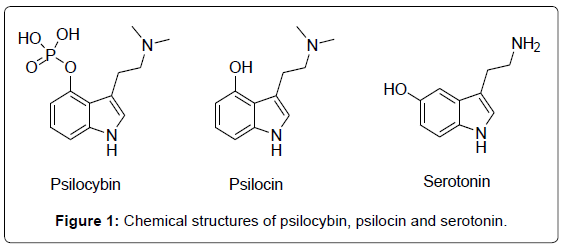
Psychoactive plants or fungi accompanied religious rituals in many ancient civilizations worldwide [1-4]. Hallucinogenic compounds contained in fungi of Psilocybe and Amanita genera have been used as recreational drugs of abuse since the beginning of the 1960s [5,6]. Although hallucinogens are thought to be safe because of relatively low physiologic toxicity and dependence potential [7,8] nowadays the context of hallucinogenic compounds consumption, mainly by young people seeking for unusual experiences, is usually negative [5,6,9]. The psychoactive principles of Psilocybe mushrooms, namely psilocybin and psilocin, were isolated and identified by Hofmann in 1958 [10]. Psilocin and psilocybin are naturally occurring indoles (Figure 1) found in several species of mushrooms [11,12] at concentrations of up to 0.5% m/m and 2% m/m, respectively [13]. Psilocybin is rapidly dephosphorylated to psilocin in vivo [14], with the latter compound being structurally related to the neurotransmitter serotonin (Figure 2) which gives rise to its comparable human metabolism [15]. This molecular similarity endows psilocin with high affinity for serotonin receptors, which blocks the release of the neurotransmitter thus giving rise to hallucinogenic effects [16]. Both psilocin and psilocybin are controlled substances in many countries, as intentional intoxication from these compounds continues to be a major problem in USA and Europe [17]. Gas chromatography cannot be recommended since the poorly volatile and heat-labile psilocybin tends to decompose by loss of the phosphate group during injection. All Liquid Chromatography (LC) methods described so far are based on RP LC [18] with conventional C18 columns. Due to the phosphate group the polarity of psilocybin is much higher compared to psilocin.
Hallucinogenic fungi taxonomically fall into several genera, the most well-known being the genus Psilocybe. Certain species of these genera synthesize psilocin and psilocybin. Since not all members of a given fungal genus contain psilocin or psilocybin, identification of an unknown fungal sample to the species level is required for legal proceedings. The determination of the species requires the analysis of several morphological characteristics. Often the samples seized by the judicial authority are dried, powdered or reduced in small pieces; consequently the morphological identification is difficult. A DNAbased approach ensures the identification of samples in the absence of morphological characteristics [19].
In this paper we describe a DNA-based approach for the taxonomic identification of mushrooms species in order to help the forensic identification of illegal samples found on the illicit market, especially when the morphological characteristics cannot be examined. Moreover the optimization of a LC/MS-MS method for the quali-quantitative analysis of psilocybin and psilocin in mushroom samples seized by the judicial authority was carried out. For the quantitative determination it was necessary to identify and synthesize a proper internal standard (IS, i.e., 5-hydroxy-N,N-diethyltryptamine), endowed with chromatographic features suitable for the analysis of the active principles.
Materials and Methods
Vegetable material
Analyses were carried out on four mushrooms seized by the judicial authority at the Malpensa airport in Northern Italy in February 2014. The weights and lengths of the vegetable materials are reported in Table 1.
| Mushroom | Weight (g) | Length (cm) |
|---|---|---|
| 1 | 2.8 | 10.0 |
| 2 | 2.2 | 9.3 |
| 3 | 2.0 | 8.2 |
| 4 | 1.3 | 6.9 |
Table 1: Characteristics of the vegetable material.
DNA extraction from growth substrate and fungi
Hundred mg of mushrooms were processed for DNA extraction by means of Ultra CleanTM Microbial DNA Isolation Kit (Mo Bio, Canada), according to manufacturer’s instructions. After DNA quantification with a Smart SpecTM Plus Spectrophotometer (Bio-Rad Laboratories, Milan, Italy) and evaluation of 260/280 ratio, 50 ng of DNA were used for species identification based on Internal Transcribed Region located between the 18S- and the 28S rDNA genes as previously well acknowledged for its ability to identify yeast to the species level [20]. The amplification was performed in a final volume of 25 μl, containing 0.5 μM of each primer (NL1: GCATATCAATAAGCGGAGGAAAAG; and NL4: GGTCCGTGTTTCAAGACGG) [20], 1 unit of DreamTaq (Fermentas, Thermo Scientific, Italy) and 50-70 ng of DNA. The reactions were performed in an automatic thermal cycler (EppendorfMastercycler, Italy) under the following conditions: initial denaturation at 94°C for 3 min; 36 cycles of 94°C for 2 min, 52°C for 1 min, 72°C for 2 min; final extension at 72°C for 7 min, holding at 4°C. The amplified products were then purified by means of UltraCleanTM PCR Clean-up DNA purification kit (Mo Bio, Canada) and finally sequenced by using primer NL1. The sequences were analyzed by use of the software Chromas2.33 (Technelysium Pty Ltd, South Brisbane, Australia) and compared to the sequences reported in the GenBank using the BLAST algorithm.
Reagents and standards
Psilocybin 1.0 mL (100 μg/mL) in methanol was purchased by Grace (Illinois, USA); Psilocin 99.3%, 1.0 mL (5 mg/mL) in methanol was from Cerilliant, (Texas, USA); 5-benzyloxytryptamine hydrochloride and all the other reagents and solvents of analytical grade were supplied by Sigma Aldrich (St. Louis, USA) and were stored as required by the manufacturer. Water (18.2 MΩ cm) was prepared by a Milly-Q System (Millipore, France).
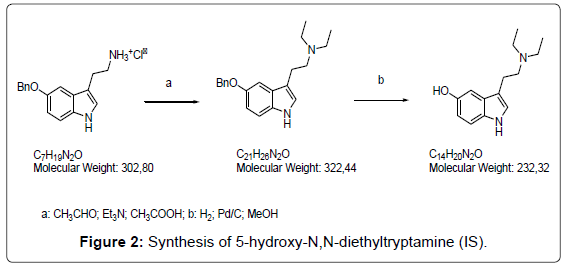
Synthesis of 5-hydroxy-N,N-diethyltryptamine
a) Triethylamine (68 μL, 0.49 mmol) was added to a suspension of 5-benzyloxytriptamine hydrochloride (150 mg, 0.49 mmol) in anhydrous methanol (7 mL) under an inert atmosphere. The mixture was cooled at 0°C and glacial acetic acid was added (112 μL, 1.96 mmol). A solution of acetaldehyde (66 μL 1.18 mmol) in methanol (7 mL) was slowly dropped (10 min). The mixture was stirred at room temperature for 2 hr until the complete transformation of the starting product (TLC: eluent: dichloromethane/methanol 8:2). The pH was adjusted to 8-9 by a 2M solution of sodium hydrogencarbonate and methanol was evaporated. The aqueous solution was extracted with dichloromethane (3 × 10 mL) and the combined organic phases were washed with water (1 × 10 mL) and brine (1 × 10 mL) and dried with anhydrous sodium sulfate. The solvent was removed under vacuum and the residue purified by a flash silica gel chromatography (eluent: dichloromethane/methanol 9:1), obtaining a pale yellow oil (86 mg, 55% yield).
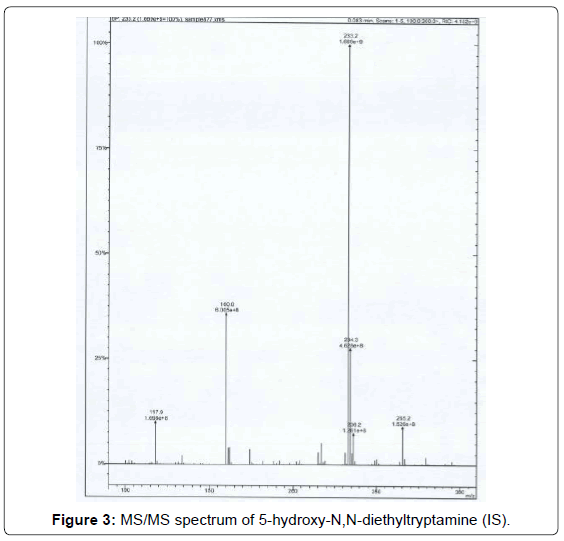
b) 5-benzyloxy N,N-diethyltriptamine (86 mg, 0.26 mmol) was dissolved in methanol (2 mL) and a catalytic amount of Pd/C 10% (8.7 mg) was added. The mixture was stirred under a hydrogen atmosphere for 24 hr and then filtered on celite. The solvent was evaporated and the residue purified by a flash silica gel chromatography (eluent: dichloromethane/methanol 8:2), obtaining a pale yellow powder (40 mg, 63% yield). In Figure 3 the MS/MS spectrum of the target compound is reported.
Preparation of the samples
Each mushroom was divided into three parts: cap, proximal part of the stem respect to the cap and distal part of the stem. From each part 100 mg for each mushroom (total 400 mg) were withdrawn and extracted with 4 mL of methanol by maceration overnight. The mixture was sonicated for 10 min and the supernatant filtered on a 0.45 μm membrane. Each extract was analyzed in triplicate adding to 300 μL of the extract 300 μL of a IS solution (10 μg/mL).
LC/MS-MS analyses
Experiments, carried out with a liquid chromatography system coupled to a mass detector, were performed on a LC-320 with an Electron Spray Ionization (ESI) source and a 320-MS triple quadrupole mass spectrometer, equipped with two 212 LC chromatographic pumps and a 410 tray cooled autosampler (Varian, Santa Clara, CA, US). The system was managed by MS Workstation software Version6.9.1 (Varian, Santa Clara, CA, US). The ESI-triple quadrupole mass spectrometer was set to perform collision induced dissociation experiments in positive ionization mode, using argon as collision gas. Particularly, Multiple Reaction Monitoring (MRM) experiments were conducted by the continuous injection at a rate of 20 μL/min of the standards of interest (2 μg/mL) into the mass spectrometer set in positive ionization mode, in methanol solutions. In order to enhance ion formation and to improve conductivity, several proves were performed to optimize ESI parameters and several proves were carried out to improve chromatographic conditions. The method was optimized for the research of the ions of interest, for example, the specific MRM transitions of each standard. In detail, once recognized the molecular ion of each standard (M + H)+, MRM experiments were performed to study the characteristic fragmentation pattern of the analytes (Table 2). ESI source settings and mass spectrometer parameters used for compound identification were Needle Voltage: +5000 V; Shield Voltage: +600 V; Nebulizing Gas (N2) Pressure: 40.00 psi; Drying Gas (N2) Pressure: 40.00 psi; Drying Gas (N2) Temperature: 100°C; Q0 Offset: +3.0 V; L4 Offset: +2.000 V; Housing Temperature: 50°C; CID Gas (Ar); Pressure: 2.00 mTorr; Electron multiplier: 1750.0 V; Scan time: 4.0 sec; Dwell time: 0.1 sec.
| Compound | Q1first mass | Q3first mass | Capillary (V) | Collision Energy (V) |
|---|---|---|---|---|
| Psilocin | 205.2 | 58.1 | 35.209 | 11.0 |
| 205.2 | 115.0 | 35.209 | 34.5 | |
| 205.2 | 160.1 | 35.209 | 14.0 | |
| 205.2 | 205.2 | 35.209 | 3.5 | |
| Psilocybin | 285.2 | 115.0 | 60.084 | 25.5 |
| 285.2 | 205.2 | 60.084 | 14.0 | |
| 285.2 | 240.0 | 60.084 | 15.0 | |
| 285.2 | 245.2 | 60.084 | 3.5 | |
| IS | 233.2 | 160.2 | 50.046 | 16.5 |
| 233.2 | 233.2 | 50.046 | 4.5 |
Table 2: MRM transitions of each analyte and IS.
Chromatographic conditions
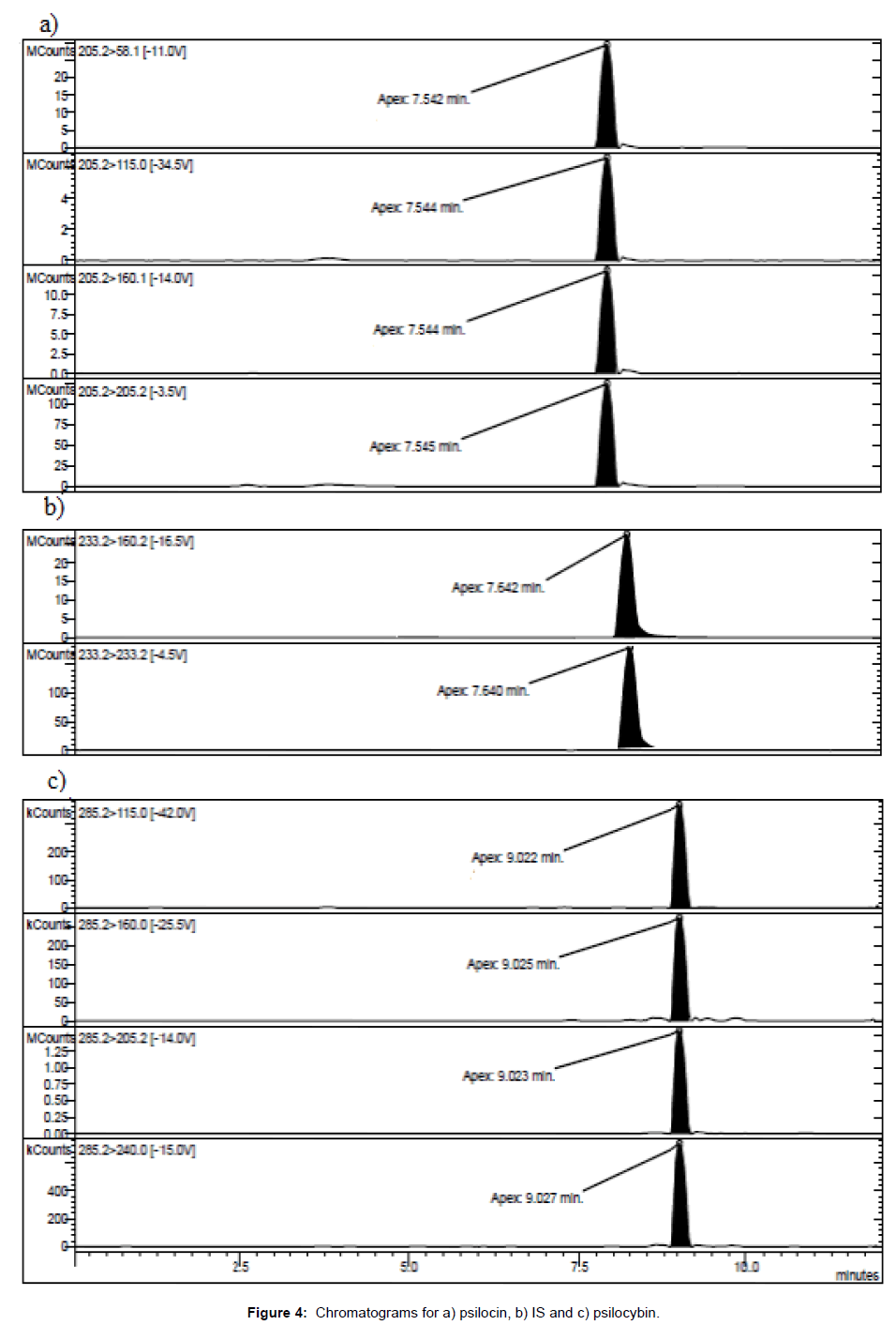
Chromatographic column: Kinetex HILIC 150 × 3.0 mm i.d. particle size 2.6 μm; pore dimension 100 A (Phenomenex™, Castel Maggiore, Italy); pre-column: Security Guard Cartridges C18 4 × 2.0 mm (Phenomenex™, Castel Maggiore, Italy); column temperature: 40°C; manifold temperature: 42°C; housing temperature: 50°C; flow rate: 200.0 μL/min; injection volume: 10 μL; injection mode: partial loop fill; solvent A: 0.1% acetic acid adjusted to pH 6 with ammonia; Solvent B: acetonitrile; mobile phase: solvents were filtered under vacuum on 0.45 μm membrane filters and degassed by immersion in ultrasonic bath for 15 minutes before column conditioning; linear gradients: 0.0-4.0 min, 85% B; 4.0-7.0 min, 85-40% B; 7.0-9.3 min 40- 85% B; 9.3-12.0 min 85% B. Retention times: psilocin 7.5 ± 0.2 min; psilocybin 9.0 ± 0.2 min.
Linearity of the analytical method
To evaluate the linearity of the analytical method standard solutions of psilocin were analyzed in an interval of concentrations from 10 μg/ mL and 100 μg/mL. In this range five solutions of non-sequential concentrations (10, 20, 40, 80, 100 μg/mL) were analyzed (n=5). The linearity equation was y=0.0139x+0.1501 with a good correlation coefficient (R²=0.9981).
To evaluate the linearity of the analytical method standard solutions of psilocybin were analyzed in an interval of concentrations from 0.625 μg/mL and 10 μg/mL. In this range five solutions of non-sequential concentrations (0.625, 1.25, 2.5, 5.0, 10 μg/mL) were analyzed (n=5). The linearity equation was y=0.0012x+0.0003 with a good correlation coefficient (R²=0.9958).
Results and Discussion
When dealing with mushrooms it is very useful to assess the genus and species of the biological materials because also the fungi are inserted in the illicit substance list. Sometimes samples seized by the judicial authority are dried or powdered and the morphological characteristics are not recognizable. Therefore an unambiguous identification by a phylogenetic approach could be very helpful, especially in the cases in which active principles could be degraded due to an incorrect storage of the vegetable material. In this context, total DNA directly extracted from small fractions of suspected hallucinogenic mushroom was used in a PCR assay in order to amplify the internal transcribed region between the 18S- and the 28SrDNA gene, and the PCR fragment obtained was subjected to sequence analysis. From all the four samples tested, sequence analysis and comparison allowed to unambiguously identify the biological materials present in the culture medium as belonging to the hallucinogenic species Psilocybe mexicana (Accession number LN830951). These results, highlighted that a molecular-based approach, characterized by a total DNA extraction from suspected culture media, amplification and sequence analysis of a discrete region of the 28S rDNA gene, could be useful to disclose the presence of hallucinogenic mushrooms even if the size of the biological material is too small to allow a morphological identification.
A suitable LC/MS-MS method was developed and tested for the analysis of the active principles of mushroom seized on the illicit market, suspected to belong to the genus Psilocybe.
For the quantitative determination it was necessary to identify and synthesize a proper internal standard (IS, i.e., 5-hydroxy-N,Ndiethyltryptamine), endowed with chromatographic features suitable for the analysis of the active principles (Figure 4).
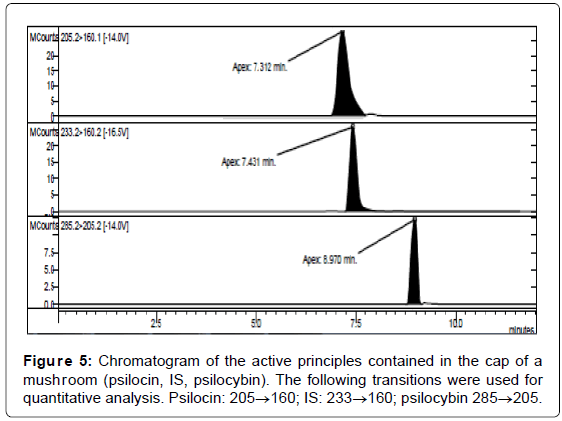
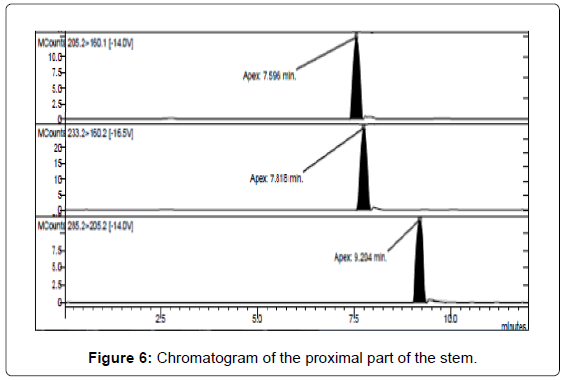
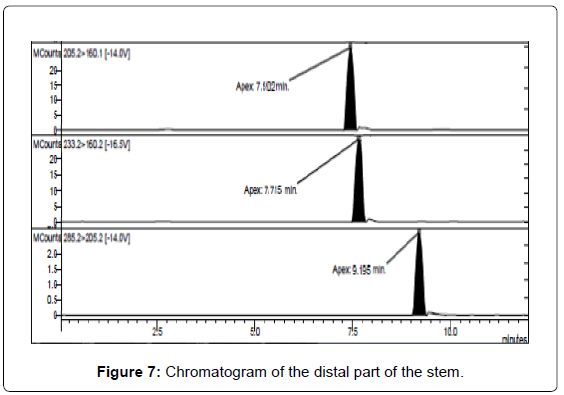
LC/MS-MS analysis of the three extracts corresponding to the three different parts of the mushrooms gave the results reported in Table 3. The concentration of psilocybin ranged from 0.5 to 1.4% while that of psilocin from 1.3 to 2.5%, confirming literature data [13]. It is possible to note that the concentration of psilocin is higher in the cap and in the distal part of the stem (near to the soil) than in the part of the stem proximal to the cap. On the other hand the concentration of psilocybin is higher in the cap and in the proximal part, being lower in the distal part of the stem (Figures 5-7). Analyses gave more reproducible results for psilocin as evidenced by the standard deviations.
| Psilocin | Psilocybin | ||||
|---|---|---|---|---|---|
| Sample | mg/mL | % (w/w) | mg/mL | % (w/w) | |
| Cap | 1 | 2.14 | 2.1 | 0.83 | 0.8 |
| 2 | 2.40 | 2.4 | 1.44 | 1.4 | |
| 3 | 2.37 | 2.3 | 1.86 | 2.0 | |
| SD | 0.14 | 0.52 | |||
| Proximal stem | 1 | 1.65 | 1.6 | 1.40 | 1.4 |
| 2 | 1.30 | 1.3 | 0.97 | 1.0 | |
| 3 | 1.36 | 1.3 | 1.39 | 1.3 | |
| SD | 0.19 | 0.25 | |||
| Distal stem | 1 | 2.54 | 2.5 | 0.50 | 0.5 |
| 2 | 2.48 | 2.4 | 0.68 | 0.6 | |
| 3 | 2.57 | 2.5 | 0.50 | 0.5 | |
| SD | 0.046 | 0.10 | |||
Table 3: Concentrations of the active principles.
Conclusion
The taxonomic identification of the biological material contained in the hallucinogenic mushrooms culture media, was carried out using a DNA-based approach, thus highlighting the usefulness of this approach in the forensic identification of illegal samples.
Moreover a LC/MS-MS method for the quali-quantitative analysis of psylocibin and psilocin was proposed and a proper internal standard (5-hydroxy-N,N-diethyltryptamine) was synthesized for the quantitative determination of the analytes in mushroom samples seized by the judicial authority.
References
- Crundwell E (1987) The unnatural history of the fly agaric. Mycologist 21:178-181.
- Furst PT (2004) Visionary plants and ecstatic shamanism. Expedition 46:26-29.
- Ott J (1976) Psycho-mycological studies of Amanita-from ancient sacramentto modern phobia. J Psychoactive Drugs8:27-35.
- Sessa BSJ (2008) Are psychedelic drug treatments seeing a comeback in psychiatry? Prog Neurol Psychiatry 12:5-10.
- Kuttner RE, Hickey RE (1970) Culture and perception: a note on hallucinogenic drugs. J Natl Med Assoc 62: 25-26.
- Pavarin RM (2006) Substance use and related problems: a study on the abuse of recreational and not recreational drugs in Northern Italy. Ann Ist Super Sanita 42: 477-484.
- Johnson M, Richards W, Griffiths R (2008) Human hallucinogen research: guidelines for safety. J Psychopharmacol 22: 603-620.
- van Amsterdam J, Opperhuizen A, van den Brink W (2011) Harm potential of magic mushroom use: a review. Regul Toxicol Pharmacol 59: 423-429.
- Riley SC, James C, Gregory D, Dingle H, Cadger M (2001) Patterns of recreational drug use at dance events in Edinburgh, Scotland. Addiction 96: 1035-1047.
- Hofmann A, Heim R, Brack A, Kobel H (1958) Psilocybin, a psychotropic substance from the Mexican mushroom Psilicybe mexicana Heim. Experientia 14: 107-109.
- Horita A, Weber LJ (1961) The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochem Pharmacol 7: 47-54.
- Horita A, Weber LJ (1962) Dephosphorylation of psilocybin in the intact mouse. Toxicol Appl Pharmacol 4: 730-737.
- Pedersen-Bjergaard S, Sannes E, Rasmussen KE, Tønnesen F (1997) Determination of psilocybin in Psilocybe semilanceata by capillary zone electrophoresis. J Chromatogr B Biomed Sci Appl 694: 375-381.
- Campbell A (2001) The Australian Illicit Drug Guide. Black Inc., Melbourne, Australia.
- Helsley S, Fiorella D, Rabin RA, Winter JC (1998) A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus. Prog Neuropsychopharmacol Biol Psychiatry 22: 649-663.
- Vollenweider FX, Geyer MA (2001) A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull 56: 495-507.
- Borowiak KS, Ciechanowski K, Waloszczyk P (1998) Psilocybin mushroom (Psilocybe semilanceata) intoxication with myocardial infarction. J Toxicol Clin Toxicol 36: 47-49.
- Stebelska K (2013) Fungal hallucinogens psilocin, ibotenic acid, and muscimol: analytical methods and biologic activities. Ther Drug Monit 35: 420-442.
- Nugent KG, Saville BJ (2004) Forensic analysis of hallucinogenic fungi: a DNA-based approach. Forensic Sci Int 140: 147-157.
- Jespersen L, Nielsen DS, Hønholt S, Jakobsen M (2005) Occurrence and diversity of yeasts involved in fermentation of West African cocoa beans. FEMS Yeast Res 5: 441-453.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 17795
- [From(publication date):
December-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 13045
- PDF downloads : 4750