Research Article Open Access
Tapentadol Prolonged Release Improves Analgesia, Functional Impairment, and Quality of Life in Patients With Chronic Pain Who Have Previously Received Oxycodone/Naloxone
Kern KU1, Krings D2, and Waldmann-Rex S2*
1Institute for Pain Medicine/Pain Practice Wiesbaden, Germany
2Medical Affairs, German Division, Grünenthal GmbH, Aachen, Germany
- *Corresponding Author:
- Susanne Waldmann-Rex
Medical Affairs, German Division, Grünenthal GmbH
Geschäftsbereich Deutschland, Aachen
Germany
Tel: +49241569-1981
E-mail: susanne.waldmann-rex@grunenthal.com
Received date: November 28, 2016; Accepted date: November 29, 2016; Published date: January 16, 2017
Citation: Kern KU, Krings D, Waldmann-Rex S (2017) Tapentadol Prolonged Release Improves Analgesia, Functional Impairment and Quality of Life in Patients with Chronic Pain who have Previously Received Oxycodone/Naloxone. J Pain Relief 6: 281. doi: 10.4172/2167-0846.1000281
Copyright: ©2017 Kern KU et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Pain & Relief
Abstract
Background: This subgroup analysis of a prospective, non-interventional study involving general practitioners/ internists evaluated tapentadol prolonged release (PR) in patients who previously received oxycodone/naloxone.
Methods: This non-interventional study, which had a 3-month observation period, included 5002 patients. Data from a subgroup of patients previously treated with oxycodone/naloxone (n=382) were included in this analysis.
Results: Back pain was the most common cause of pain, and mixed pain (nociceptive and neuropathic) predominated. Oxycodone/naloxone pretreatment was most commonly combined with non-opioids (78.3%) or coanalgesics (56.0%). The most common reasons for starting tapentadol PR were insufficient analgesia (87.4%) and impaired quality of life (70.2%). Switching to tapentadol PR (final average daily dose, 252.9 mg) resulted in a mean pain reduction of 3.41 points on an 11-point numerical rating scale (baseline, 7.29 ± 1.40; end of observation, 3.88 ± 1.86; descriptive P value ≤ 0.001; n=373). The percentage of patients who did not require additional analgesics (i.e., those on tapentadol PR monotherapy) increased from 24.6% at the start of treatment to 33.5% at the final visit. Coanalgesic use also decreased from the start of treatment to the final visit (antidepressants: 46.6% to 39.4%; anticonvulsants: 24.6% to 15.9%). Significant improvements were achieved in pain-related functional impairment and Short Form-12 summary scores. Tapentadol PR was well tolerated.
Conclusion: Patients who previously received oxycodone/naloxone benefit from tapentadol PR therapy; clinically relevant improvements in analgesia, functionality, and quality of life were documented. Furthermore, analgesic comedication could be reduced during tapentadol PR treatment, reducing medication burden and potentially leading to improved compliance.
Keywords
Severe chronic pain; Tapentadol PR; Everyday practice; Pain management; Health-related quality of life
Abbreviations
ADRs: Adverse Drug Reactions; CI: Confidence Interval; CNS: Central Nervous System; CR: Controlled Release; DGS questionnaire: German Pain Questionnaire; EQ-5D: EuroQol-5Dimension; MOR: µ-opioid Receptor; NIS: Non- Interventional Study; NRI: Noradrenaline Reuptake Inhibition; NRS: Numerical Rating Scale; NSAID: Non-Steroidal Anti-Inflammatory Drug; PR: Prolonged Release; SD: Standard Deviation; SF-12: Short Form-12; SF-36: Short Form-36
Introduction
Chronic pain impairs physical function and quality of life, and causes emotional disorders like anxiety and depression increasing in intensity commensurate with the degree of chronification. Besides effective pain reduction, restoring or improving functionality and health-related quality of life are increasingly relevant parameters of successful pain management.
Tapentadol prolonged release (PR) is a centrally acting analgesic for the management of severe chronic pain and has been available since 2010 as Palexia® prolonged release. Tapentadol combines an opioidergic and a noradrenergic component of action in one molecule (MOR-NRI). This results synergistically in potent analgesia with a good tolerability profile, especially due to the reduced incidence of opioid-typical gastrointestinal (eg. nausea, vomiting) and central nervous system (eg. dizziness) undesirable effects [1].
Efficacy and tolerability in the treatment of severe chronic pain of variable etiology were documented in clinical trials [2-4] and have also been confirmed in daily medical practice in Germany [5,6]. Based on the Short Form-36 (SF-36) and EuroQol-5 dimension (EQ-5D) questionnaires, clinical studies showed significant improvements in quality of life on tapentadol PR compared with both placebo and oxycodone CR. Significant improvements in physical functionality, vitality, social functioning, and other parameters were detected in patients receiving tapentadol PR compared to oxycodone CR [2].
A non-interventional study (NIS) investigated the change in functionality and associated health-related quality of life of more than 5000 patients after starting treatment with tapentadol PR in daily general medical practice [7]. The present subgroup analysis from this study examines the data of patients previously treated with a fixed oxycodone/naloxone combination (Targin®). In contrast to tapentadol (1 molecule with 2 mechanisms of action), this combination consists of a potent opioid (oxycodone) with an opioid antagonist (naloxone), which is intended to counteract opioid-induced constipation [8]. In 2012, this fixed combination, with 13.6 million defined daily doses, accounted for nearly one-third of all oxycodone prescriptions in Germany [9].
The presented subgroup analysis investigates the change-over from oxycodone/naloxone to tapentadol PR under routine conditions of daily practice, and specifically whether and how patients previously treated with the fixed combination benefit from tapentadol PR therapy.
Materials and Methods
From the dataset of the prospective NIS (effectiveness population, n=5002), [7] all patients were selected whose therapy contained a fixed oxycodone/naloxone combination immediately before study onset. The data from these 382 patients are presented below.
The prospective NIS was performed between September 2011 and May 2013 in Germany in accordance with Section 4, Subsection 23, Paragraph 3, and Section 67, Subsection 6 AMG (German Medicines Act) of the Federal Republic of Germany. The NIS was registered at the BfArM (Federal Institute for Drugs and Medical Devices), KBV (National Association of Statutory Health Insurance Physicians), and the National Associations of Social Health Insurers as required by law. In addition, the research project was reviewed in advance by an ethics committee (in accordance with Section 15 of the Association Professional Code of Conduct).
Office-based general practitioners, primary care physicians, and internal medicine specialists with a homogeneous geographic distribution participated in the study. In accordance with the indication, adult patients with severe chronic pain (eg. back pain, gonarthrosis, coxarthrosis, and tumor pain) could be enrolled if, in the opinion of the physician, they could only be adequately treated with opioid-containing analgesics. The patients had to be able to answer the SF-12 questionnaires.
Data were documented in the case report form at 3 time points: 1) at treatment initiation (baseline examination), 2) in the interim (after about 4-6 weeks), and 3) at the final visit (at the end of observation), over a period of about 3 months, taking into account the individual treatment duration. Tapentadol PR was dosed in accordance with the Summary of Product Characteristics, adapted to the individual pain intensity and tolerability, and depending on prior treatment. All treatment decisions were completely at the physician's discretion.
The properties, intake, and mean daily dose of the previous medication were to be taken into account when switching from another opioid. The exact dosage of the previous medication was not included in the questions. After starting treatment, the dose was to be adjusted by means of the usual opioid conversion tables such that adequate analgesia was achieved and undesirable effects were minimized under close monitoring by the prescribing physician. In case of inadequate pain relief, the dose of tapentadol PR could be increased by 2 � 50 mg/day, up to a maximum dose of 2 � 250 mg/day. In contrast to randomized clinical studies with closely defined inclusion and exclusion criteria and treatment protocols, this study design allows an insight into routine therapy under practice conditions in a patient population reflecting the reality of medical care.
Data recording
Initially, demographic and socioeconomic data and concomitant diseases were recorded. Besides pain diagnosis, pain type, and duration, the medicinal and non-medicinal analgesic therapies before starting on tapentadol PR were also documented. Any analgesic used in addition to oxycodone/naloxone was recorded as rescue medication. Also recorded were the main reasons for changing treatment. If the physician established the indication for the use of tapentadol PR after recording the medical history, performing the examination, and recording the findings in a patient with severe chronic pain, the patient could be enrolled in the observation phase.
The patient's individual treatment goal was documented on a goal attainment scale. This goal attainment scale is used to define treatment goals and should be defined in cooperation with the patient [10,11] The individual patient's treatment goal could be related to quality of life, physical functionality, mental well-being, independence, social activities, or ability to work.
The mean pain intensity over the last 3 days was recorded on an 11- point numerical rating scale (NRS-3; with 0=no pain to 10=worst imaginable pain). Functionality was recorded by rating 4 aspects using an 11-point numerical rating scale: patient's difficulty with 1) bending over, kneeling, squatting, 2) shopping, 3) washing or getting dressed and 4) performing leisure activities, where 0=never any difficulty and 10=always difficult.
At all 3 recording time points, besides the mean pain intensity and functionality, tapentadol PR dosages, as well as analgesic and concomitant medication, were documented by the physician. Any analgesic used in addition to tapentadol PR was recorded as rescue medication during the observation period.
Any changes made in pain management were recorded when reviewing the therapy and at the end of observation. At the final visit (after about 3 months or at discontinuation of treatment), the patient estimated achievement of therapy goals by categorization (much better than expected/better than expected/as expected/worse than expected/ much worse than expected) and rated the pain management with tapentadol PR compared to the previous treatment. If observation was discontinued prematurely, the reasons were to be recorded.
Adverse drug reactions (ADRs) were to be recorded throughout the observation period (questioning at visits, reports by the patients). An explicit causality assessment was not performed by the physician. Completion of the ADR report form implied that the physician presumed the existence of a causal relationship between symptoms that occurred and treatment with tapentadol PR (ie. that an ADR was present).
Patient questionnaire
At baseline examination and at the end of observation, patients were asked to estimate their own health-related quality of life using the SF-12 questionnaire (time window: 4 weeks retrograde). The SF-12 is a validated generic instrument that measures 8 dimensions and 2 physical and mental health component summary scores; low summary scores imply poor health, and high summary scores imply good health. The 8 subscales comprise the following dimensions: physical functionality, physical role function, pain, general health perception, vitality, social function, emotional role function, and mental well-being [12]. The time required to complete the questionnaire is about 2 minutes [13], which makes the SF-12 particularly suitable for practical use.
Statistical methods
The case report forms completed by physicians were processed by factum GmbH (Offenbach, Germany) using the DMSys® data management program (version 5.1; double data entry), and all data were checked for completeness, consistency, and plausibility. The effectiveness analysis was performed by factum GmbH using the SPSS statistics package (version 15.0.0).
For the assessment scales to record mean pain intensity and functional impairments, descriptive statistics (i.e., mean, median, standard deviation [SD]), as well as 95% confidence intervals (CIs) for the mean difference, were calculated. Descriptive P values were also calculated for the changes during the course of the study using Wilcoxon signed-rank tests.
The safety and tolerability analysis was performed by PHARMSOFT Dr. B. Rodust GmbH (Ascheberg, Germany). ADRs were coded with the Medical Dictionary for Regulatory Activities (MedDRA®, version 16.1). For the incidence of ADRs, the 95% confidence interval was determined.
Results
Patient selection and demographic characteristics
Altogether, 382 patients of the total NIS population had been treated by prior therapy with a fixed oxycodone/naloxone combination. Patients (58.6% women, 41.4% men) were on average 66.8 (22-94) years old; 62 patients (16.2%) were employed, 14 (3.7%) were looking for work, 279 (73.0%) were retired, 21 patients (5.5%) were in a retirement application procedure. Fifty-seven patients (14.9%) had a care level of 1 (average need for assistance for = 90 minutes/day, with basic care needs for = 45 minutes/day), 24 patients (6.3%) had a care level of 2 (i.e., average need for assistance for = 180 minutes/day, with basic care needs for =120 minutes/day), and 1 patient (0.3%) had a care level of 3 (i.e., average need for assistance = 300 minutes/day, with basic care needs for = 240 minutes/day; must also require regular basic care at night).
One additional patient (0.3%) had a care level of 0 (i.e., had limited everyday skills and requires general supervision, but doesn�t meet the time requirements for level 1), 297 patients (77.8%) had no care level, and data were missing for 2 patients (0.5%). Eight patients (2.1%) were nursing home residents.
Three hundred and seventeen patients (82.9%) had concomitant diseases, most frequently cardiovascular (56.5%) and metabolic (41.6%) diseases and mental illnesses (35.8%, n=147), including 118 patients with documented depression (30.9%).
Pain diagnosis
The most common pain diagnosis was back pain (85.1%), in most cases due to intervertebral disc degeneration (53.4%) and/or spondylarthrosis (39.0%), or spinal stenosis (35.6%), in 239 patients (73.5%) associated with radicular radiation. 250 patients had other documented causes of pain (with multiple causes of pain possible for each patient), predominantly gonarthrosis and coxarthrosis (n=105 [27.5%] and 96 [25.1%], respectively).
Tumor pain was present in 32 patients (8.4%); 49 patients (12.8%) had diabetic neuropathy. A mixed pain type (clinical assessment by treating physician) was reported most frequently (74.6%, n=285). In 37 cases (9.7%), there was a predominantly nociceptive type of pain; and in 43 cases (11.3%), there was a predominantly neuropathic type of pain.
More than half of patients (51.1%; n=195) had pain for longer than 2 years, affecting 27 (43.6%) of the 62 employed patients. One hundred and twelve patients (29.3%) had been hospitalized for pain management in the previous 6 months), 49 of these patients (43.8%) were hospitalized several times.
For patients with hospitalizations due to pain during the last 6 months (n=79), the mean hospitalization period was 16.22 days (minimum 2 days and maximum 66 days, with a median of 14 days). Only 6 of 62 employed persons (9.7%) had no pain-related inability to work in the previous quarter year, while 45 employed patients (72.6%) were off work for a mean of 27.04 days (median 12 days, maximum 92 days) in the previous quarter year.
Previous analgesic management
The previous oxycodone/naloxone treatment was administered as long-term medication without rescue medication in 72.3% of patients (n=276). The procedure for treating patients was decided by the treating physicians based on a clinical assessment of the achieved effect and the undesirable effects. It was not the subject of the study, but the procedure resembled that used for later management with tapentadol.
Seventeen patients (4.5%) had additionally received oxycodone as monotherapy, 18 (4.7%) received transdermal fentanyl, and 22 (5.8%) received a different potent opioid (morphine, hydromorphone, buprenorphine) in addition to oxycodone/naloxone.
About one-third of the previous treatments (29.3%; n=112) also included weak opioids as long-term medication: 64 cases (16.8%) of tilidine/naloxone, 61 cases (15.9%) of tramadol, 13 cases (3.4%) of both. In 299 patients (78.3%) oxycodone/naloxone had been combined with (at least) 1 non-opioid, usually metamizole (42.4%; n=162) and NSAIDs (42.2%; n=161). One hundred and three patients (26.9%) had received analgesic rescue medication, mainly non-opioids (17.8%; n=68).
Co-analgesics (antiepileptics and antidepressants) supplemented the previous treatment of 56.0% of patients (n=214). Antidepressants had been received by 178 patients (46.6%), usually amitriptyline (22.0%; n=84) or citalopram (13.1%; n=50). Antiepileptics had been prescribed for 94 patients (24.6%), usually pregabalin (16.0%; n=61) or gabapentin (7.9%; n=30). Forty-nine patients (12.8%) received antiemetics, 98 patients (25.6%) received laxatives, and 25 patients (6.5%) received both. Dosages of the substances of the previous treatment were not recorded.
On this previous therapy, mean pain intensity at treatment initiation was 7.31 ± 1.39 NRS points (n=381; median, 7.0 points). The painrelated impairment of function for 4 recorded activities in the last week of the previous treatment is shown in (Table 1).
| Patient has difficulties in- | Time point | Mean | SD | Median | 95% CI | Na |
|---|---|---|---|---|---|---|
| Bending, kneeling, stooping | Previous treatment | 7.79 | 1.9 | 8 | 373 | |
| Final | 4.64 | 2.52 | 4 | |||
| Difference | -3.15 | 2.22 | -3.00 | -3.37, -2.92 | ||
| Shopping | Previous treatment | 7.13 | 2.31 | 7 | 373 | |
| Final | 4.15 | 2.76 | 4 | |||
| Difference | -2.98 | 2.31 | -3.00 | -3.22, -2.75 | ||
| Washing or getting dressed | Previous treatment | 5.92 | 2.47 | 6 | 373 | |
| Final | 3.24 | 2.34 | 3 | |||
| Difference | -2.67 | 2.33 | -3.00 | -2.91, -2.44 | ||
| Performing leisure activities | Previous treatment | 7.47 | 2.1 | 8 | 373 | |
| Final | 4.34 | 2.52 | 4 | |||
| Difference | -3.13 | 2.42 | -3.00 | -3.38, -2.89 |
Table 1: Changes in pain-related impairments of functionality for different activities of all patients with data on previous treatment (last week of previous treatment) and at the final visit (last week on tapentadol PR treatment); the difference represents the change between the previous treatment and the end of observation.
Reasons for starting tapentadol PR
In almost all patients, the insufficient analgesia of the previous treatment (87.4%) and impaired quality of life (70.2%) were frequently reported as reasons for starting tapentadol PR. Insufficient effecttolerability ratio (30.4%) or intolerance of the previous medication (34.3%) were other (in some cases additional) reasons for switching treatment in one-third of patients.
Drug interactions with concomitant medication and lacking compliance were (additionally) listed 28 times (7.3%) each. The patients (n=379) attempted to achieve pain relief with a mean NRS target value of 3.27 points (median, 3.0 points). The individual treatment goals agreed upon between the physician and patient usually related to quality of life (74.6%, n=285) and physical function (48.4%, n=185).
Tapentadol PR dosage during the study course
The mean daily dose at the start of treatment was 175.72 ± 93.16 mg tapentadol PR, 233.10 ± 101.13 mg at the follow-up visit, and 252.90 ± 109.90 mg at the final visit. The mean documented duration of treatment was 95.03 days (8-178 days), 340 patients (89.0%) stated that they wished to continue treatment after the end of observation. Treatment was discontinued in 42 patients (10.9%), reasons included inadequate analgesia, hospitalization or intolerance (20, 10 and 5 patients respectively). Only 1 (0.3%) of the 382 patients terminated the treatment within the first 10 days of the tapentadol PR treatment.
Supplementary analgesic treatment during the study course
The prescription of additional analgesics decreased throughout the study period. At the start of tapentadol PR treatment, 68.1% (n=260) were receiving additional long-term analgesic treatment, while 24.6% (n=94) received only tapentadol PR, and a further 6.0% (n=23) received only rescue medication in addition to tapentadol PR. The long-term analgesic medication prescribed in addition to tapentadol PR consisted of another strong opioid in 41 patients (10.7%), a weak opioid in 33 patients (8.6%), and tapentadol was combined with nonopioids in 237 patients (62.0%).
The further reduction of additional analgesic therapy during the study period produced a situation in which 54.9% (n=210) of all patients were still receiving only 1 additional pain medication at the end of observation. Tapentadol PR monotherapy was documented at the final visit in 33.5% (n=128) of patients, while 8.1% (n=31) additionally received only 1 analgesic rescue medication. The proportion of patients prescribed co-analgesic antidepressant medication decreased from 46.6% (n=178/382) at treatment initiation to 39.4% (n=145/368) at the final visit, while the proportion of patients with co-analgesic anticonvulsant treatment decreased from 24.6% (n=94/382) to 15.9% (n=59/369).
The prescription of adjuvants (antiemetic, laxatives) also decreased from 23.7% (90/379 patients) at treatment initiation to 19.0% (70/368 patients) at the final visit. At the end of observation, only 7.1% (26/368) of patients were receiving antiemetics, and 14.9% (55/368) were receiving laxatives (3.0% [11/368] of patients were receiving both).
Analgesia
Pain intensity decreased from a mean ± SD of 7.29 ± 1.40 NRS points at treatment initiation (on oxycodone/naloxone to 3.88 ± 1.86 NRS points at the end of 3 months of observation (on tapentadol PR), corresponding to a mean reduction of pain intensity by 3.41 ± 1.89 NRS points (n=373). At the interim visit, a mean ± SD decrease to 4.52 ± 1.72 NRS points (n=360) was already observed.
The mean target pain intensity was 3.27 NRS points (minimum 0 points, maximum 9 points), with 108 patients (28.5%) stating a target value of 2 or less NRS points, and 10 patients stating 7, 8 and 9 NRS points as the attempted target value.
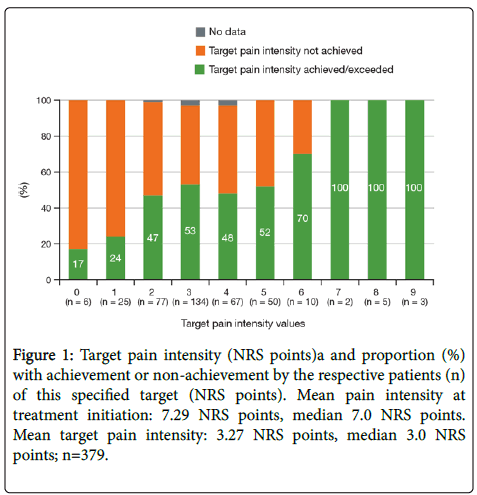
The personally attempted pain reduction was achieved or exceeded by 189 patients (49.5%). At the initial mean pain intensity of 7.29 NRS points, the mean attempted pain reduction corresponds to a decrease of 4.02 NRS points. In fact, a mean reduction in pain intensity of 3.41 NRS points was achieved (about 85% of the target reduction). The relationship between the intended and achieved pain reduction was as expected (Figure 1).
Figure 1: Target pain intensity (NRS points)a and proportion (%) with achievement or non-achievement by the respective patients (n) of this specified target (NRS points). Mean pain intensity at treatment initiation: 7.29 NRS points, median 7.0 NRS points. Mean target pain intensity: 3.27 NRS points, median 3.0 NRS points; n=379.
NRS: Numerical rating scale. aTarget pain intensity was defined by the patient and physician together prior to starting tapentadol PR treatment as an intended mean pain intensity score to be achieved by switching to tapentadol PR.
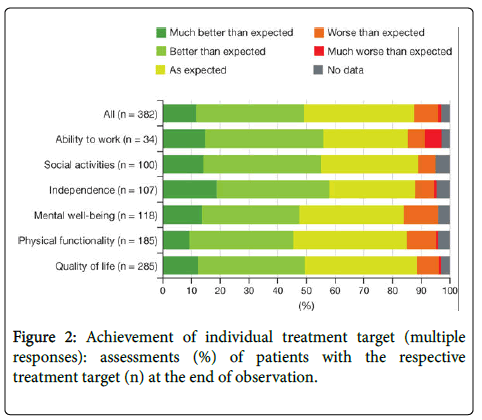
Achieved individual treatment goals
At the end of observation, patients assessed the achievement of their individually agreed treatment goals as generally very positive (Figure 2). Forty-four patients (11.5%) stated that achievement of their treatment goal was �very much better� than expected, 143 (37.4%) stated �better� than expected, and 147 (38.5%) stated �as expected,� while 36 patients (9.4%) stated it was �worse� or �much worse� than expected. For treatment goals in the areas of independence, social activities, and ability to work, more than half of patients achieved their goals �much better� or �better� than expected.
Although many patients positively rated the achievement of goals in the area ability to work, this category also included the highest percentage of patients who were dissatisfied with their goal achievement (�worse� and �much worse� than expected). However, the number of patients with a goal relating to their ability to work is the smallest (8.9%; n=34), which is due to the low number of employed patients in the study population (n=62).
Improvement of pain-related functional impairments
The pain-related functional impairments that had been recorded at the start of the study, during the last week of the oxycodone/naloxone pretreatment, had decreased significantly at the end of observation for all 4 studied activities. Improvements of more than 3.1 NRS points were achieved in each case for bending, kneeling, and crouching, as well as in the performance of leisure activities. Improvements of just under 3 NRS points (-2.98 points) were documented for difficulties in shopping.
The fourth investigated activity, difficulty in washing or getting dressed, was comparatively least impaired by pain (mean initial 5.9 NRS points) at treatment initiation; here too, a significant improvement was determined with a reduction by 2.67 NRS points at the end of observation (Table 1).
Quality of life
For 306 of the 382 total patients (79.8%), completely filled out, evaluable SF-12 questionnaires were available for both time points, at treatment initiation (last 4 weeks of previous treatment) and at the final visit (last 4 weeks of tapentadol PR treatment).
The physical component summary score includes the following subscales: physical functionality (2 items), physical role function (2 items), pain (1 item), and general health (1 item).
The mental component summary score includes the following subscales: vitality (1 item), social function (1 item), emotional role function (2 items), and mental health (2 items). For both the physical and mental component summary scores significant improvements from baseline were documented by the patients at the end of observation (Table 2).
| SF-12 | Mean | SD | Median | N |
|---|---|---|---|---|
| Physical component summary score | ||||
| Initial | 26.17 | 5.43 | 25.13 | 306 |
| Final | 36.49 | 8.14 | 37.26 | 306 |
| Difference | *10.32 | 7.74 | 10.01 | 306 |
| Mental component summary score | ||||
| Initial | 34.41 | 10.02 | 33.06 | 306 |
| Final | 45.32 | 9.94 | 47.43 | 306 |
| Difference | **10.91 | 10.93 | 10.69 | 306 |
Table 2: Change in SF-12 summary scales of all patients with data at treatment initiation (time window: last 4 weeks of previous therapy) and at the final visit (time window: last 4 weeks of tapentadol PR therapy); the difference represents the change between previous treatment and end of observation.
All items showed positive changes, with an improvement of at least 1 scale point in median values of the respective item subscale being reached in 11 of the 12 items (exception: item 5/physical role function; question: Did you have any difficulties with work or other everyday occupational or home activities because of your physical health last week? I could only do certain things: yes=1/no=2. Initial value 1.05; final value 1.47; median at both time points 1.00).
Tolerability and treatment assessment
In the subgroup of patients (n=382) who were previously treated with oxycodone/naloxone, altogether 19 ADRs occurred in 11 patients (2.9%) on tapentadol In the subgroup of patients (n=382) who were previously treated with oxycodone/naloxone, altogether 19 ADRs occurred in 11 patients (2.9%) on tapentadol PR. No ADRs were reported for 371 patients (97.1%). None of the 19 ADRs were serious. Most ADRs corresponded to the known side effect spectrum of tapentadol PR (15; 78.9%). The most commonly reported ADR was nausea (n=3; 0.79%). Four ADRs (21.1%) were classified as unexpected: nervous system disorder (not further specifiable in reporting, n=2; 0.52%), alopecia (n=1; 0.26%), palpitations (n=1; 0.26%; not known for tapentadol PR at the time of performing the study, but still included in the label).
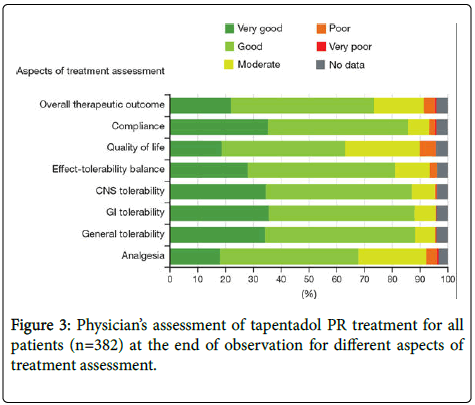
At the end of observation, a therapy assessment was performed for each patient for analgesia, general, gastrointestinal, and CNS tolerability, and for further aspects such as quality of life and compliance. In a few cases, treatment was not rated as positive in these different aspects Figure 3.
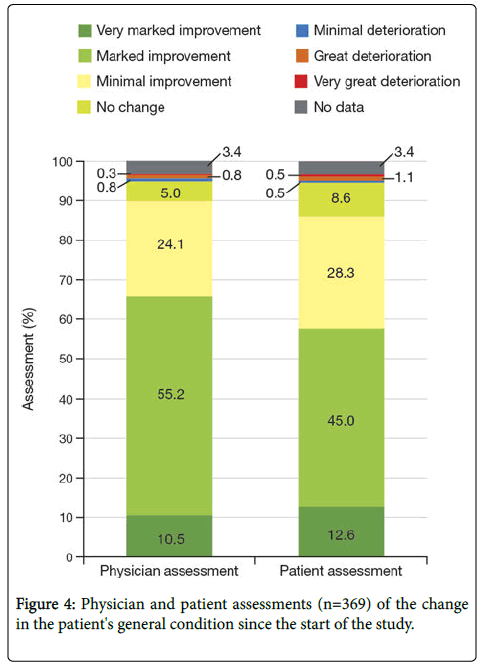
Twenty-three patients (6.0%) were hospitalized due to pain in the observation period while taking tapentadol PR. On average, 1.19 hospital admissions were recorded for 21 of these 23 patients. The mean total duration of hospital stays due to pain was 11.5 days. At the end of the study, the physician and patient were to separately assess the patient's general condition compared to the start of the study. Physicians observed positive changes (very marked, marked, or slight improvements) in 89.8% of patients, while the patients� selfassessments were slightly below this level (85.9%). Minimal, marked, or very marked deteriorations in general condition were observed by physicians in 7 patients (1.8%), and no changes were observed by physicians in 19 patients (5.0%). Six patients (1.6%) saw worsening of their own condition, and 33 patients (8.6%) saw no change in their general condition compared to the start of the study (Figure 4).
Discussion
The study on which the subgroup analysis is based investigates not only the effectiveness and safety of use of tapentadol PR in the routine treatment of severe chronic pain, but also changes in patient�s functionality and health-related quality of life. Thus, central parameters are pain intensity, achievement of individual treatment goals, dimensions of functionality, and the patient's self-assessment of quality of life using the SF-12. The subgroup considered here represents the 382 patients who received prior treatment with a fixed combination consisting of oxycodone (a potent opioid) and naloxone (an opioid antagonist).
Chronic non-specific back pain accounts for the main proportion of the population's pain diagnoses, with only a small number of patients having only 1 cause of pain. Back pain often also has a neuropathic component; the neuropathic pain component dominates the pain syndrome in 37% of cases, as demonstrated by a cohort study of unselected back pain patients [13]. In the present population, just fewer than 75% of the patients showed a mixed type of pain, as assessed by the treating physician.
High disease burden
Demographic characteristics, concomitant diseases, and pain history describe a patient population with in most cases advanced painful disease and a high individual disease burden. The high proportion of 30% with accompanying depression is particularly notable. The fact that approximately 30% of the patients had a mean pain-related hospitalization period of 16 days, as well as work absenteeism in 45 of 62 employed persons, which accounted for a mean 27 days per quarter, indicates the socioeconomic burden.
Previous management
The previous management of the subgroup reflects an overall high analgesic demand, although the substance dosages used were not recorded. The frequency of multiple substance combinations, especially the frequency of the combination of different opioids, which is not recommended, may be seen as evidence of the high pressure for therapeutic action. Notwithstanding the partially pronounced analgesic polymedication, it was not able to provide an acceptable pain reduction in the study population, as demonstrated by the mean pain intensity of 7.31 NRS points at treatment initiation and the predominance of ineffective prior analgesic treatment reported as a reason for starting tapentadol PR. This is also emphasized by the initially identified pain-related functional impairments, which in some cases caused the patient considerable difficulties in performing different everyday activities.
Treatment change-over
In the current subgroup analysis, the primary reason for changing treatment was a lack of efficacy with oxycodone/naloxone. A lack of tolerability with prior oxycodone/naloxone treatment was the contributory reason for approximately one-third of changes in treatment. On one hand, the fact that 70% of the patients were also switched due to insufficient quality of life with prior treatment confirms the individual consequences of chronic, inadequately treated pain for those affected. On the other hand, this explanation also shows that the treating physicians perceive their patients� impaired quality of life as relevant and extremely burdensome. A 5:1 dose ratio of tapentadol PR to oxycodone CR has previously been demonstrated to provide comparable analgesic efficacy [2,14,15] and a similar dose conversion ratio was generally used by clinicians for conversion from oxycodone/naloxone to tapentadol PR in the current noninterventional study.
Although no data on the dosage of the previous medication is available in this non-interventional study, we nevertheless know from other non-interventional studies, in which previous dosages were also evaluated (GRT; data unpublished to date), that the mean oxycodone/ naloxone dose covered the entire range of 10 mg to >80 mg/day.
Impaired quality of life
The physicians� perception is verified by the SF-12 data, documented directly by the patient for the first time in this noninterventional study with tapentadol PR. The scale of quality of life impairment is made clear by a comparison with the validation sample of the German Pain Questionnaire, which includes the SF-12, and with the German Norm Sample [16]. The validation sample for the DGS questionnaire (German Pain Questionnaire) is based on data from 1086 pain patients, with a mean pain intensity of 6.65 NRS-3 points (ie. 0.66 points lower than in the subgroup population) in the previous 4 weeks. The validation sample reaches a mean value of 30.5 points for the SF-12 physical component summary score and of 44.7 points for the mental component summary score, which are considerably lower values than those reported for the German norm sample: 49.6 points (physical component summary score) and 52.3 points (mental component summary score).
The subgroup presented here initially documented even much lower values than the validation sample, namely only 26.17 (physical) and 34.41 (mental) points.
The authors of the DGS validation report indicate that values of less than 29 points in the physical component summary score and less than 44 points in the mental component summary score are also to be regarded as �a problem� for pain patients [16]. Based on this interpretation, the baseline situation of the subgroup presented here is to be regarded as particularly poor at baseline in terms of impaired quality of life, even in a population of pain patients.
Successful pain management with tapentadol PR
The 3-month treatment with tapentadol PR led to a significant reduction in mean pain intensity from 7.29 (on oxycodone/naloxone) to a mean 3.88 NRS points (on tapentadol PR), with marked pain relief to 4.52 NRS points already documented after 4 to 6 weeks. This pain reduction of 3.41 points after 3 months of treatment was achieved with a mean dose of 252.9 mg/day tapentadol PR, with a simultaneous reduction of concomitant analgesics, co-analgesics, and adjuvants compared to the baseline situation. In a previous randomized, doubleblind, placebo- and active-controlled phase 3 study of tapentadol PR in moderate to severe chronic low back pain, a similar reduction in pain intensity of 2.9 points over 15 weeks of treatment was observed at a mean dose of 313.2 mg [14]. In a separate open-label, phase 3b study of tapentadol PR for severe chronic low back pain with or without a neuropathic pain component (a population similar to that in the current sub study), a reduction in pain intensity of 3.9 points over 12 weeks was observed at a mean daily dose of 311.2 mg [17]. Although a pain target of an absolute value of 0 or 1 on an 11-point NRS is very ambitious in chronic pain patients, the findings that 1 of 6 patients aiming for complete freedom from pain (NRS 0) and 6 of 25 patients aiming for NRS 1 actually achieved this objective shows that it is not impossible.
Pain reduction and improvement in quality of life
In addition to reduced pain intensity in the form of NRS values, the improvements in function also represent a patient-relevant result. Parallel to pain reduction, all 4 investigated functionalities showed significant improvements. For example, in the present study, better analgesia was associated with better mobility and easier performance of daily activities.
According to their own perception, the majority of patients also benefited in terms of quality of life because the treatment success of tapentadol PR was reflected not only in the pain reduction and the improvement of pain-related impairments, but also in the SF-12 recorded by patients.
While quality of life before starting the study was impaired to an extent that it can already be described as a problem (mean 26.17 points in the physical component summary score and 34.41 points in the mental component summary score), both values improved significantly to a mean 36.49 points in the physical component summary score and 45.32 points in the mental component summary score at the end of the study, thus improving from the previously highly impaired level [16].
The marked improvement in the mental component summary score is remarkable in view of the high comorbidity of the patient population, for 30% of which depression was documented. Above and beyond the analgesic effect, treatment with tapentadol PR was also able to provide a patient benefit in this respect.
In modern pain medicine, restoring or stabilizing the individual quality of life of chronic pain patients is regarded as a priority therapeutic goal. In this study, well-documented improvements in quality of life and also of patients� general well-being in patients with tapentadol PR treatment, above and beyond the pain reduction, may be an expression of the special mechanism of action of this molecule. This is further confirmed by the fact that it was possible to achieve a relevant reduction in additional long-term analgesic therapy, with the result that more than one-third of the patients did not need additional analgesics at the end of observation. Decreases in the use of coanalgesics were also observed over the course of treatment with tapentadol PR. The reductions in analgesic and co-analgesic use associated with tapentadol PR treatment may be associated with improved compliance with therapy related to reduce pill burden.
Specifically, in indications with a high proportion of mixed type of pain (nociceptive plus neuropathic), tapentadol can exert its moleculespecific properties exceeding a â��pure opioid effectâ�� with the benefits of both µ-opioid receptor agonism (MOR) and noradrenaline reuptake inhibition (NRI), two synergistically acting mechanisms of action are simultaneously brought to bear in a single molecule. Tapentadol PR has shown strong efficacy in clinical studies in different chronic pain conditions [2-4]
Tolerability
The success of a switch in pain management depends not only on the desired analgesia, but also on good tolerability. Here again, data of the subgroup analysis showed tapentadol PR to be beneficial: the fact that 97.1% of patients previously treated with oxycodone/naloxone showed no ADRs after switching treatment shows the generally good tolerability of tapentadol PR treatment. This is also highlighted by the fact that during the change-over phase (ie. in the first 10 days after switching treatment), none of the patients terminated treatment and ADRs occurred in only 11 of 382 patients during the observation period.
Limitations
This subgroup analysis is based on data from a prospective, noninterventional trial under everyday conditions. This subgroup comprised patients who were previously treated unsatisfactorily with oxycodone/naloxone. The opioid was given in a fixed combination with the µ-antagonist naloxone. This combination accounts for about one-third of all oxycodone prescriptions in Germany.
The absence of a randomized control group is a fundamental problem of a non-interventional study, and cannot be completely remedied through comparison with a different sub-population or with the total population. The deviating outline conditions of noninterventional studies in daily practice and randomized clinical studies have already been discussed previously. However, the different study designs are particularly suitable to complement each other, and therefore combine findings from routine practice with data from clinical studies that relate to patients with predefined inclusion and exclusion criteria and controlled outline conditions. It is increasingly accepted that non-interventional studies, despite corresponding limitations, offer a relevant gain in knowledge when implementation to scientific standards is guaranteed [18]. Non-interventional studies, such as the one from which the data presented here originate, reflect daily clinical practice routine and conditions. Non-interventional studies differ greatly from the specifications of clinical study protocols, which especially aim at pain therapy with the test substance as a monotherapy, despite different inclusion and exclusion criteria. This procedure differs fundamentally from daily practice in which a combination of analgesics is the rule, even though these may occasionally include combinations which should ideally be avoided (e.g., the combination of several opioids).
In the study reported here, the quality of life data documented by the patient using SF-12 are a special feature corresponding to a modern, patient benefit-oriented treatment in daily practice.
Summary for Practice
The results of this subgroup analysis from daily practice document a statistically significantly improved analgesia and tolerability of tapentadol PR in patients who had insufficient pain relief and/or inadequate quality of life on previous treatment with oxycodone/ naloxone. The clinical effectiveness of treatment with tapentadol PR enabled partial reduction of the concomitant analgesic medication, and also led to a significantly improved quality of life, as documented by the patients themselves, and an improvement in pain-related daily impairments.
Acknowledgement
The authors thank all physicians who participated in the study, as well as Dr. med. Astrid Anderson-Hillemacher for editing the manuscript and Shannon O�Sullivan of MedErgy (Yardley, USA) for editorial support.
References
- Tzschentke TM, Christoph T, Schröder W, Englberger W, De Vry J, et al. (2011) Tapentadol: with two mechanisms of action in one molecule effective against nociceptive and neuropathic pain. Preclinical overview. Schmerz 25: 19-25.
- Lange B, Kuperwasser B, Okamoto A, Steup A, Häufel T, et al. (2010) Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain. AdvTher 27: 381-399.
- Schwartz S, Etropolski M, Shapiro DY, Okamoto A, Lange R, et al. (2011) Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin 27: 151-162.
- Wild JE, Grond S, Kuperwasser B, Gilbert J, McCann B, et al. (2010) Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract 10: 416-427.
- Schwittay A, Schumann C, Litzenburger B, Schwenke K (2013) Tapentadol prolonged release for severe chronic pain: results of a noninterventional study involving general pratitioners and internists. J Pain Palliat Care Pharmacother 27: 225-234.
- Agbalaka A, Schwenke K, Litzenburger B (2012) Tapentadol prolonged release for the treatment of severe chronic tumor pain in routine clinical practice. MMW Fortschr Med 154: 123-130.
- Lange T, Krings D, Waldmann-Rex S (2015) Clinical practice data regarding tapentadol prolonged release treatment for severe chronic pain � improvement of analgesia, functional competence and quality of life in particular under tapentadolmonotherapy. MMW Fortschr Med 156: 12-21.
- MundiPharma GmbH (2013) Targin (oxycodone hydrochloride and naloxone hydrochloride dihydrate) prescribing information. Limburg, Germany.
- Schwabe U, Paffrath D (2012) Drug prescription report 2012.
- Malec JF (1999) Goal attainment scaling in rehabilitation. NeuropsycholRehabil 9: 253-275.
- Kiresuk TJ, Sherman RE (1968) Goal attainment scaling: a general method for evaluating comprehensive community mental health programs. Community Ment Health J 4: 443-453.
- Ware JE, Kosinski M, Keller SD (1996) A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34: 220-233.
- Freynhagen R, Baron R, Gockel U, Tölle TR (2006) painDETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 22: 1911-1920.
- Buynak R, Shapiro DY, Okamoto A, Van Hove I, Rauschkolb C, et al. (2010) Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. Expert OpinPharmacother 11: 1787-1804.
- Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, et al. (2010) Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig 30: 489-505.
- Pfingsten M, Nagel B, Emrich O, Seemann H, Lindena G, et al. (2012) DeutscherSchmerz-Fragebogen�Handbuch, �berarbeitung.
- Steigerwald I, Müller M, Davies A, Samper D, Sabatowski R, et al. (2012) Effectiveness and safety of tapentadol prolonged release for severe, chronic low back pain with or without a neuropathic pain component: results of an open-label, phase 3b study. Curr Med Res Opin 28: 911-936.
- BfArM (2013) NichtinterventionelleklinischePrüfungen von Arzneimitteln (Anwendungsbeobachtungen).
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 4952
- [From(publication date):
January-2017 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 3909
- PDF downloads : 1043