Targeting Soluble Epoxide Hydrolase for Temporal Lobe Epilepsy
Received: 08-Jun-2016 / Accepted Date: 05-Jul-2016 / Published Date: 11-Jul-2016
Abstract
Epilepsy is a common brain disorder characterized by a chronic predisposition to generate spontaneous seizures. It remains unknown regarding the mechanisms for epilepsy formation. Prolonged seizures trigger significant neuroinflammatory responses in experimental and human temporal lobe epilepsy, which have been speculated to be mediating the epileptogenesis. Currently, extensive evidence suggests that anti-inflammatory approaches might offer a promising alternative therapeutic strategy especially when antiepileptic medications are ineffective. By diminishing the anti-inflammatory activities of epoxyeicosatrienoic acids (EETs), soluble epoxide hydrolase (sEH) has been considered a potential therapeutic target for epileptic seizures. Here we review evidences concerning the relevance of neuroinflammarion to the pathophysiology of epilepsy, the physiological functions of EETs-sEH metabolism in central nervous system, and the linkage of EET-sEH pathway to neuroinflammation and neuromodulation. Several contemporary studies have contributed to defining the importance of sEH inhibition in modulating inflammatory responses and abnormal hyperexcitability related to epilepsy. Notwithstanding some discrepancies have been noted between different experimental models or between pharmacological inhibition and genetic deletion of sEH, the involvement of sEH in the generation and progression of epilepsy suggests that sEH may be an attractive target for therapeutic intervention.
Keywords: Soluble epoxide hydrolase; Epoxyeicosatrienoic acids; Epilepsy; Neuroinflammation; Brain hyperexcitability
5516Introduction
Temporal lobe epilepsy (TLE), one of the most prevalent neurological syndromes, is the episodes of abnormal excessive and synchronous electrical discharges occurring in temporal lobe of the brain. Medical intractability is an important clinical problem in TLE. Further surgical treatment is needed for the patients with TLE who still have seizure attacks under adequate medications [1]. Although numerous possible mechanisms underlying epileptogenesis have been addressed, there remains still largely unknown about how to successfully treat the pathogenesis of epilepsy.
More recent studies draw attention to the contributions of brain inflammation and immune processes in the progression of epilepsy. Accumulating evidence suggests that both innate and adaptive immune responses activated by seizures contribute to the generation and progression of epilepsy and to epileptogenic pathologies [2-4]. Epileptic pathological alterations, comprising neuronal cell death, aberrant reactive gliosis, axonal sprouting [5], enhanced neurogenesis [6] in hippocampal formation and the dysfunction of blood brain barrier [7], that leading to network hyperexcitability and decreased seizure threshold, have been proposed to be associated with inflammatory reaction [3,8], implying an etiological role of neuroinflammation for central pathogenesis in epileptogenesis and ictogenesis [9,10]. Accordingly, further understanding of the mechanisms underlying epileptogenesis-related inflammatory reactions is paramountly important for the development of therapeutic strategies against epilepsy.
Recently, anti-inflammatory approaches to epilepsy management have been considered as a promising alternative therapeutic strategy when antiepileptic medications are ineffective [11,12]. Numerous anti-inflammatory targets for epilepsy prevention and treatment have been demonstrated in both clinical and experimental studies [13-15]. Some specific anti-inflammatory drugs coupled with anticonvulsant activity demonstrated in experimental studies, such as inhibitors of interleukin-1β (IL-1β)-converting enzyme /caspase-1 and antagonists of IL-1β receptors [16], cyclooxygenase-2 (COX-2) inhibitors [17] as well as antagonists of toll-like receptors [18,19], may have therapeutic potential in proinflammatory processes in the epileptic brain [20].
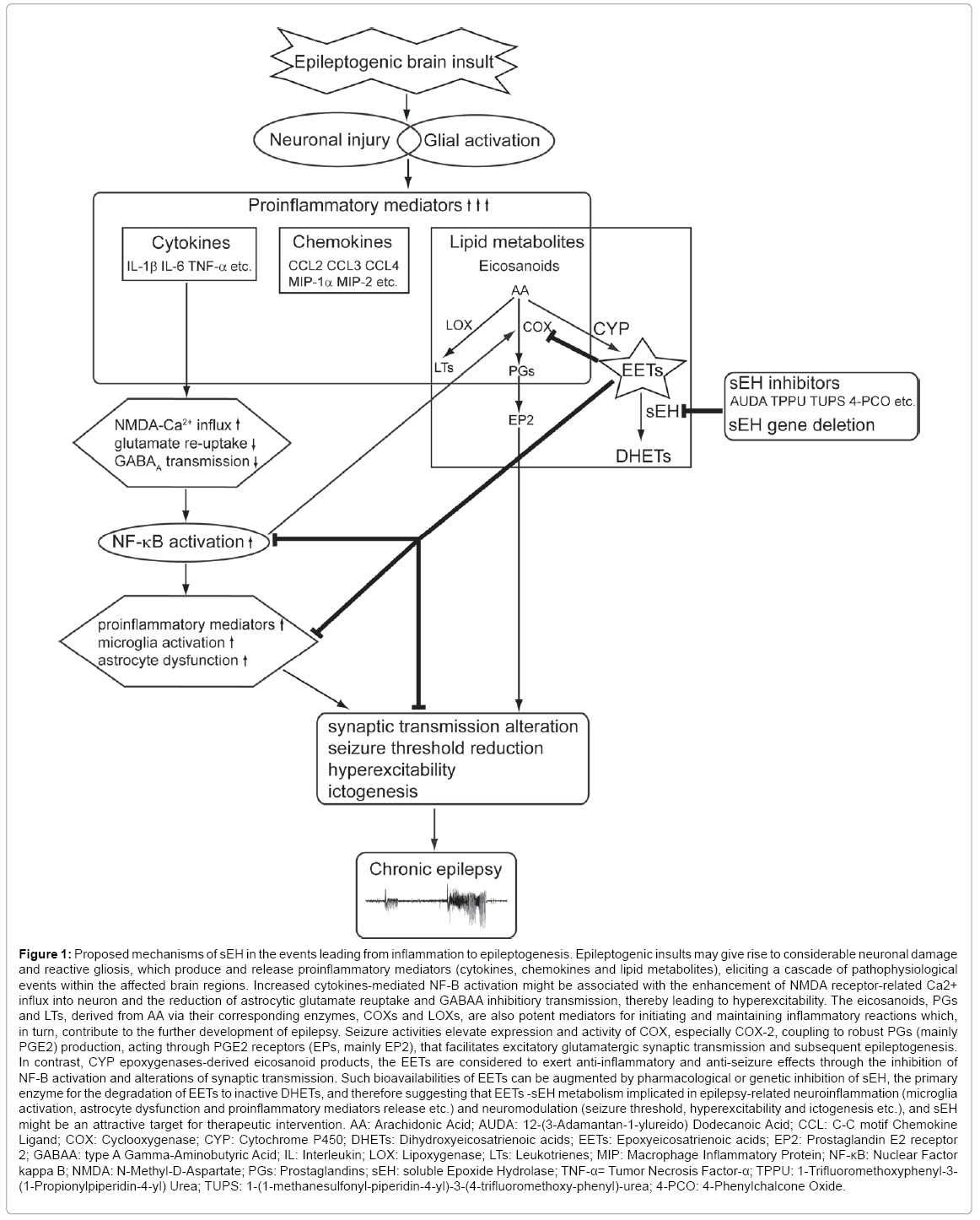
A potential target enzyme for anti-inflammatory treatment of epilepsy, soluble epoxide hydrolase (sEH), has been characterized to participate in regulating inflammatory responses and brain excitability in epileptic animal models in current studies [21-23]. sEH, a phase I xenobiotic metabolizing enzyme broadly distributed in mammalian tissues [24,25], is the major enzyme in the metabolic conversion and degradation of anti-inflammatory epoxy-fatty acid to their corresponding inactive dihydroxy-fatty acid [26]. Inhibition of sEH enzymatic activity either by pharmacological inhibitors or genetic deletion stabilizes bioactivity of the arachidonic acid (AA) derived epoxyeicosatrienoic acids (EETs) and therefore enhances their anti-inflammatory and neuroprotective effects [27-29]. In addition, recent evidences indicate that sEH and EETs appear to be involved in modulating neuronal activity [30], synaptic neurotransmission [31] and axon outgrowth [32], which are germane to the development of epilepsy [5,33]. In this review, the recent advances are summarized with emphasis on the fundamentally mechanisms of sEH-dependent EETs metabolic pathway in neuroinflammation and abnormal hyperexcitability in epilepsy (Figure 1).
Figure 1: Proposed mechanisms of sEH in the events leading from inflammation to epileptogenesis. Epileptogenic insults may give rise to considerable neuronal damage and reactive gliosis, which produce and release proinflammatory mediators (cytokines, chemokines and lipid metabolites), eliciting a cascade of pathophysiological events within the affected brain regions. Increased cytokines-mediated NF-B activation might be associated with the enhancement of NMDA receptor-related Ca2+ influx into neuron and the reduction of astrocytic glutamate reuptake and GABAA inhibitiory transmission, thereby leading to hyperexcitability. The eicosanoids, PGs and LTs, derived from AA via their corresponding enzymes, COXs and LOXs, are also potent mediators for initiating and maintaining inflammatory reactions which, in turn, contribute to the further development of epilepsy. Seizure activities elevate expression and activity of COX, especially COX-2, coupling to robust PGs (mainly PGE2) production, acting through PGE2 receptors (EPs, mainly EP2), that facilitates excitatory glutamatergic synaptic transmission and subsequent epileptogenesis. In contrast, CYP epoxygenases-derived eicosanoid products, the EETs are considered to exert anti-inflammatory and anti-seizure effects through the inhibition of NF-B activation and alterations of synaptic transmission. Such bioavailabilities of EETs can be augmented by pharmacological or genetic inhibition of sEH, the primary enzyme for the degradation of EETs to inactive DHETs, and therefore suggesting that EETs -sEH metabolism implicated in epilepsy-related neuroinflammation (microglia activation, astrocyte dysfunction and proinflammatory mediators release etc.) and neuromodulation (seizure threshold, hyperexcitability and ictogenesis etc.), and sEH might be an attractive target for therapeutic intervention. AA: Arachidonic Acid; AUDA: 12-(3-Adamantan-1-ylureido) Dodecanoic Acid; CCL: C-C motif Chemokine Ligand; COX: Cyclooxygenase; CYP: Cytochrome P450; DHETs: Dihydroxyeicosatrienoic acids; EETs: Epoxyeicosatrienoic acids; EP2: Prostaglandin E2 receptor 2; GABAA: type A Gamma-Aminobutyric Acid; IL: Interleukin; LOX: Lipoxygenase; LTs: Leukotrienes; MIP: Macrophage Inflammatory Protein; NF-κB: Nuclear Factor kappa B; NMDA: N-Methyl-D-Aspartate; PGs: Prostaglandins; sEH: soluble Epoxide Hydrolase; TNF-α= Tumor Necrosis Factor-α; TPPU: 1-Trifluoromethoxyphenyl-3- (1-Propionylpiperidin-4-yl) Urea; TUPS: 1-(1-methanesulfonyl-piperidin-4-yl)-3-(4-trifluoromethoxy-phenyl)-urea; 4-PCO: 4-Phenylchalcone Oxide.
Neuroinflammation in epilepsy
Neuroinflammation is an underlying component of a diverse range of neurodegenerative diseases and their associated neuropathology, which has been identified in epilepsy-related tissue from both experimental and clinical evidence [34,35]. Significant inflammatory responses triggered by seizures are robust glial activation and the release of multiple inflammatory mediators, including cytokines, chemokines and lipid metabolites from injured neurons and reactive glial cells [10,36], triggering the pro-inflammatory signaling cascades around the affected brain regions [8,37-42].
There have been a considerable number of studies addressed that the proinflammatory cytokines, IL-1β, interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are major inflammatory factors implicated in epilepsy [9,43,44]. Pronounced overexpression of proinflammatory cytokines has been observed in patients and experimental models with epilepsy [9,39], and is considered to be pro-epileptogenic in large variety of epileptic animal models [43].
IL-1β exerts proconvulsive effects via enhancement of N-methyl-D-aspartate (NMDA) receptor-mediated Ca2+ influx into neuron and inhibition astrocytic glutamate reuptake [45], which potentiate excitotoxicity [46] and seizure generation [47]. IL-1β also blocks type A gamma-aminobutyric acid (GABAA) receptor function in hippocampal neurons, thus possibly reducing inhibitory transmission that leading to hyperexcitability [48].
The action of IL-6 and TNF-α is complex and their influence on seizures has been addressed to be either pro-convulsive or anti-convulsive, based on the experimental designs and epilepsy models [49-56]. Increased seizure susceptibility has been reported in mice with IL-6 gene deletion [53]. It has also been demonstrated that neonatal rats treated with IL-6 display delayed onset of hyperthermia-induced convulsions and decreased seizure severity [57], supporting an anticonvulsant role of this cytokine. Moreover, proconvulsant action of IL-6 in the initiation and propagation of seizures has been described in pentylenetrtrazol (PTZ)-induced generalized seizures [58] and in transgenic mice with IL-6 overexpression [52].
Implication of TNF-α in the modulation of neuronal activity was confirmed by in vitro and in vivo models of neuronal hyperexcitability and excitotoxicity. TNF-α induces rapid recruitment of α-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and endocytosis of GABAA receptors, enhancing AMPA-dependent excitatory postsynaptic current and decreasing GABAA transmission, which may exacerbate excitotoxic damage of hippocampal pyramidal cells [59,60]. In amygdala-kindled rats, the duration of epileptiform discharges was increased after 5.0 mg/kg TNF-α administration [50]. In contrast, relative low dose of TNF-α (2.5 and 15.0 pmol/25 g mouse) intrahippocampal injected into mice potently attenuated seizures [49]. Similar to IL-6, TNF-α possesses dichotomous properties in epilepsy [9,44,61], which may be derived from either its concentrations [62] or two distinct TNF-signaling pathways mediated by its different receptor in the brain, p55 and p75 [63]. Studies using TNF-α receptor knockout mice demonstrated that the potentiation of seizure activity and excitotoxicity by TNF-α involves p55 receptor, whereas the anticonvulsant activity was mediated by p75 receptor [49,64]. In Shigella-mediated seizures, TNF-α at low concentrations is proconvulsive, but exerts an anticonvulsive effect at higher concentrations [51], which may be associated with the activation of p55 and p75 pathway, respectively [44,65].
In inflamed brain, non-esterified arachidonic acid (AA) is released from phospholipids and converts to corresponding bioactive eicosanoids, including prostaglandins (PGs), leukotrienes, and EETs by COXs, lipoxygenases and cytochrome P450 (CYP) epoxygenases, respectively [66-68]. In the central nervous system (CNS), eicosanoids have been found to express in neurons, astrocytes, cerebral vascular endothelial cells and cerebrospinal fluid and to participate in synaptic function, cerebral blood flow (CBF) regulation, apoptosis, angiogenesis, and gene expression under physiological conditions [27,69-71]. They also play a crucial role for initiating and maintaining the inflammatory responses in neurological disorders [66].
In addition to pro-inflammatory cytokines, up-regulation of COX-2, the key enzyme required for PGs biosynthesis, has been shown to contribute to the mechanisms leading to neuroinflammation, neuronal damage and aberrant neurogenesis in brain regions susceptible to seizures and epilepsy [8,39,72-80]. Manipulation of COX-2 activity with inhibitors or genetic deletion has been shown either to attenuate or exacerbate epileptogenesis [74,76,80,81], which likely depend on the different profiles of PGs production and their specific actions in the models of seizure and epilepsy [17,66].
PGE2 is one of the key downstream products of COX-2 in the brain, which has been recognized as a pro-epileptogenic factor via the activation of its G protein-coupled receptor EP2 [82-85]. Robust COX-2-coupled PGE2 production following seizures not only facilitates inflammatory processes but also modulates synaptic transmission [86] that may reduce seizure threshold and lead to subsequent epileptogenesis [87]. For example, electroencephalography recordings of rats with methylmalonate-induced seizures displayed short latency for seizure onset and high amplitude of spikes after intraventricular injection of PGE2, indicating a role for PGE2 in potentiating ictogenesis [88]. Furthermore, blockade of interaction of PGE2 and EP2 alleviated neuronal injury, brain inflammation and seizure severity caused by pilocarpine-induced status epilepticus (SE) [84,85]. These evidences point to a role for the interaction of COX-2 - coupled PGE2 and EP2 in epileptogenesis, and therefore targeting the downstream effector molecules in COX-2 signaling cascade might serve as alternative therapeutic approaches for epilepsy [10,17,88,89].
Epoxyeicosatrienoic acids and soluble epoxide hydrolase metabolism in the brain
In contrast to the proinflammatory eicosanoids synthesized from AA by acting through COXs and lipoxygenases, CYP epoxygenases-derived eicosanoid products, the EETs are anti-inflammatory [90,91], which confer several important biological effects to the vascular, neuronal and renal system [25,92]. Recent evidences have suggested that EETs signaling may play distinctly different roles in CNS function compared to that of peripheral tissues [27,92,93]. Expending beyond the functions in the regulation of the cerebral vasculature, EETs have been demonstrated to modulate neuronal pain processing in the brainstem [94], to suppress the ischemia-evoked inflammatory responses in the brain circulation [95], to exert the neuroprotective effects that alter microglia activation during ischemic injury and excitotoxicity [23,96], to regulate neuropeptide and neurotransmitter release [97,98], as well as to affect synaptic neurotransmission and plasticity [31,32,99]. The EETs are predominately catalyzed by sEH to dihydroxyeicosatrienoic acids (DHET) with reduced biological activity, thus inhibition of sEH has been considered as a potential approach for enhancing the bioavailability and beneficial effects of EETs [26,93,100,101].
sEH is a bifunctional enzyme, which has been shown to hydrolyze EETs to DHET with a C-terminus hydrolase domain and involve in the biosynthesis of cholesterol and protein isoprenylation via an Nterminus lipid phosphatase domain [102]. Immunolocalization of sEH in the human and rodent brain has been described in non-vascular and vascular regions, with region-specific and cell-specific differences [98,103,104]. The expression of sEH has been noted in the soma and processes of neuronal cells, oligodendrocytes, and astrocytes [98,105-107] in the brain parenchyma, as well as in the vascular smooth muscle cells of arterioles and microvessels in the brain vasculature [104], suggesting that sEH might implicate in EETs-mediated multiple neuronal functions and cellular signaling throughout the brain in addition to the link of cerebral vascular function [103].
The significance of sEH related epoxyeicosanoid pathway in cerebral circulation has been established in a number of studies [27,93]. Within the cerebral circulation, EETs are derived from vascular endothelium and astrocyte [32,108], which contributes to CBF regulation via neurovascular coupling [28,109]. Endothelial EETs exert the vasomotor effects through interacting with vascular smooth muscle transient receptor potential vanilloid-4 (TRPV4) channels that elicit Ca2+-sparks and then activate large conductance, Ca2+-activated K+ (BKCa) channel, resulting in hyperpolarization and thus vasorelaxion [110,111]. Moreover, astrocytic EETs are released from astrocytic endfeet abutting cerebral capillaries and vessels, modulating neuronal activity that coupling to local regulation of CBF [112]. Accordingly, elevations in brain EETs levels offer beneficial effects on cerebrovascular diseases [113-115] and several studies focus on the implications of sEH epoxide hydrolase activity in the vascular function and non-vascular neuroprotctive and anti-inflammatory effects as well as the pharmacological and genetic manipulations of sEH for disease treatment [29,106,114,116].
Moreover, emerging evidence shows that EETs- sEH pathway in the brain exerts a variety of biological functions other than neurovascular modulation and protective effects on ischemic injury [27,93,117]. Various impacts of sEH in the brain have been documented, ranging from the modulation of neurophysiological and neuroimmunological properties to the involvement of the pathogenesis of several neurological diseases [21-23,31,32,92,96,118,119].
Recently, Ren et al. reported that both genetic and pharmacological inhibition of sEH confers resilience to repeated social defeat stress [118]. They found that the potent sEH inhibitor, 1- trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU), not only enhanced neuronal plasticity associated with depression in vitro but also displayed rapid antidepressant effects in both inflammation and social defeat stress models of depression. Furthermore, elevated expression of sEH was found in the brain of chronically stressed mice with depression-like behaviors and depressed patients, which might be involved in the pathogenesis of certain psychiatric diseases, including depression, bipolar disorder, and schizophrenia. After repeated social defeat stress, increased expression of brain-derived neurotrophic factor (BDNF) and phosphorylation of its receptor tyrosine-related kinase B (TrkB) were found in the prefrontal cortex, hippocampus of sEH knockout (sEH KO) mice and these mice did not show depression-like behavior, suggesting that sEH plays a key role in the pathophysiology of depression through modulating BDNF-TrkB signaling in the prefrontal cortex and hippocampus resulting in stress resilience, and that its inhibitors could be potential therapeutic or prophylactic drugs for depression. Moreover, the physiological effects of sEH inhibition on synaptic function and learning memory formation have also been determined [31]. Administration with the selective sEH inhibitor, 12-(3- adamantan-1-yl-ureido) dodecanoic acid (AUDA), induced an enhancement of synaptic neurotransmission and plasticity in the prefrontal cortex, which was associated with increased expression of postsynaptic glutamatergic NMDA subunits NR1, NR2A, NR2B and AMPA subunits GluR1, GluR2, and the enhanced synaptic long-term potentiation which through the ERK phosphorylation mediated by these postsynaptic glutamatergic receptors, indicating a role of sEH in modulation of glutamatergic neurotransmission and synaptic efficacy in the prefrontal cortex.
In addition to glutamatergic neurotransmission, it has been demonstrated by Dr. Hammock and co-workers that GABAergic neurotransmisstion also affected by sEH modulation [22]. Inhibition or genetic deletion of sEH was found to impede convulsive seizures instigated by GABAA antagonists, picrotoxin and PTZ, but not seizures evoked through other mechanisms. The anti-convulsive effects of sEH inhibition on GABA mediated neurotransmission to delay onset of seizures and seizure related excitability have been shown to be achieved through the augmentation of epoxy fatty acids levels, and specifically EETs in the brain, which suppress the tonic component of seizures and its related excitatory signaling.
They also worked on the antidotal properties of sEH inhibition for tetramethylenedisulfotetramine (TETS), the potent convulsant poison that acts by inhibiting the functions of GABAA receptor and found that combined administration of high dose GABAA receptor positive allosteric modulator, diazepam (5 mg/kg, i.p.) and a small molecule sEH inhibitor, 1-(1-methanesulfonyl-piperidin-4-yl)-3-(4- trifluoromethoxy-phenyl)-urea (TUPS, 1 mg/kg, ip, starting 1 h after diazepam and repeated every 24 h) prevented mortality and altered the signs of neuroinflammation in the hippocampus of mice with acute TETS intoxication, in which significantly decreased microglial activation and enhanced reactive astrogliosis were observed. Their findings suggested that sEH inhibition targets pathogenic mechanisms of acute TETS intoxication relevant to neuroinflammatory responses to protect the brain more efficaciously and may lead to better therapeutic outcomes in combination with antiseizure agents [23].
Pharmacological and genetic intervention of sEH inhibition on neuroinflammation and brain excitability of epilepsy
The anti-ictogenesis and anti-inflammatory effects of sEH inhibition on the models of TLE other than the models of seizures caused by GABAA receptor antagonism have also been documented [21]. The involvement of sEH in neuroinflammation, seizure generation and subsequent epileptogenesis was investigated using two mouse models of TLE established by pilocarpine-induced SE and electrical amygdale kindling in both wild-type (WT) C57BL/6 mice and sEH knockout (sEH KO) mice. The results demonstrated herein showed that seizure related neuroinflammation and ictogenesis were attenuated by pharmacological inhibition of sEH enzymatic activity but not by sEH genetic deletion.
In WT mice subjected to pilocarpine-induced SE, two different types of sEH inhibitors, AUDA and TPPU attenuated up-regulated pro-inflammatory cytokines, IL-1β and IL-6 in the hippocampus via the reduction of EETs degradation and IκB phosphorylation. Furthermore, the effectiveness of AUDA in terms of anti-ictogenesis properties resulted in decreased frequency and duration of spontaneous motor seizures in the pilocarpine-SE mice and increased seizure-induction threshold of the fully kindled mice.
Similar to WT mice, however, sEH KO mice revealed significantly increased expression of hippocampal IL-1β and IL-6 as well as decreased levels of EETs and EET/14,15-DHET ratio in response to SE. Moreover, sEH KO mice required lower dose of pilocarpine (sEH KO mice: 295 mg/kg, i.p.; WT mice: 325 mg/kg, i.p.) to entered convulsive SE and fewer stimulations to achieve fully kindled seizures. They displayed a marked acceleration of kindling development with a shorter latency to seizure onset and a lower after discharge threshold, indicating that sEH KO mice were more vulnerable to seizure induction than WT mice.
Notably, contrary to the previous findings in experimental cerebral ischemia models [95] and convulsive seizures elicited by GABA antagonists [22], these results demonstrated that genetic deletion of sEH did not exert an anti-inflammatory or anti-ictogenic effect in either the pilocarpine-induced SE model system or in the electrical kindling model system, which in contrast to the beneficial effects provided by pharmacological inhibition of sEH enzymatic activity with inhibitors.
The discrepant findings of pharmarcological inhibition and genetic deletion of sEH in SE-induced cytokine expressions and ictogenesis might be due to alternative mechanisms for EETs hydrolysis other than sEH in sEH KO mouse brain. Interestingly, the levels of EETs and the ratio of EETs to 14,15-DHET in the hippocampus were found to be not statistically different between WT and sEH KO mice under normal and epileptic conditions, suggesting that brain hydrolase activities were not largely affected by sEH genetic deletion. The residual hydrolase activity in sEH KO brain might be compensated by other epoxide hydrolases (EHs), in particular microsomal EH activity, which is assumed to account for much of EETs hydrolysis in the sEH KO null mice [120].
Additionally, a number of compensatory mechanisms involving complex changes in signaling pathways have been identified in the sEH null mice, including CYP4A mediated elevated production of 20- hydroxyeicosatetraenoic acid contributing to maintenance homeostatic blood pressure [121] and the enhanced effects of upregulation of aortic A2A adenosine receptors (AR), CYP2J, and PPAR coupled with downregulation of A1AR and PPARα on the modulation of adenosineinduced vascular responses [122]. Accordingly, the differential expression of A1 AR and A2A AR in the mice lacking sEH raises the possibility that brain excitatory transmission and metabolic activity controlled by these receptors [123] might alter, resulting in increased seizure facilitation in sEH KO mice. Also, it should be considered the implication of N-terminal lipid phosphatase activity of sEH in modulating brain activity though its precise physiological function and potential substrates in the brain remains to be elucidated at present.
Taken together, the anti-inflammatory and anti-ictogenic effects of sEH inhibition on epileptic animal models are effective in pharmacological but not genetic manipulation suggesting an involvement of sEH in seizure generation and subsequent alterations of EETs-sEH metabolism and neuroinflammatory responses, which may have clinical therapeutic potential for epilepsy in the future, particularly when treating TLE.
Concluding Remarks
Epilepsy is a complex brain disorder characterized by a chronic predisposition to generate spontaneous seizures, the cause (pathogenic mechanisms) and effective interventions of which remain to be elucidated to date. More recent studies devoted to clarify the pathophysiological role of inflammation in the mechanisms involved in persistent excitability changes that may contribute to epileptogenesis and sought to identify the potential therapeutic targets and exploit novel approaches to curing epilepsy. Given the impact of sEH and EETs on a number of inflammatory or inflammation-linked diseases [26,124,125], there is emerging evidence that both pharmacological and genetic manipulations of sEH provide salutary outcomes of diseases management, thereby making sEH an attractive target for neuroinflammation of epilepsy.
The anti-inflammatory and neuromodulatory activities provided by of sEH inhibition through elevating EETs level have been determined in central and peripheral nervous system [31,32,94,117,119,126], considered as therapeutic strategies for several neurological diseases, including seizures and epilepsy. However, interestingly, some discrepancies for anti-inflammatory and anti-ictogenesis effects of sEH inhibition on different models of seizures and epilepsy have been noted, and the inconsistencies between pharmacological inhibition and genetic deletion of sEH are shown [21]. Despite the precise role of sEH in the pathophysiology of brain hyperexcitability currently remains uncertain, these findings provide evidences that sEH participates in the generation and progression of seizures and epilepsy. Further comprehensive studies in various types of seizures and epilepsy derived from experimental models and human subjects are required to clarify the physiological functions of the two functional sEH domains in the brain and to expand our knowledge of the metabolic alterations of sEH-EETs in the epileptic brain and therapeutic implication.
Conflict of Interest
All authors declare that there are no conflicts of interest.
Acknowledgements
This study was supported in part by research grants from the Taipei Veterans General Hospital (V96S4-010, V96C1-123, V97S4-011, V97C1-034, V98S4-001, V98C1-095, V99S4-004, V99C1-156, V100E6-003, V100C-146, V102E9-003, V102C-101, V103E9-003, V103C-062, V104E9-004, V104C-068), from the Ministry of Science and Technology (NSC-96-2628-B-010-030-MY3, NSC-99-2628- B-010-011-MY3, NSC 102-2628-B-010-008-MY3), and from the Ministry of Education, Aim for the Top University Plan (102AC-B22, 102AC-B7), Taipei, Taiwan.
References
- Spencer SS (2002) When should temporal-lobe epilepsy be treated surgically? Lancet Neurol 1: 375-382.
- Choi J, Koh S (2008) Role of brain inflammation in epileptogenesis. Yonsei Med J 49: 1-18
- Jankowsky JL, Patterson PH (2001) The role of cytokines and growth factors in seizures and their sequelae. Prog Neurobiol 63: 125-149.
- Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7: 31-40.
- Jessberger S, Parent JM (2015) Epilepsy and Adult Neurogenesis. Cold Spring Harb Perspect Biol 7.
- van Vliet EA, Aronica E, Gorter JA (2015) Blood-brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol 38: 26-34.
- Vezzani A, Friedman A, Dingledine RJ (2013) The role of inflammation in epileptogenesis. Neuropharmacology 69: 16-24.
- Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 22: 797-803.
- Shimada T, Takemiya T, Sugiura H, Yamagata K (2014) Role of inflammatory mediators in the pathogenesis of epilepsy. Mediators Inflamm. 2014: 901902.
- Dedeurwaerdere S, Friedman A, Fabene PF, Mazarati A, Murashima YL, et al. (2012) Finding a better drug for epilepsy: antiinflammatory targets. Epilepsia 53: 1113-1118.
- Yu N, Liu H, Di Q (2013) Modulation of Immunity and the Inflammatory Response: A New Target for Treating Drug-resistant Epilepsy. Curr Neuropharmacol. 11: 114-127.
- Walker L, Sills GJ (2012) Inflammation and epilepsy: the foundations for a new therapeutic approach in epilepsy? Epilepsy Curr. 12: 8-12.
- Loscher W, Klitgaard H, Twyman RE, Schmidt D (2013) New avenues for anti-epileptic drug discovery and development. Nat Rev Drug Discov. 12: 757-776.
- Serrano E, Kanner AM (2015) Recent treatment advances and novel therapeutic approaches in epilepsy. F1000prime reports 7: 61
- Vezzani A, Balosso S, Maroso M, Zardoni D, Noe F, et al. (2010) ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy. Curr Opin Investig Drugs 11: 43-50
- Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, et al. (2014) Cyclooxygenase-2 in epilepsy. Epilepsia 55: 17-25.
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T (2011) IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 25: 1281-1289.
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, et al. (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nature 16: 413-419.
- Vitaliti G, Pavone P, Mahmood F, Nunnari G, Falsaperla R (2014) Targeting inflammation as a therapeutic strategy for drug-resistant epilepsies: an update of new immune-modulating approaches. Hum Vaccin Immunother. 10: 868-875.
- Hung YW, Hung SW, Wu YC, Wong LK, Lai MT, et al. (2015) Soluble epoxide hydrolase activity regulates inflammatory responses and seizure generation in two mouse models of temporal lobe epilepsy. Brain Behav Immun. 43: 118-129.
- Inceoglu B, Zolkowska D, Yoo HJ, Wagner KM, Yang J, et al. (2013) Epoxy fatty acids and inhibition of the soluble epoxide hydrolase selectively modulate GABA mediated neurotransmission to delay onset of seizures. PloS one 8: e80922.
- Vito ST, Austin AT, Banks CN, Inceoglu B, Bruun DA, et al. (2014) Post-exposure administration of diazepam combined with soluble epoxide hydrolase inhibition stops seizures and modulates neuroinflammation in a murine model of acute TETS intoxication. Toxicol Appl Pharmacol. 281: 185-194.
- Enayetallah AE, Grant DF (2006) Effects of human soluble epoxide hydrolase polymorphisms on isoprenoid phosphate hydrolysis. Biochem Biophys Res Commun 341: 254-260.
- Pillarisetti S, Khanna I (2012) Targeting soluble epoxide hydrolase for inflammation and pain - an overview of pharmacology and the inhibitors. Inflamm Allergy Drug Targets 11: 143-158.
- Morisseau C, Hammock BD (2013) Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 53: 37-58.
- Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, et al. (2010) Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat 91: 68-84.
- Iliff JJ, Alkayed NJ (2009) Soluble Epoxide Hydrolase Inhibition: Targeting Multiple Mechanisms of Ischemic Brain Injury with a Single Agent. Neurol 4: 179-199.
- Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, et al. (2009) Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol 174: 2086-2095.
- Sellers KW, Sun C, Diez-Freire C, Waki H, Morisseau C, et al. (2005) Falck JR, Hammock BD, Paton JF, Raizada MK: Novel mechanism of brain soluble epoxide hydrolase-mediated blood pressure regulation in the spontaneously hypertensive rat. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 19: 626-628.
- Wu HF, Yen HJ, Huang CC, Lee YC (2015) Soluble epoxide hydrolase inhibitor enhances synaptic neurotransmission and plasticity in mouse prefrontal cortex. J Biomed Sci 22: 94.
- Abdu E, Bruun DA, Yang D, Yang J, Inceoglu B, et al. (2011) Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem 117: 632-642.
- O'Dell CM, Das A, Wallace Gt, Ray SK, Banik NL (2012) Understanding the basic mechanisms underlying seizures in mesial temporal lobe epilepsy and possible therapeutic targets: a review. J Neurosci Res. 90: 913-924.
- Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, et al. (2008) Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 29: 142-160.
- Matin N, Tabatabaie O, Falsaperla R, Lubrano R, Pavone P, et al. (2015) Epilepsy and innate immune system: A possible immunogenic predisposition and related therapeutic implications. Hum Vaccin Immunother. 11: 2021-2029.
- Hopkins SJ, Rothwell NJ (1995) Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci 18: 83-88.
- Friedman A, Dingledine R (2011) Molecular cascades that mediate the influence of inflammation on epilepsy. Epilepsia 52 Suppl 3: 33-39.
- Ravizza T, Balosso S, Vezzani A (2011) Inflammation and prevention of epileptogenesis. Neurosci Lett 497: 223-230.
- Silveira G, de Oliveira AC, Teixeira AL (2012) Insights into inflammation and epilepsy from the basic and clinical sciences. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 19: 1071-1075.
- Vezzani A1, Aronica E, Mazarati A, Pittman QJ (2013) Epilepsy and brain inflammation. See comment in PubMed Commons below Exp Neurol 244: 11-21.
- Yang T, Zhou D, Stefan H (2010) Why mesial temporal lobe epilepsy with hippocampal sclerosis is progressive: uncontrolled inflammation drives disease progression? J Neurol Sci 296: 1-6.
- Galic MA, Riazi K, Pittman QJ (2012) Cytokines and brain excitability. Front Neuroendocrinol 33: 116-125.
- Li G, Bauer S, Nowak M, Norwood B, Tackenberg B, et al. (2011) Cytokines and epilepsy. Seizure 20: 249-256.
- Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC (2000) Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 7: 153-159.
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, et al. (2003) Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M: Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. The Journal of neuroscience : the official journal of the Society for Neuroscience 23: 8692-8700.
- Vezzani A, Baram TZ (2007) New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr 7: 45-50.
- Wang S, Cheng Q, Malik S, Yang J (2000) Interleukin-1beta inhibits gamma-aminobutyric acid type A (GABA(A)) receptor current in cultured hippocampal neurons. J Pharmacol Exp Ther 292: 497-504.
- Balosso S, Ravizza T, Perego C, Peschon J, Campbell IL, et al. (2005) Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol 57: 804-812.
- Shandra AA, Godlevsky LS, Vastyanov RS, Oleinik AA, Konovalenko VL, et al. (2002) The role of TNF-alpha in amygdala kindled rats. Neurosci Res 42: 147-153.
- Yuhas Y, Weizman A, Ashkenazi S (2003) Bidirectional concentration-dependent effects of tumor necrosis factor alpha in Shigella dysenteriae-related seizures. Infect Immun 71: 2288-2291.
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, et al. (1993) Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A 90: 10061-10065.
- De Luca G, Di Giorgio RM, Macaione S, Calpona PR, Costantino S, et al. (2004) Susceptibility to audiogenic seizure and neurotransmitter amino acid levels in different brain areas of IL-6-deficient mice. Pharmacology, biochemistry, and behaviour 78: 75-81.
- Erta M, Quintana A, Hidalgo J (2012) Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8: 1254-1266.
- Penkowa M, Molinero A, Carrasco J, Hidalgo J (2001) Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience 102: 805-818.
- Samland H, Huitron-Resendiz S, Masliah E, Criado J, Henriksen SJ, et al. (2003) Campbell IL: Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 73: 176-187.
- Fukuda M, Morimoto T, Suzuki Y, Shinonaga C, Ishida Y (2007) Interleukin-6 attenuates hyperthermia-induced seizures in developing rats. Brain Dev 29: 644-648.
- Kalueff AV, Lehtimaki KA, Ylinen A, Honkaniemi J, Peltola J (2004) Intranasal administration of human IL-6 increases the severity of chemically induced seizures in rats. Neurosci Lett 365: 106-110.
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, et al. (2002) Control of synaptic strength by glial TNFalpha. Science 295: 2282-2285.
- Stellwagen D, Beattie EC, Seo JY, Malenka RC (2005) Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci 25: 3219-3228.
- Rao RS, Prakash A, Medhi B (2009) Role of different cytokines and seizure susceptibility: a new dimension towards epilepsy research. Indian J Exp Biol 47: 625-634.
- Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, et al. (1999) Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci U S A 96: 8721-8726.
- Allan SM, Rothwell NJ (2001) Cytokines and acute neurodegeneration. Nat Rev Neurosci 2: 734-744.
- Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, et al. (2005) Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. The Journal of neuroscience : the official journal of the Society for Neuroscience 25: 6734-6744.
- Grell M, Wajant H, Zimmermann G, Scheurich P (1998) The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci U S A 95: 570-575.
- Phillis JW, Horrocks LA, Farooqui AA (2006) Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev 52: 201-243.
- Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, et al. (2004) Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem 88: 1168-1178.
- Shimizu T, Wolfe LS (1990) Arachidonic acid cascade and signal transduction. J Neurochem 55: 1-15.
- Wolfe LS (1982) Eicosanoids: prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. See comment in PubMed Commons below J Neurochem 38: 1-14.
- Leslie JB, Watkins WD (1985) Eicosanoids in the central nervous system. See comment in PubMed Commons below J Neurosurg 63: 659-668.
- Murphy S, Pearce B (1988) Eicosanoids in the CNS: sources and effects. Prostaglandins Leukot Essent Fatty Acids 31: 165-170.
- Kawaguchi K, Hickey RW, Rose ME, Zhu L, Chen J, et al. (2005) Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in CA3 hippocampus. Brain Res 1050: 130-137.
- Manabe Y, Anrather J, Kawano T, Niwa K, Zhou P, et al. (2004) Prostanoids, not reactive oxygen species, mediate COX-2-dependent neurotoxicity. Ann Neurol 55: 668-675.
- Polascheck N, Bankstahl M, Löscher W (2010) The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp Neurol 224: 219-233.
- Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, et al. (2011) Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. The Journal of neuroscience : the official journal of the Society for Neuroscience 31: 14850-14860.
- Takemiya T, Maehara M, Matsumura K, Yasuda S, Sugiura H, et al. (2006) Prostaglandin E2 produced by late induced COX-2 stimulates hippocampal neuron loss after seizure in the CA3 region. Neurosci Res 56: 103-110.
- Das A, Wallace G, Holmes C, McDowell ML, Smith JA, et al. (2004) Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience 220: 237-246.
- Desjardins P, Sauvageau A, Bouthillier A, Navarro D, Hazell AS, et al. (2003) Induction of astrocytic cyclooxygenase-2 in epileptic patients with hippocampal sclerosis. Neurochem Int 42: 299-303.
- Rumià J, Marmol F, Sanchez J, Carreño M, Bargalló N, et al. (2012) Eicosanoid levels in the neocortex of drug-resistant epileptic patients submitted to epilepsy surgery. Epilepsy Res 99: 127-131.
- Jung KH, Chu K, Lee ST, Kim J, Sinn DI, et al. (2006) Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis 23: 237-246.
- Toscano CD, Kingsley PJ, Marnett LJ, Bosetti F (2008) NMDA-induced seizure intensity is enhanced in COX-2 deficient mice. Neurotoxicology 29: 1114-1120.
- Oliveira MS, Furian AF, Rambo LM, Ribeiro LR, Royes LF, et al. (2008) Modulation of pentylenetetrazol-induced seizures by prostaglandin E2 receptors. Neuroscience 152: 1110-1118.
- Oliveira MS, Furian AF, Royes LF, Fighera MR, Fiorenza NG, et al. (2008) Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res 79: 14-21.
- Jiang J, Ganesh T, Du Y, Quan Y, Serrano G, et al. (2012) Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A 109: 3149-3154.
- Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, et al. (2013) Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A 110: 3591-3596.
- Yang H, Chen C (2008) Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des 14: 1443-1451.
- Chen C, Bazan NG (2005) Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol 93: 929-941.
- Salvadori MG, Banderó CR, Jesse AC, Gomes AT, Rambo LM, et al. (2012) Prostaglandin E(2) potentiates methylmalonate-induced seizures. Epilepsia 53: 189-198.
- Ganesh T (2014) Prostanoid receptor EP2 as a therapeutic target. J Med Chem 57: 4454-4465.
- Schneider C, Pratt DA, Porter NA, Brash AR (2007) Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem Biol 14: 473-488.
- Smith WL, Murphy RC (2002) The eicosanoids: cyclooxygenase, lipoxygenase, and epoxygenase pathways. Biochemistry of lipids, lipoproteins and membranes 4.
- Pillarisetti S, Khanna I (2015) A multimodal disease modifying approach to treat neuropathic pain--inhibition of soluble epoxide hydrolase (sEH). Drug Discov Today 20: 1382-1390.
- Harris TR, Hammock BD (2013) Soluble epoxide hydrolase: gene structure, expression and deletion. Gene 526: 61-74.
- Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, et al. (2008) Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A 105: 18901-18906.
- Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, et al. (2008) Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front Biosci 13: 2833-2841.
- Wang J, Fujiyoshi T, Kosaka Y, Raybuck JD, Lattal KM, et al. (2013) Inhibition of soluble epoxide hydrolase after cardiac arrest/cardiopulmonary resuscitation induces a neuroprotective phenotype in activated microglia and improves neuronal survival. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 33: 1574-1581.
- Iliff JJ, Fairbanks SL, Balkowiec A, Alkayed NJ (2010) Epoxyeicosatrienoic acids are endogenous regulators of vasoactive neuropeptide release from trigeminal ganglion neurons. J Neurochem 115: 1530-1542.
- Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M (2009) Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience 163: 646-661.
- Sisignano M, Park CK, Angioni C, Zhang DD, von Hehn C, et al. (2012) 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience 32: 6364-6372.
- Imig JD, Hammock BD (2009) Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov 8: 794-805.
- Morisseau C, Hammock BD (2005) Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol 45: 311-333.
- Newman JW, Morisseau C, Harris TR, Hammock BD (2003) The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci U S A 100: 1558-1563.
- Bianco RA, Agassandian K, Cassell MD, Spector AA, Sigmund CD (2009) Characterization of transgenic mice with neuron-specific expression of soluble epoxide hydrolase. Brain Res 1291: 60-72.
- Sura P, Sura R, Enayetallah AE, Grant DF (2008) Distribution and expression of soluble epoxide hydrolase in human brain. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 56: 551-559.
- Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, et al. (2007) Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci 27: 4642-4649.
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, et al. (2007) Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab 27: 1931-1940.
- Iliff JJ, Close LN, Selden NR, Alkayed NJ (2007) A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol 92: 653-658.
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, et al. (1996) Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27: 971-979.
- Koehler RC, Gebremedhin D, Harder DR (2006) Role of astrocytes in cerebrovascular regulation. J Appl Physiol (1985) 100: 307-317.
- Graier WF, Simecek S, Sturek M (1995) Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol 482 : 259-274.
- Hu S, Kim HS (1993) Activation of K+ channel in vascular smooth muscles by cytochrome P450 metabolites of arachidonic acid. Eur J Pharmacol 230: 215-221.
- Higashimori H, Blanco VM, Tuniki VR, Falck JR, Filosa JA (2010) Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am J Physiol Cell Physiol. 299: 1068-1078.
- Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, et al. (2008) Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol 294: H2838-2844.
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, et al. (2008) Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke 39: 2073-2078.
- Nelson JW, Young JM, Borkar RN, Woltjer RL, Quinn JF, et al. (2014) Role of soluble epoxide hydrolase in age-related vascular cognitive decline. Prostaglandins & other lipid mediators 113-115: 30-37.
- Siler DA, Berlow YA1, Kukino A1, Davis CM1, Nelson JW1, et al. (2015) Soluble Epoxide Hydrolase in Hydrocephalus, Cerebral Edema, and Vascular Inflammation After Subarachnoid Hemorrhage. Stroke 46: 1916-1922.
- Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD (2007) Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat 82: 42-49.
- Ren Q, Ma M, Ishima T, Morisseau C, Yang J, et al. (2016) Gene deficiency and pharmacological inhibition of soluble epoxide hydrolase confers resilience to repeated social defeat stress. Proceedings of the National Academy of Sciences of the United States of America 113: 1944-1952.
- Strauss K, Gruzdev A, Zeldin DC (2013) Altered behavioral phenotypes in soluble epoxide hydrolase knockout mice: effects of traumatic brain injury. Prostaglandins & other lipid mediators 104-105: 18-24.
- Luria A, Morisseau C, Tsai HJ, Yang J, Inceoglu B, et al. (2009) Alteration in plasma testosterone levels in male mice lacking soluble epoxide hydrolase. Am J Physiol Endocrinol Metab 297: E375-383.
- Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, et al. (2007) Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem 282: 2891-2898.
- Nayeem MA, Pradhan I, Mustafa SJ, Morisseau C, Falck JR, et al. (2013) Adenosine A2A receptor modulates vascular response in soluble epoxide hydrolase-null mice through CYP-epoxygenases and PPARgamma. Am J Physiol Regul Integr Comp Physiol 304: 23-32.
- Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA (2011) Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808: 1380-1399.
- Thomson SJ, Askari A, Bishop-Bailey D (2012) Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med 2012: 605101.
- Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, et al. (2005) Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A 102: 9772-9777.
- Wagner K, Inceoglu B, Gill SS, Hammock BD (2011) Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: novel mediators of pain reduction. J Agric Food Chem 59: 2816-2824.
Citation: Wen Hung Y, Lin YY (2016) Targeting Soluble Epoxide Hydrolase for Temporal Lobe Epilepsy. J Clin Exp Neuroimmunol 1:109.
Copyright: ©2016 Wen Hung Y et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 12071
- [From(publication date): 10-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 11133
- PDF downloads: 938