Commentary Open Access
Targeting Functionality of Cancer Cell-Derived Extracellular Vesicles to Immune Cells of the Tumor Microenvironment
Hinrich P Hansen*, Katrin S Reiners and Elke Pogge von StrandmannDepartment of Internal Medicine I, University Clinic Cologne, Cologne, Germany
- *Corresponding Author:
- Hinrich P Hansen
Department of Internal Medicine I
University Clinic Cologne
Cologne, Germany
Tel: +49 221 5808
Email: h.hansen@unikoeln. de
Received Date: April 17, 2015; Accepted Date: May 15, 2015; Published Date: May 18, 2015
Citation: Hansen HP, Reiners KS, Strandmann EPV (2015) Targeting Functionality of Cancer Cell-Derived Evs to Immune Cells of the Tumor Microenvironment. J Clin Exp Pathol 5:226. doi: 10.4172/2161-0681.1000226
Copyright: ©2015 Jeffrey Hansen HP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
In most cases, cancer cells cannot proliferate alone. They receive support form stromal cells and recruit and reprogram non-malignant immune cells not to damage the cancer cells but to support the tumor growth. Classical Hodgkin lymphoma (cHL) is a good example, as its growth critically depends on tumor-supporting immune cells. The affected lymphoid tissue contains very few disseminated malignant Hodgkin- and Reed-Sternberg (H-RS) cells, which are outnumbered by a massive infiltrate of lymphocytes, fibroblasts and innate immune cells. [1]. The H-RS cells need this environment to survive and proliferate because they are usually not detectable in the peripheral blood and they have difficulties to grow in immune-deficient mice [2]. H-RS cells manipulate the bystander cells for better development of their malignant phenotype and evasion of the host defense [3]. Among the infiltrated immune cells, the eosinophils and mast cells play an important role and their cell count indicates a negative prognostic feature [4,5]. In vitro, the direct interaction with cancer cells communicates survival in cancer cells and the production of cancersupporting factors in immune cells. In the last years, extracellular vesicles (EVs) emerged as important vehicles to shuttle between different the cell types and communicate as a surrogate cellular crosstalk in trans [6,7].
Commentary
In most cases, cancer cells cannot proliferate alone. They receive support form stromal cells and recruit and reprogram non-malignant immune cells not to damage the cancer cells but to support the tumor growth. Classical Hodgkin lymphoma (cHL) is a good example, as its growth critically depends on tumor-supporting immune cells. The affected lymphoid tissue contains very few disseminated malignant Hodgkin- and Reed-Sternberg (H-RS) cells, which are outnumbered by a massive infiltrate of lymphocytes, fibroblasts and innate immune cells. [1]. The H-RS cells need this environment to survive and proliferate because they are usually not detectable in the peripheral blood and they have difficulties to grow in immune-deficient mice [2]. H-RS cells manipulate the bystander cells for better development of their malignant phenotype and evasion of the host defense [3]. Among the infiltrated immune cells, the eosinophils and mast cells play an important role and their cell count indicates a negative prognostic feature [4,5]. In vitro, the direct interaction with cancer cells communicates survival in cancer cells and the production of cancer-supporting factors in immune cells. In the last years, extracellular vesicles (EVs) emerged as important vehicles to shuttle between different the cell types and communicate as a surrogate cellular cross-talk in trans [6,7].
Extracellular Vesicles
In multicellular organisms, cells have to influence each other to coordinate individual functionalities and to adapt to changing environmental conditions. In the traditional view, they communicate by direct cell contact or by the release of soluble molecules that diffuse into the environment and bind to the corresponding receptors on cells in the neighbourhood [8]. EVs provide an alternative intercellular communication platform. They are small plasma membrane-surrounded particles, which are released in variable amounts by virtually all cell types. EVs navigate shorter or longer distances across the body and, because they are tethered with characteristic markers of the donor cell, are able to select the target cell. In addition, they are suggested as serum biomarker to detect primary tumor mutations and serve as early indicators to monitor drug efficacy under anti-cancer therapy [9].
Although the mechanism of the target cell selection and the subsequent down-stream effects are not fully resolved, three interaction principles have been described so far. After a rolling and binding phase EVs i) fuse with the target cell and transfer mRNA, miRNA, lipids and proteins from the donor cell, ii) are taken up by phagocytosis, clathrin-mediated endocytosis or pinocytosis, or iii) interact in a cell contact-like fashion [10]. Thus, EVs have features of humoral and direct cell communication, as they act over distance like soluble factors but with the impact of multivalent interactions, similar to a direct cell contact or a virus uptake.
EVs play a critical role in many physiological and pathological processes, ranging from antigen presentation to inflammation [11,12]. In particular, tumor tissue contains high amounts of EVs and there is evidence that EVs have a pivotal functions in cancer cell growth and progression by modulating the microenvironment of the cancer cell, including immune evasion and stromal cell activation [8,13].
Healthy cells release principally two types of EVs, namely the exosomes and ectosomes, the latter also referred to as microvesicles or oncosomes [10]. Both are enriched in cholesterol and lipid raft proteins, they expose tetraspanins, such as CD9, CD63, CD81 and high concentrations of phosphatidylserine on the outer leaflet of the membrane. The vesicle types differ in the site of biogenesis, timing of release and diameter. Exosomes are small, rather homogeneous in diameter (50-100 nm) and appear as cup-shaped structures in transmission electron microscopy. They are generated as intraluminal vesicles (ILVs) by budding into late endosomes. Filled with vesicles, the endosomes are referred to as multivesicular bodies (MVB), which fuse with the plasma membrane releasing vesicles that are termed exosomes as soon as delivered to the extracellular environment. Ectosomes are larger (>100 nm) and generated by outward budding from the plasma membrane. Despite their different site of production, the EV assembly is similar, involving components of the endosomal sorting complex required for transport (ESCRT). Also the protein and nucleic acid composition is rather dependent on the type and demands of the releasing cell than on the vesicle type. Because the functionality of exosomes and ectosomes is generally strongly overlapping and they are difficult to distinguish in body fluids many publications use the more general term extracellular vesicle instead.
CD30 Functionality
Suspended Hodgkin cell release a mixture of larger and smaller EVs into the cell supernatant in vitro [14]. These EVs express CD30, which is a type II transmembrane receptor of the TNF receptor superfamily (TNFRSF8) and selectively expressed on the malignant cells in cHL tissue. CD30 crosslinking by its ligand (CD30L, CD153 or TNFSF8) initiates survival signaling involving TRAF adaptors and activation of the NF-κB signaling pathway, which eventually results in the transcription of various cytokines [15,16]. This cell activation is counter-regulated by the ectodomain cleavage of CD30, which is strongly enhanced upon CD30 cross-linking [17]. Cleavage is performed by metalloproteinases of the ADAM family, leaving the endoplasmic trunk and releasing the ectodomain (sCD30) into the environment. Thus, sCD30 and uncleaved CD30 on EVs are the released forms of CD30. In the serum of patients the concentration of soluble CD30 serves as an independent prognostic marker of the disease. However, since the standard CD30 ELISA is not able to discriminate between sCD30 and EV-associated CD30, the percentage of CD30 on EVs in the serum of patients is unresolved.
CD30L is a type I transmembrane protein and able to transduce a signal into the cell. It is expressed on the surface of typical bystander cells in cHL-affected tissue, such as mast cells, eosinophils and neutrophils [2]. This so-called reverse signaling is not only stimulated by CD30+ cancer cells but also by their EVs in a concentration and CD30-dependent manner [14]. Granulocytes and mast cell stimulation follows a unique pathway as it does not cause any degranulation of cytotoxic molecules but initiates the biosynthesis and release of tumor- supporting chemokines such as IL-8 (CXCL8), MIP-1a (CCL3) and MIP-1b (CCL4) by mast cells and granulocytes, which contribute to the recruitment of proinflammatory cells into the affected tissue [18]. Such effects were not caused by sCD30. The data indicate that EVs are able to substitute for a direct cell contact by presenting CD30 to a distant recipient cell in an immobilized and concentrated manner and that sCD30 serves as competitor [14]. Thus, a high local concentration of EV-CD30 stimulates, whereas a high local concentration of sCD30 inhibits reverse signaling in CD30L+ bystander cells.
Protrusions and EVs in HL
Classical HL is a tissue-associated disease, where the malignant H-RS cells and the bystander cells are embedded in the matrix of the affected lymph node. In vitro, Hodgkin cells release more CD30 by metalloproteinase-dependent ectodomain shedding (sCD30) than CD30 on the surface of EVs (EV-CD30) [14]. Because sCD30 is a competitor of the EV-CD30, the question arises how the in situ communication with immune cells is facilitated under such competitive conditions. Investigating the CD30/CD30L cross-talk as a model, it could be demonstrated that cancer cell EVs are not randomly released, when donor and target cells were together embedded in matrigel. Instead, cancer cell EVs are targeted to the recipient cells, where they accumulate and form a high local concentration of membrane-associated CD30, high enough to cause polarization of the corresponding ligand in the target cells. This EV enrichment at the target cell may explain its functionality in the competitive environment created by excessive concentrations of the non-functional ectodomain of CD30 (sCD30). Why EVs are not randomly distributed but targeted to a meaningful direction is not entirely resolved. Confocal microscopy showed that the tumor cells form long tubulin-based protrusions with tiny actin containing branches. CD30+ EVs are associated with these protrusions to reach the target cell. Thus, the cancer cell-derived filaments are most likely responsible for EV targeting. Because such EVs highways are also detectable in sections of the nodular sclerosing and mixed-cellularity types of cHL suggests that the guided EV transport occurs also in situ.
Are EVs a Benefit for the cHL-affected Tissue?
Most cancer cells do not grow independently but need the direct supportive contact to a non-malignant cell. They often influence stromal cells to create a cancer and cancer stem cell-supportive niche involving soluble and membrane-anchored stem/stromal cell-derived factor 1 (SDF-1) and the chemokine receptor CXCR4. Fibroblast-derived exosomes drive the breast cancer cell invasive behavior involving the Wnt-planar cell polarity (PCP) signaling [19]. In cHL this mechanism plays probably not a major role because the fibroblast count is extremely variable and not a general prognostic marker. Instead, the infiltration of certain immune cells is more relevant. The immune cell infiltration is enormous, often reducing the percentage of H-RS cells to almost 1%. Among them, the eosinophil and mast cell counts have prognostic value. They are effective through CD30 signaling. Also macrophages seem to play a role in CSF-R-based chemo resistance [20]. Despite the important function of these cells, they are rarely observed in close vicinity in cHL-affected tissue. Instead, rosettes of silenced or regulatory T cells and scattered B cells surround the H-RS cells and prevent a direct contact with the above innate immune cells.
Direct cell contact in trans is possible by an enlargement of the cell surface, either by sprawled and long protrusions or by the release of signaling proteins on EVs. Both represent functional parts of the donor cell membrane and are able to reach and interact with distant cells. EVs are expected to follow random diffusion through the tissue and finally enter the circulation but the radius of action of protrusions is probably limited. Similar to nerve growth, they may be directed to the target cell by guidance cues. Then, in the vicinity of the selected cell, they may cumulate signaling proteins, thus creating a robust interaction with the corresponding binding partner. In 3D culture of H-RS and mast cells both mechanisms were observed. Because EVs and protrusions co-localize, it is not impossible that the mechanisms synergize. Extracellular matrix and basal membranes provide a resistance for migrating EVs. Therefore it is tempting to speculate that protrusions prepare the pathway for EVs in extracellular matrix not to randomly diffuse but to enrich at the target cells, which are selected by the protrusions.
Model
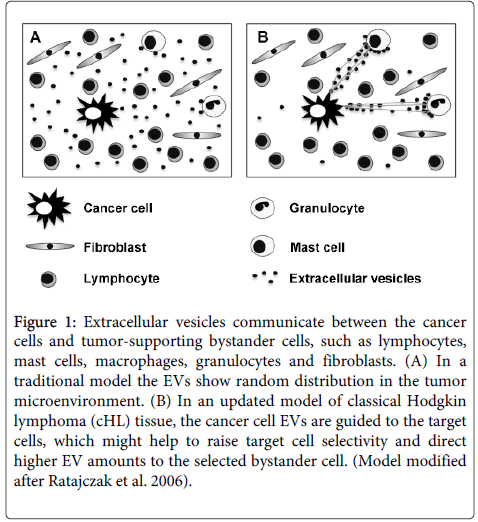
In conclusion, we suggest a model of cancer and bystander cell communication in cHL tissue. cHL-affected lymphoid tissue contains generally only a low percentage of driver cells, such as the malignant H-RS cells, infiltrating mast cells, eosinophils and tumor-associated macrophages [21]. They are disseminated in tumor tissue and release various growth factors and EVs for the tumor-promoting cross-talk in trans. In contrast to soluble factors, which loose functionality with a concentration gradient starting from the donor cell, EVs are guided to the corresponding target cells by a network of protrusions. Such a model avoids distance-dependent EV dilution and enables an enrichment and sustained functionality at the target cell, even throughout longer distance within the tissue. In addition to earlier models [8] that regard the tumor microenvironment randomly soaked with growth factors and EVs, we would like to suggest that the EV distribution in the cHL microenvironment is organized and guided by a network of filaments (Figure 1).
Figure 1: Extracellular vesicles communicate between the cancer cells and tumor-supporting bystander cells, such as lymphocytes, mast cells, macrophages, granulocytes and fibroblasts. (A) In a traditional model the EVs show random distribution in the tumor microenvironment. (B) In an updated model of classical Hodgkin lymphoma (cHL) tissue, the cancer cell EVs are guided to the target cells, which might help to raise target cell selectivity and direct higher EV amounts to the selected bystander cell. (Model modified after Ratajczak et al. 2006).
Acknowledgement
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG; Grant No. HA2432/5-1).
References
- Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A (2010) The classical Hodgkin's lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol 221: 248-263.
- Küppers R, Engert A, Hansmann ML (2012) Hodgkin lymphoma. J Clin Invest 122: 3439-3447.
- Steidl C, Connors JM, Gascoyne RD. (2011) Molecular Pathogenesis of Hodgkin's Lymphoma: Increasing Evidence of the Importance of the Microenvironment. J ClinOncol 29: 1812-1826.
- Molin D1, Edström A, Glimelius I, Glimelius B, Nilsson G, et al. (2002) Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. Br J Haematol 119: 122-124.
- Von Wasielewski R, Seth S, Franklin J, Fischer R, Hubner K, et al. (2000) Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood 95: 1207-1213.
- Peinado H, AleÄkoviÄ? M, Lavotshkin S, Matei I, Costa-Silva B, et al. (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18: 883-891.
- Benito-Martin A, Di Giannatale A, Ceder S, Peinado H (2015) The new deal: a potential role for secreted vesicles in innate immunity and tumor progression. Front Immunol 6: 66.
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ (2006) Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20: 1487-1495.
- Shao H, Chung J, Balaj L, Charest A, Bigner DD, et al. (2012) Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med 18: 1835-1840.
- Cocucci E1, Meldolesi J2 (2015) Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol .
- Théry C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9: 581-593.
- Lai FW, Lichty BD, Bowdish DM (2015) Microvesicles: ubiquitous contributors to infection and immunity. J LeukocBiol 97: 237-245.
- Webber J, Yeung V, Clayton A (2015) Extracellular vesicles as modulators of the cancer microenvironment. Semin Cell DevBiol 40: 27-34.
- Hansen HP, Engels HM, Dams M, PaesLeme AF, Pauletti BA, et al. (2014) Protrusion-guided extracellular vesicles mediate CD30 trans-signalling in the microenvironment of Hodgkin's lymphoma. J Pathol 232: 405-414.
- Duckett CS, Gedrich RW, Gilfillan MC, Thompson CB (1997) Induction of nuclear factor kappaB by the CD30 receptor is mediated by TRAF1 and TRAF2. Mol Cell Biol 17: 1535-1542.
- Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, et al. (1993) CD30 antigen, a marker for Hodgkin's lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 73: 1349-1360.
- Eichenauer DA, Simhadri VL, von Strandmann EP, Ludwig A, Matthews V, et al. (2007) ADAM10 inhibition of human CD30 shedding increases specificity of targeted immunotherapy in vitro. Cancer Res 67: 332-338.
- Fischer M, Harvima IT, Carvalho RF, Möller C, Naukkarinen A, et al. (2006) Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest 116: 2748-2756.
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, et al. (2012) Exosomes mediate stromal mobilization of autocrineWnt-PCP signaling in breast cancer cell migration. Cell 151: 1542-1556.
- Steidl C1, Diepstra A, Lee T, Chan FC, Farinha P, et al. (2012) Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood 120: 3530-3540.
- Steidl C1, Lee T, Shah SP, Farinha P, Han G, et al. (2010) Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med 362: 875-885.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14334
- [From(publication date):
June-2015 - Jul 18, 2025] - Breakdown by view type
- HTML page views : 9726
- PDF downloads : 4608