Targeting Demalication and Deacetification Methods: The Role of Carboxylic Acids Transporters
Received: 17-Nov-2017 / Accepted Date: 28-Nov-2017 / Published Date: 05-Dec-2017 DOI: 10.4172/2168-9652.1000224
Abstract
As weak organic acids, carboxylic acids partially dissociate in aqueous systems, like wine, establishing equilibrium between uncharged molecules (undissociated form) and their anionic form, according to the medium pH and their pKa. This property influences yeasts cell-behaviour, particularly the mechanisms by which the molecules can cross biological membranes. Occasionally wines may present an excessive amount of organic acids. In the mouth they will seem unbalanced and sometimes excessive sourness diminishes their quality. Moreover, these acids originated from grapes or from the fermentation process itself, negatively affect wine yeasts, yeast fermentation process and the final wine quality. Two of those acids are L-malic acid and acetic acid. The first one affects the wine mainly in his tastiness, making it much to sour; the second one, being a volatile compound, besides the excessive sourness, also imprints the wine with an unpleasant vinegar flavour. One approach to solving this problem is biological deacidification by Saccharomyces and non-Saccharomyces wine yeasts. To these biological processes of wine acidity bio-reduction we can call wine bio-demalication (malic acid bio-degradation) and wine biodeacetification (acetic acid consumption by yeasts).
Keywords: Malic acid; Acetic acid; Carboxylic acids transport; Wines bio-deacidification
General Overview
The chemical grape composition, mainly in organic acids, influences final wine quality and tastiness. Though some acids are formed during wine fermentation, is during this biological process that the winemakers must act, to produce a wine with the appropriated acidic balance. For instances, acetic acid, formed during yeast metabolism (fermentation) and also, among others, during the metabolism of acetic and lactic acids, has a negative impact on yeast fermentation performance and affects wines quality when present above his detection threshold [1].
Among the strategies used to lower high acidity in dry wines biological deacidification by yeast and bacteria, are considered the most natural ones. Malolactic fermentation (MLF) is the common method of biological deacidification or demalication, since lactic acid bacteria (LAB) have the ability to consume the malic acid and convert it to lactic [2], softer in the mouth than the malic acid. Non- Saccharomyces yeasts species like Schizosaccharomyces pombe and Lachancea thermotolerans also possess the ability to degrade malic acid. The first one converts it into ethanol through malo-ethanolicdeacidification [3,4] while L . thermotolerans produces lactic acid, allowing the wine to achieve its potential acidity and tastiness. Both bio-demalication processes avoid the use of LAB strains and the final wines are fruity and contain less acetic acid and biogenic amines [5]. However, these non-Saccharomyces yeasts present poor alcohol tolerance, and it is convenient to use them in combination with Saccharomyces cerevisiae strains, in order to complete wine fermentation.
In an attempt to develop a biological strategy to reduce the acetic acid concentration in musts and wines, many works have been publish since the pioneer work of Ribéreau-Gayon et al. [6]. In their work they described a process consisting in refermenting the acidic wines by mixing them with the solid remains of grapes from a finished wine fermentation, freshly crushed grapes or musts. During these refermentation processes acetic acid is consumed by yeasts [7]. Bearing in mind the work of Ribéreau-Gayon et al. [6], we have made some studies using indigenous and commercial wine yeasts, and we have found that the studied strains were able to consume acetic acid during alcoholic fermentation and directly in acidic wines, without the need to had sugar [7,8].
Nevertheless, for these two carboxylic acids (malic and acetic acids) to be consumed by yeast, they need to enter the yeast cell trough plasma membrane. This mini-review pretends to elucidate about how L-malate and acetic acid enter the yeast cell membrane bearing in mind that carboxylic acids can either be transported into the cells, to be used as nutrients, or extruded in response to acid stress conditions [9].
Yeast cell transporters important to demalication activity
The yeast S. cerevisiae has long been known as a poor metabolizer of extracellular malate, due to the lack of a mediated transport system for the acid [10]. Moreover, the malic enzyme, located in the mitochondria, has low substrate affinity (Km=50 mM) [11,12] and under fermentation conditions, is regulated by the fermentative glucose metabolism that causes mitochondrial deterioration [13]. These biochemical characteristics make the yeast demalication activity has being strain dependent [14-16].
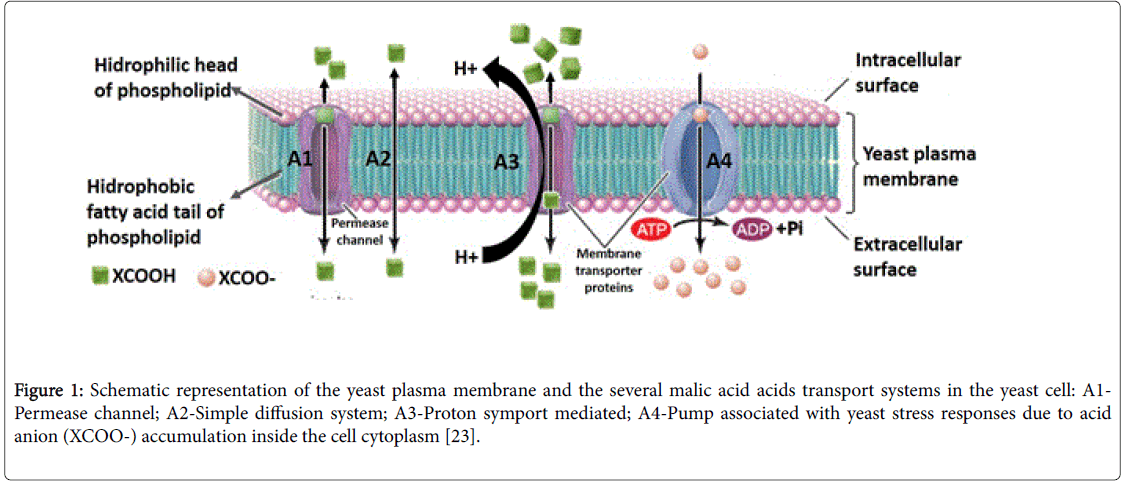
As mentioned above, the ability of a yeasts strain to degrade extracellular L-malate is dependent on the efficient transport of the dicarboxylic acid into the cell. In Kluyveromyces lactis the transport of this acid is carrier-mediated [17] as it is also in In Z. bailii , able to transport malic acid by facilitated diffusion (Figure 1, A1). In this later strain, malic acid transport is induced by glucose and repressed by fructose [18].
In S. cerevisiae L-malate enters the cells by simple diffusion (Figure 1, A2), while in the yeasts Candida utilis , Candida sphaerica, Hansenula anomala and Kluyveromyces marxianus the transport of malate is performed by a proton symport/induced (Figure 1, A3) and glucose repressed system [11,19-21]; Schizosaccharomyces pombe also possesses a proton symport system (maelp) (Figure 1, A3) [11]. Recently, in the article Vilela [22], we have made some considerations about these transport systems.
Figure 1: Schematic representation of the yeast plasma membrane and the several malic acid acids transport systems in the yeast cell: A1- Permease channel; A2-Simple diffusion system; A3-Proton symport mediated; A4-Pump associated with yeast stress responses due to acid anion (XCOO-) accumulation inside the cell cytoplasm [23].
After entering the yeast cell L-malate must enter the yeast mitochondria. The Dic1p dicarboxylates carrier protein was isolated and studied by Lancar-Benba et al. [24]. This carrier transports malate (Km=0.56 mM) [25] succinate or malonate. The main function of the Dic1p carrier is to transport dicarboxylates from the cytoplasm into the mitochondria, being an anaplerotic transporter for the Krebs cycle [26].
Another mitochondrial aspartate/glutamate carriers named Agc1p [26], as also being studied. This transporter plays a role in the malate/ aspartate shuttle, and this role is critical for growth on acetate and fatty acids.
Odc1p and Odc2p carriers (61% identical to each other) transport and kinetic properties show that they are isoforms of a novel mitochondrial transporter. They transport oxoadipate and oxoglutarate and the corresponding dicarboxylates and malate by a counterexchange mechanism [27]. The expression of Odc1p is strongly repressed by glucose and galactose, whereas Odc2p is expressed on all carbon sources that were tested [27]. So, under respiratory conditions Odc1p is the major carrier, and in the presence of glucose and anaerobiosis Odc2p is the one that dominates. An important physiological role of the oxodicarboxylate carrier in yeast is to export oxoglutarate, in exchange for malate, to the cytoplasm [26].
Saccharomyces transporter proteins that facilitate deacetification process
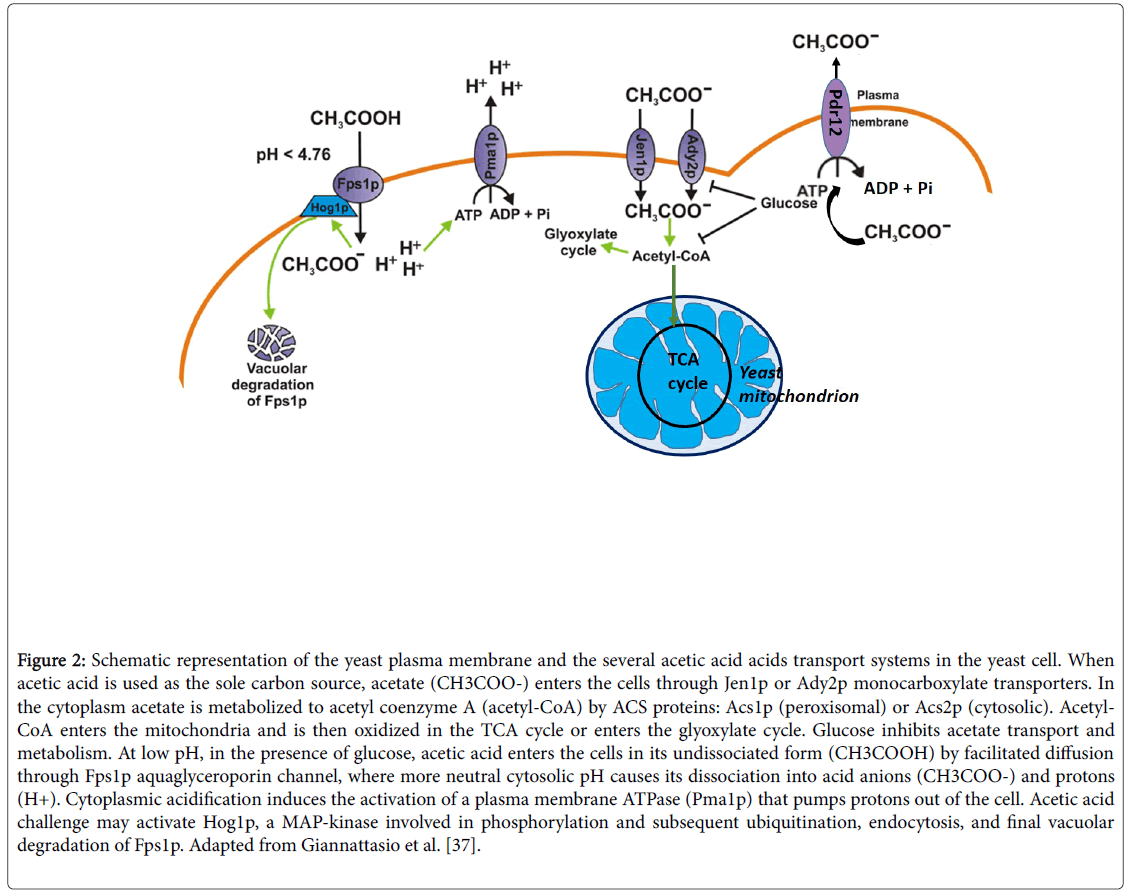
S. cerevisiae is able to metabolize acetic acid during a refermentation process [6,28]. Several works have being done about this subject [1,7,8,28,29] and they all agree that excessive volatile acidity can be removed by refermentation with an appropriate S. cerevisiae wine yeast. The transport of acetate into the yeast cell is an imperative step for its metabolism [1]. In glucose-repressed yeast cells, at low pH, acetic acid enters mainly by simple diffusion [30]. However, Jen1p, a transport carrier studied by Casal et al. [31], required for the uptake of lactate in S. cerevisiae is also able to transport acetate (Figure 2).
Figure 2: Schematic representation of the yeast plasma membrane and the several acetic acid acids transport systems in the yeast cell. When acetic acid is used as the sole carbon source, acetate (CH3COO-) enters the cells through Jen1p or Ady2p monocarboxylate transporters. In the cytoplasm acetate is metabolized to acetyl coenzyme A (acetyl-CoA) by ACS proteins: Acs1p (peroxisomal) or Acs2p (cytosolic). Acetyl- CoA enters the mitochondria and is then oxidized in the TCA cycle or enters the glyoxylate cycle. Glucose inhibits acetate transport and metabolism. At low pH, in the presence of glucose, acetic acid enters the cells in its undissociated form (CH3COOH) by facilitated diffusion through Fps1p aquaglyceroporin channel, where more neutral cytosolic pH causes its dissociation into acid anions (CH3COO-) and protons (H+). Cytoplasmic acidification induces the activation of a plasma membrane ATPase (Pma1p) that pumps protons out of the cell. Acetic acid challenge may activate Hog1p, a MAP-kinase involved in phosphorylation and subsequent ubiquitination, endocytosis, and final vacuolar degradation of Fps1p. Adapted from Giannattasio et al. [37].
At pH 4.5 passive diffusional flux of undissociated acetic acid into the cell can be mediated by Fps1, an aquaglyceroporin membrane channel.This aquaglyceroporin can be destabilized by direct Hog1 mitogen-activated protein kinase (MAPK) phosphorylation, thus rendering stress resistance to the yeast cell, acquired through this channel loss. Hog1 MAPK is quickly activated in yeast exposed to toxic levels of acetic acid. This Hog1 then phosphorylates the plasma membrane aquaglyceroporin, Fps1. A phosphorylation that results in Fps1 degradation in the vacuole (Figure 2) [32].
Later, it was found that the protein Ady2p was vital for acetate transport in acetic acid-grown cells [33]. Jen1p mediates the transport of lactate, pyruvate, acetate and propionate whereas Ady2p mediates the transport of acetate, propionate, formate and lactate, being both induced by non-fermentable carbon sources, and repressed in the presence of glucose [34].
Exposure of Saccharomyces cerevisiae to sorbic acid strongly induces two plasma membrane proteins, one of which was identified as the ATP-binding cassette (ABC) transporter Pdr12 [35]. It was later found that overexpression of PDR12 increased tolerance to acids with longer chain length, such as sorbic, propionic and levulinic, whereas deletion of the gene increased tolerance to the shorter acetic and formic acid [36]. The induction of a weak acid efflux pump (Pdr12) poses potential problems for homeostasis maintenance in cells adapted to these acids, like acetic acid, unless there is also a synchronized system restricting free diffusional entry of the undissociated acid. Pma1p is the proton-translocating plasma membrane ATPase (Figure 2) [35].
Final Remarks
Malolactic fermentation by Lactic Acid Bacteria strains has been the traditional method, used by winemakers, to perform biological demalication. On the other hand, during malolactic fermentation subproducts such as acetic acid and biogenic amines can be produced, imprinting the wines with unpleasant and even unhealthy characteristics. The use of wine yeast strains in the demalication process is one of the promising enological steps in improving wine quality. The same goes for biological deacetification. The right selection of yeast strains determines the performance of deacetification process, leading to higher quality wine. One of the steps in improving biological demalication and deacetification is studying the mechanisms involved in yeasts carboxylic acids transporters, and the way they operate according to the yeast cell response to environmental changes, as carbon source availability, extracellular pH and acid stress conditions.
Acknowledgement
This work is supported by the Chemistry Research Centre of Vila Real (CQ-VR). Additional thanks to the Project NORTE-01-0145- FEDER-000038 (I&D INNOVINE&WINE–Innovation Platform of Vine & Wine).
References
- Vilela-Moura A, Schuller D, Mendes-Faia A, Silva RF, Chaves SR, et al. (2011) The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape-musts and wines – Mini review. Appl Microbiol Biotechnol 89: 271-280.
- Inês A, Tenreiro T, Tenreiro R, Mendes-Faia A (2008) Review: The lactic acid bacteria of wine- Part I: Science Téc. Vitiv v.23: 81-96
- Van Rooyen TJ, Tracel RP (1987) Biological Deacidification of musts induced by yeasts or malolactic bacteria and the effect on wine quality. S Afr J Enol Vitic 8: 60-69.
- Camarasa C, Bidard F, Bony M, Barre P, Dequin S (2001) Characterization of Schizosaccharomyces pombe Malate Permease by Expression in Saccharomyces cerevisiae. Appl Environ Microbiol 67: 4144-4151
- Benito A, Calderón F, Palomero F, Benito S (2015) Combined use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 20: 9510-9523
- Ribéreau-Gayon J, Peynaud E, Ribéreau-Gayon P, Sudraud P (1975). Les mécanismes des fermentations. In: Chez Dunod (eds) Traité d`oenologie, sciences et techniques du vin, Tome 2, Dunod, Paris, pp: 511-556.
- Vilela-Moura A, Schuller D, Mendes-Faia A, Côrte-Real M (2008) Reduction of volatile acidity of wines by selected yeast strains. Appl Microbiol Biotechnol 80: 881-890.
- Vilela-Moura A, Schuller D, Mendes-Faia A, Côrte-Real M (2010) Effects of acetic acid, ethanol and SO2 on the removal of volatile acidity from acidic wines by two Saccharomyces cerevisiae commercial strains. Appl Microbiol Biotechnol 87:1317-1326.
- Casal M, Queirós O, Talaia G, Ribas D, Paiva S (2016) Carboxylic acids plasma membrane transporters in Saccharomyces cerevisiae. Adv Exp Med Biol 892: 229-251
- Salmon JM (1987) L-Malic acid permeation in resting cells of anaerobically grown Saccharomyces cerevisiae. Biochim Biophys Acta 901:30-34.
- Saayman M, Viljoen-Bloom M (2006) The biochemistry of malic acid metabolism by wine yeasts - A review. S Afr J Enol Vitic 27: 113-122.
- Volschenk H, van Vuuren HJJ, Viljoen-Bloom M (2003) Malo-ethanolic fermentation in Saccharomyces and Schizosaccharomyces. Curr Genet 43: 379-391.
- Saayman M, van Zyl WH, Viljoen-Bloom M (2006) Cloning, characterization and heterologous expression of the Candida utilis malic enzyme gene. Curr Genet 49: 248-258.
- Redzepovic S, Orlic S, Majdak A, Kozina B, Volschenk H, et al. (2003) Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int J Food Microbiol 83: 49-61
- Pretorius IS, Bauer FF (2002) Meeting the consumer challenge through genetically customized wine-yeast strains. Trends Biotechnol 20: 426-432.
- Rodriguez SB, Thornton RJ (1990) Factors influencing the utilization of L-malate by yeasts. FEMS Microbiol Lett 60: 17-22.
- Zmijewski MJ, Macquillan AM (1975) Dual effects of glucose on dicarboxylic acid transport in Kluyveromyces lactis. Can J Microbiol 21: 473-480.
- Baranowski K, Radler F (1984) The glucose dependent transport of l-malate in Zygosaccharomyces bailii. Antonie Van Leeuwenhoek 50: 329-340.
- Côrte-Real M, Leão C, Van Uden N (1989) Transport of L(−)malic acid and other dicarboxylic acids in the yeast Candida sphaerica. Appl Microbiol Biotechnol 31: 551-555
-  Côrte-Real M, Leão C (1990) Transport of malic acid and other dicarboxylic acids in the yeast Hansenula anomala. Appl Environ Microbiol 56: 1109-1113.
- Queirós O, Casal M, Althoff S, Moradas-Ferreira P, Leão C (1998) Isolation and characterization of Kluyveromyces marxianus mutants deficient in malate transport. Yeast 14: 401-407.
- Vilela A (2017) Biological demalication and deacetification of musts and wines: Can wine yeasts make the wine taste better? Fermentation 3: 1-14
- Casal M, Paiva S, Queirós O, Soares-Silva I (2008) Transport of carboxylic acids in yeasts. FEMS Microbiol Rev 32: 974-994
- Lancar-Benba J, Foucher B, Saint-Macary M (1996) Characterization, purification and properties of the yeast mitochondrial dicarboxylate carrier (Saccharomyces cerevisiae). Biochimie 78: 195-200
- Palmieri L, Palmieri F, Runswick MJ, Walker JE (1996) Identification by bacterial expression and functional reconstitution of the yeast genomic sequence encoding the mitochondrial dicarboxylate carrier protein FEBS Lett 399: 299-302
- Â Palmieri F, Agrimi G, Blanco E, Castegna A, Di Noia M-A, et al. (2006) Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim Biophys Acta 1757: 1249-1262.
- Palmieri L, Agrimi G, Runswick MJ, Fearnley IM, Palmieri F, et al. (2001) Identification in Saccharomyces cerevisiae of two isoforms of a novel mitochondrial transporter for 2-oxoadipate and 2-oxoglutarate. J Biol Chem 276: 1916-1922.
- Vilela-Moura A, Schuller D, Falco V, Mendes-Faia A, Côrte-Real M (2010) Effect of refermentation conditions and micro-oxygenation on the reduction of volatile acidity by commercial S. cerevisiae strains and their impact on the aromatic profile of wines. Int. J Food Microbiol 141: 165-172
- Vasserot Y, Mornet F, Jeandet P (2010) Acetic acid removal by Saccharomyces cerevisiae during fermentation in oenological conditions. Metabolic consequences. Food Chem 119: 1220-1223
- Casal M, Cardoso H, Leão C (1996) Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology 142: 1385-1390.
- Casal M, Paiva S, Andrade RP, Gancedo C, Leão C (1999) The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. J Bacteriol 181: 2620-2623.
- Mollapour M, Piper PW (2007) Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27: 6446-6456
- Paiva S, Devaux F, Barbosa S, Jacq C, Casal M (2004) Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast 21: 201-210,
- Pacheco A, Talaia G, Sá-Pessoa J, Bessa D, Gonçalves MJ, et al. (2012) Lactic acid production in Saccharomyces cerevisiae is modulated by expression of the monocarboxylate transporters Jen1 and Ady2. FEMS Yeast Res 12: 375-381.
- Piper P, Mahé Y, Thompson S, Pandjaitan R, Holyoak C, et al. (1998). The pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast. EMBO J 17: 4257-4265.
- Nygård Y, Mojzita D, Toivari M, Penttilä M, Wiebe MG et al (2014) The diverse role of Pdr12 in resistance to weak organic acids. Yeast 31: 219-232
- Giannattasio S, Guaragnella N, Ždralević M, Marra E (2013). Molecular mechanisms of Saccharomyces cerevisiae stress adaptation and programmed cell death in response to acetic acid. Front Microbiol 4: 1-7.
Citation: Vilela A (2017) Targeting Demalication and Deacetification Methods: The Role of Carboxylic Acids Transporters. Biochem Physiol 6:224. DOI: 10.4172/2168-9652.1000224
Copyright: 2017 Vilela A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use,distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4672
- [From(publication date): 0-2017 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 4014
- PDF downloads: 658