Research Article Open Access
Tapentadol Prolonged Release as Used in Clinical Practice in Patients with Severe Chronic Tumor Pain
Schwenke K2*, Agbalaka A1 and Litzenburger B31Practice for Specialized Pain Therapy, Kassel, Germany
2Director of Medical Affairs, Knowledge Management, Grunenthal GmbH, Aachen, Germany
3Medical Manager, Medical Affairs, Knowledge Management, Grunenthal GmbH, Aachen, Germany
- *Corresponding Author:
- Karla Schwenke
Grunenthal GmbH
Germany Operations
52099 Aachen, Germany
Tel: +49 241 569-1381
Fax: +49 241 569-2875
E-mail: karla.schwenke@grunenthal.com
Received date: Octomber 09, 2014, Accepted date: February 23, 2015, Published date: March 03, 2015
Citation: Schwenke K, Agbalaka A, Litzenburger B (2015) Tapentadol Prolonged Release as Used in Clinical Practice in Patients with Severe Chronic Tumor Pain. J Palliat Care Med 5:211. doi: 10.4172/2165-7386.1000211
Copyright: © 2015 Schwenke K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provid`ed the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Objective: This subgroup analysis of a non-interventional study that included general practitioners and internists, assessed efficacy and safety of tapentadol prolonged release (Palexia® retard) as used in routine clinical practice in Germany for the treatment of severe chronic tumor pain. Study design: Data of all patients in the study cohort who were exclusively diagnosed with ‘tumor pain’ (n=143) were included in this analysis. Data collection during the 3-month observation period included previous analgesic and concomitant treatment, tapentadol PR dosage, pain intensity, sleep and quality of life parameters and tolerability of tapentadol PR. Results: A total of 96.5% of all patients with tumor pain had already received analgesic long-term treatment before initiation of tapentadol PR therapy, 49.0% of those had received strong opioids. Switching to tapentadol PR resulted in a mean pain reduction of 3.8 points from 7.1 ± 1.4 at baseline to 3.3 ± 1.9 at end of observation (NRS-11, 11-point pain scale; descriptive p value<0.001). At end of observation, 67.4% of the patients had experienced a clinically relevant pain relief of >50%, and 89.9% of the patients attained either their desired pain reduction and/or an additional individual treatment goal; both goals had been predefined at start of tapentadol PR treatment. This was accompanied by a significant decrease in pain-related impairments of daily activities and an improvement in quality of life (descriptive p value<0.001) with an overall good tolerability of tapentadol PR. Treatment with tapentadol PR was assessed positively by physicians and patients. Conclusions: In this routine clinical practice non-interventional study, treatment with tapentadol PR resulted in effective and well-tolerated relief of severe tumor pain and improvement of pain-related impairments of daily activities and quality of life. Tapentadol PR, an innovative effective analgesic, may thus provide an alternative treatment option in the management of tumor pain.
Keywords
Clinical practice; Pain management; Quality of life; Severe chronic pain; Tapentadol PR; Tumor pain
Abbreviations
ADR: Adverse Drug Reaction; ICD: International Classification of Diseases Code; NRS Scale: Numerical Rating Scale; Tapentadol PR: Tapentadol Prolonged Release; WHO: World Health Organization
Introduction
In Germany each year, more than 450,000 people are diagnosed with malignant tumors, which for many patients are associated with persistent, often severe pain, impairment of daily life and enormous loss of quality of life [1]. The prevalence of tumor pain increases with disease progression; at the time of diagnosis about 28% of patients suffer from pain; in the advanced stage, the rate is 70-90% [2]. In more than 80% of the patients in the advanced stage of the disease, the pain is caused primarily by direct tumor infiltration [3]. Despite numerous guidelines on pain management in tumor patients, often inadequate therapy is administered [4]. Traditionally, oral administration of morphine is regarded as the gold standard of pain treatment; however, this has been debated for some time [5-7]. Successful pain management with opioids requires a balance between effective analgesia and good tolerability, which is often difficult to achieve with conventional opioids [8].
Since October 2010, tapentadol prolonged release (Tapentadol PR, Palexia® retard), a centrally acting analgesic for the treatment of severe chronic pain that combines two mechanisms of action in one molecule, has been available in Germany. Tapentadol combines an opioidergic and noradrenergic active component to synergistically produce a potent analgesic effect with a good tolerability profile, in particular through a reduced incidence of opioid-typical gastrointestinal and central nervous system adverse effects [9]. Tapentadol is a strong analgesic corresponding to the WHO stage III.
The indication varies across countries. In Germany, where the study was conducted, the indication for tapentadol prolonged release is for severe chronic pain that can only be treated with opioid analgesics. The broad efficacy across different pain conditions, including cancer pain, and the good tolerability in the treatment of severe chronic pain has been documented in clinical studies using oxycodone and morphine as comparators [10-15] and recently in the first non-interventional study [16]. The data presented below are from a subgroup analysis of this non-interventional study and document the effectiveness of pain treatment in cancer patients with tapentadol PR in routine clinical practice in Germany.
Materials and Methods
In the presented subgroup analysis of a prospective, non-interventional study of the efficacy and safety of tapentadol PR for the treatment of severe chronic pain in routine practice [16], the data of all patients with the sole pain diagnosis ‘tumor pain’ (n=143), were examined. To ensure an exclusive investigation of the efficacy of tapentadol PR in tumor pain, patients who had another pain diagnosis in addition to tumor pain, were not considered in the analysis. The following describes the methodology of the overall study in which the data of 3134 patients with severe chronic pain that could be only adequately managed with opioid analgesics were included in the efficacy analysis.
This study was carried out between October 2010 and June 2011 by general practitioners, family physicians, internists based in Germany and in accordance with the German Medicinal Products Act (AMG). In accordance with statutory requirements, a notification was delivered to the Federal Institute for Drugs and Medical Devices, the National Association of Statutory Health Insurance Physicians, and the Central Federal Association of Health Insurance Funds. A non-interventional study allows an assessment of medication use in actual practice, in a diverse patient population, which more closely corresponds to treatment practice than when following a selected group of patients in randomized clinical trials with narrowly defined inclusion criteria and treatment protocols.
The patients were observed over a period of approximately three months; all treatment decisions were solely at the discretion of the physician. Tapentadol PR was used according to the prescribing information, adapted and titrated to the individual severity of pain being treated and the ability to monitor the patient. Depending on the prior therapy, treatment could be started with 2 × 50 to 2 × 250 mg/day, whereby a dose adjustment in case of insufficient analgesia within three days was recommended [17].
Data Collection and Statistical Evaluation
At three data collection time points; baseline visit (start of therapy), treatment assessment after four to six weeks, and the end of observation after about three months - the treating physician documented the data collected in an observation sheet [16].
During the baseline visit, the demographic data of the patients, existing comorbidities and prior analgesic therapy were recorded by the physician in the observation sheet, along with the pain diagnosis and the reason for switch to Tapentadol PR. At the three data collection time points (as described above), the physician documented the respective tapentadol dose, analgesic and other concomitant medications, and asked the patients about their average pain intensity in the last three days, the diurnal course of pain over the last 24 hours, as well as disturbances in sleep quality, quality of life, social activities, independence and libido in the last four weeks.
The patients described their pain intensity and any pain-related impairments using a numerical 11-point scale (NRS scale) on which they could rate the intensity of the pain from 0=no pain to 10=maximum pain imaginable and the severity of the impairment from 0=no impairment and 10=maximum impairment imaginable [16].
During the baseline visit the physician and patient mutually agreed to the treatment goals that should be achieved over the treatment period. In addition to the desired reduction in pain intensity (NRS pain scale), additional realistically achievable individual treatment goals in the areas of quality of life, physical functioning, psychological well-being, independence, social activities, ability to work and the like were selected and rated by the patient at the end of the observation period as "much better than expected," "better than expected," "as expected,” "worse than expected,” and "much worse than expected" [16].
Over the entire course of the study, the occurrence of adverse drug reactions (ADRs) was monitored by the physician (query at visits, spontaneous reports by patients) to assess the tolerability of Tapentadol PR and documented on an ADR documentation form. An explicit causality assessment was not performed by the physician. The fact that the ADR documentation form was filled out implied that the physician assumed a causal link between the occurring symptoms and treatment with Tapentadol PR, i.e. suspected an ADR [16]. At the end of the observation period, the physician and patient evaluated the treatment with Tapentadol PR.
The observation sheets filled in by the physicians were processed (double data entry) by factum GmbH (Offenbach) using the data management program DMSys® (version 5.1) and all data were checked for completeness, consistency and plausibility. The efficacy analysis was performed by factum GmbH using the statistical program SPSS (version 15.0.0).
For the rating scales for the assessment of the average pain intensity, sleep quality, quality of life, social activities, independence and libido, descriptive p-values for the changes during the course of the study were calculated using Wilcoxon rank tests. The safety and tolerability analysis was done by PHARMSOFT Dr. B. Rodust GmbH (Ascheberg). ADRs were coded with the “Medical Dictionary for Regulatory Activities” (MedDRA®, version 14.0) [16].
Results
Patient demographics
A total of 269 of the 3134 patients in the total cohort with efficacy data indicated the diagnosis "tumor pain." In this sub-analysis, data from 143 patients for whom no other pain diagnosis besides tumor pain was present, were recorded (4.6% of the total cohort). On average, the 78 women (54.6%) and 65 men (45.5%) were 68.5 ± 11 years old; 71.3% of the tumor-pain patients had comorbidities. The most commonly mentioned were cardiovascular disorders (42.7%), mental disorders (23.1%), and metabolic disorders (19.6%).
Total 140 patients (97.9%) had an ICD-10 coding for the cause of pain diagnosis. The most frequent primary localizations were the gastrointestinal tract (n=26, including the pancreas), the urogenital tract (n=18), breast (n=12) and lungs (n=7). The coding of the 47 patients with unclassified and unspecified pain (R52) was ‘chronic unmanageable pain’ (R52.1) in 40 patients and ‘other chronic pain’ (R52.2) in seven patients.
The most commonly documented type of pain was mixed pain (85.3%); 4.9% of the patients had predominantly nociceptive pain and only one patient (0.7%) had predominantly neuropathic pain. The pain lasted up to three months in 39.2% of patients, for three to twelve months in 40.6%, and for more than a year in 20.3%.
Analgesic pre-therapy
The majority of the 143 tumor-pain (96.5%) patients had received a long-term therapy predominantly consisting of several analgesics. Four patients (2.8%) had no prior pain therapy; for one patient, there was no information. The strongest analgesic long-term medications that patients received as immediate pre-treatment were WHO-III analgesics in 49.0% (n=70) of patients and WHO I/II analgesics in 48.3% (n=69) of patients. Fentanyl was the most frequently prescribed strong opioid (47.1% of the 70 WHO-III pre-treated patients) followed by oxycodone/naloxone (18.6%) and morphine (17.1%). As weak opioids, tramadol (59.7% based on the 62 patients who were pre-treated with weak opioids) and tilidine/naloxone (43.5%) were used in the long-term therapy. In addition, 81.8% of all tumor pain patients received non-opioids as long-term medication; the most common medications were metamizole (76.1% of the 117 patients who were treated with non-opioids) and non-steroidal anti-inflammatory drugs (NSAIDs; 35%).
Overall, 36.4% of all tumor pain patients received antidepressants and 11.2% received antiepileptics before starting treatment with tapentadol PR. In addition, laxatives were documented for 28.7% of patients and anti-emetics for 34.3%. Almost 30% of all patients (29.4%) received as-needed analgesics in addition to the above-described long-term therapy.
Reasons for switch to tapentadol PR
Inadequate analgesia (89.5%) and a poor quality of life (58.7%) for tumor patients were the most common reasons for the switch to tapentadol PR for these patients. In addition, insufficient general tolerability (23.8%), inadequate balance between efficacy and tolerability (21.0%), lack of compliance (6.3%) and interactions with concomitant medications (3.5%) were mentioned.
Dosing during the study
In the majority of tumor-pain patients (67.8%), treatment with tapentadol PR was initiated at 2 × 50 mg/day; 27.3% received a starting dose of 2 × 100 mg/day, and 4.9% received at least 2 × 150 mg/day. The strong opioid used most frequently as previous therapy was transdermal fentanyl at a dose of 50-75 µg/h. This corresponds to a morphine equivalent of 120-160 mg/day.
At the start of treatment, the mean daily dose for all patients was 140.6 ± 71.4 mg and amounted to 206.8 ± 105.1 mg at the end of the titration phase. In 28.0% of tumor-pain patients, the titration was done within three days and in 34.3% of patients, within four to seven days; a titration phase of one to two weeks was documented for 18.9% and more than two weeks for 14.0% (no information for 4.9%) of patients. On average, the daily dose at the end of the observation period was 223.1 ± 111.4 mg. At that time, the most frequently documented doses were 2 × 50 mg/day (31.5% of patients), 2 × 100 mg/day (30.1%) and 2 × 150 mg/day (23.1%). Patients on WHO III pre-therapy received an average of 77 mg/day more tapentadol PR than patients on WHO-I/II pre-therapy (262.1 ± 117.5 mg vs. 185.1 ± 90.9 mg). The median treatment duration was 88.5 days; in 87.4% of patients the therapy was continued following the therapy assessment after four to six weeks and in 69.2% of all patients the therapy was continued after the end of the observation period.
Supplemental analgesic therapy
It was possible to reduce administration of a long-term medication in addition to tapentadol PR during the observation period. At the start of therapy, 73.4% of tumor pain patients received an additional long-term therapy (9.8% strong opioids, 15.4% weak opioids, 63.6% non-opioids; multiple indications possible). At end of the observation, 44.1% of patients received additional therapy (8.4% strong opioids, 7.7% weak opioids, 37.8% non-opioids, multiple combinations possible) and 31.5% of patients received only tapentadol PR for the duration of analgesic treatment. At the beginning of the trial, 32.9% (47/143) of patients received additional analgesic break-through pain medication vs. 23.8% (34/143) at the final visit.
The number of patients with prescriptions for antidepressants along with long-term tapentadol therapy decreased from 34.3% at the start of therapy with tapentadol PR to 30.1% at the end of the observation period. For 15 patients, no data on use of antidepressant treatment were available.
Analgesia
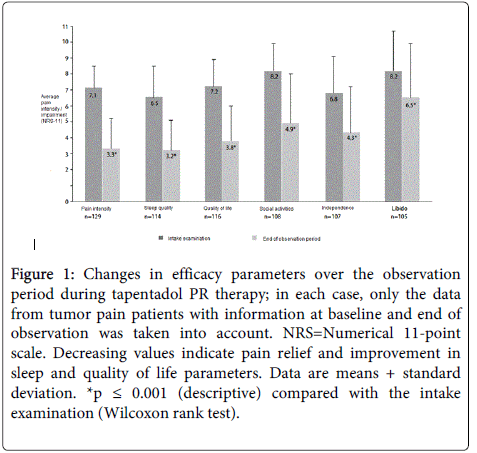
During the observation period, the pain intensity of the tumor-pain patients, which was 7.1 ± 1.4 on average at the baseline visit, was reduced by 3.8 points to 3.3 ± 1.9 during tapentadol PR therapy (Figure 1). At end of the observation period (based on available data for n=129) at least a 50% pain reduction was achieved in 67.4% of the tumor-pain patients. In the tumor-pain patients previously treated in accordance with WHO III, 67% experienced a reduction in pain (based on available data for n=65).
Figure 1: Changes in efficacy parameters over the observation period during tapentadol PR therapy; in each case, only the data from tumor pain patients with information at baseline and end of observation was taken into account. NRS=Numerical 11-point scale. Decreasing values indicate pain relief and improvement in sleep and quality of life parameters. Data are means + standard deviation. *p ≤ 0.001 (descriptive) compared with the intake examination (Wilcoxon rank test).
Pain relief was also observed over the diurnal course. The proportion of tumor pain patients with persistent pain and pain attacks decreased significantly (39.9% before therapy, 7.7% at the end of the observation period). Accordingly, an increase in the proportion of patients with pain-free intervals (from 9.1% to 40.6%) was documented.
Improved sleep and quality of life
Sleep improved by 3.4 points to 3.2 ± 1.9 and quality of life improved to 3.8 ± 2.2, social activities by 3.3 points to 4.9 ± 3.1, independence by 2.5 points to 4.3 ± 2.9, and libido by 1.7 points to 6.5 ± 3.4 (Figure 1). The frequency of nighttime awakening due to pain also decreased. Whereas the majority of patients (74.1%) at the start of observation woke up at least twice a night, at the end of the observation period, this proportion was only 21.7%. The proportion of patients with an undisturbed night's sleep increased from 5.6% to 17.5%.
Achieved treatment goals
Table 1 summarizes the proportion of tumor pain patients who achieved their target pain intensity and/or their agreed additional individual treatment goal. The target pain intensity of the patients at the baseline visit was a mean of 2.9 ± 1.4 points on the 11-point NRS pain scale: The distribution of the individual data showed that most patients (89.5%) wanted to achieve pain intensity with a value of NRS 4 or lower with the tapentadol PR therapy. At the end of the observation period, the mean pain intensity was 3.3 ± 1.9; this corresponds to a deviation of only 0.4 points from the therapy goal set at baseline. A total of 60.5% of all tumor pain patients achieved the intended pain reduction determined at the start of therapy.
| Patients with tumor pain | WHO-III pre-therapy | WHO-I/II pre-therapy | |
|---|---|---|---|
| Desired pain reduction | 60.5% (n=129) | 56.9% (n=65) | 62.3% (n=61) |
| Additional individual treatment goal | 94.9% (n=117) | 94.9% (n=59) | 94.6% (n=55) |
| Response rate (combined)* | 89.9% (n=129) | 89.2% (n=65) | 90.2% (n=61) |
Table 1: Proportion of tumor-pain patients who had achieved their expected treatment goals at the end of observation; *achieved the desired pain reduction and/or the additional individual treatment goal
The most commonly agreed additional individual treatment goals were in the areas of quality of life (84.6%), psychological well-being (33.6%) and physical functioning (25.2%). At the end of the observation period, 94.9% of tumor-pain patients had achieved or exceeded their individual treatment goals: 12.0% judged the pain as "much better than expected," 46.2% indicated "better than expected," and 36.8% indicated "as expected." Overall, by end of the observation period and regardless of prior WHO III or WHO I/II therapy, 89.9% of patients achieved the desired reduction in pain intensity they determined at the start of treatment and/or a further previously agreed individual treatment goal.
Tolerability
For seven (4.9%) of the 143 patients, ten ADRs were reported: dizziness, loss of appetite, nausea (two patients), syncope, diarrhea, vestibular vertigo, blurred vision, confusion, and severe pruritus without rash, mainly on the trunk. All seven patients discontinued tapentadol PR therapy prematurely, six due to an ADR. Overall, tapentadol PR was well-tolerated. No ADRs were reported in 136 patients (95.1%).
Therapy assessment
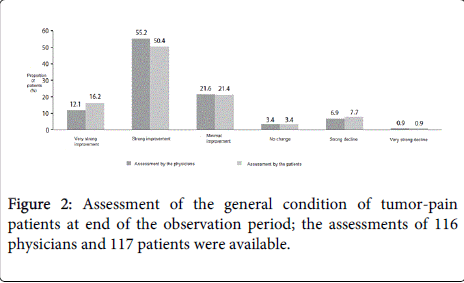
The majority of the treating physicians assessed the various aspects of tapentadol PR therapy, including analgesia effectiveness, and the balance between efficacy and tolerability, positively (good/very good) (Table 2). The assessment by both physicians and patients regarding the change in general condition since the start of therapy was comparable (Figure 2). The majority of both groups evaluated the general condition of the patients as ‘much improved’ to ‘very much improved’ (67.2% of physicians and 66.7% of patients).
| Assessment very good/good | |
|---|---|
| Analgesia | 101 (70.6%) |
| General tolerability | 104 (72.7%) |
| Gastrointestinal tolerability | 96 (67.1%) |
| CNS tolerability | 108 (75.5%) |
| Balance between efficacy and tolerability | 103 (72.0%) |
| Quality of life | 83 (58.0%) |
| Compliance | 106 (74.1%) |
| General therapy success | 95 (66.4%) |
Table 2: Assessment by treating physicians of different aspects of the tapentadol therapy at the end of observation period (n=143 tumor pain patients); Figures indicate proportion of patients (%).
Comparison to the total cohort of the non-interventional study
Compared to the total cohort [14] of the non-interventional study, the tumor pain patients, on average, were slightly older with a higher proportion of men (Table 3). The pain lasted up to three months in 39.2% of the tumor-pain patients; in 40.6% it lasted three to twelve months; and 20.3% had pain for more than a year. This differs significantly from the total cohort in which the majority of patients (61.9%) had pain for more than one year.
| Tumor patients(n=143) | Total cohort(n=3134) | |
|---|---|---|
| Demographic | ||
| Age (years) | 68.5 ± 11 | 66.8 ± 13.6 |
| Men | 45.5% | 39.9% |
| Women | 54.6% | 60.0% |
| Duration of pain | ||
| ≤3 months | 39.2% | 12.7% |
| >3-12 months | 40.6% | 25.1% |
| >1 year | 20.3% | 61.9% |
| Strongest medication in previous therapy | ||
| WHO III | 49% | 42.5% |
| WHO I/II | 48.3% | 55.9% |
| Tapentadol dose (mg/day) | ||
| At start of therapy | 140.6 ± 71.4 | 131.6 ± 62.7 |
| At end of observation period | 223.1 ± 111.4 | 203.7 ± 102.4 |
| Patients receiving tapentadol long-term therapy at the end of treatment | 31.5% | 34.8% |
| Pain intensity (NRS 0-10) | ||
| At baseline | 7.1 ± 1.4 | 7.0 ± 1.5 |
| At end of observation period | 3.3 ± 1.9 | 3.1 ± 1.8 |
| Pain reduction at end of observation period ≥ 50% | 67.4% | 72.1% |
| Achieved treatment goals | ||
| Desired pain reduction | 60.5% | 65.8% |
| Additional individual treatment goal | 94.9% | 89.7% |
| Response rate (combined)* | 89.9% | 89.4% |
Table 3: Selected comparison data for tumor pain patients and total cohort; Data are means ± standard deviation or proportion of patients (%). Percentages may not add to 100% due to missing information in the questionnaire or number rounding. *Achievement of the desired pain reduction and/or the additional individual treatment goal.
More tumor-pain patients than patients in the total cohort received WHO III analgesics as strongest analgesic long-term medications for immediate pre-treatment (49.0% vs. 42.5%). The proportion of non-opioids used as analgesic long-term therapy was comparable (81.8% vs. 82.8% in the total cohort). However, prescription of the non-opioid metamizole, which is among the most frequently used medications in palliative care in Germany [18], was significantly higher (76.1% versus 49.5% for the total cohort). Laxatives (28.7% vs. 20.0%) and antiemetics (34.3% vs. 12.8%) were also prescribed at a higher rate compared with the total cohort.
The average pain intensity at the baseline visit was comparable: sleep and quality of life parameters, however, were more negatively affected in tumor-pain patients than in patients from the total cohort: 6.5 ± 2.0 vs. 6.1 ± 2.0 for sleep quality, 7.2 ± 1.7 vs. 6.8 ± 1.7 for quality of life, 8.2 ± 1.7 vs. 7.2 ± 2.1 for social activities, 6.8 ± 2.3 vs. 5.9 ± 2.4 for independence and 8.2 ± 2.5 vs. 6.5 ± 3.2 for libido. Despite these overall less favorable conditions, a reduction in both intensity of pain and in pain-related impairments occurred with tapentadol PR, which was comparable to the total cohort. 5.2% more tumor-pain patients than patients in the total cohort reached their individual treatment goal.
Discussion
The present subgroup analysis of the first non-interventional tapentadol PR study showed, for the first time, the utility of tapentadol PR as used in routine practice, in patients with severe chronic tumor pain. There are however some limitations to this analysis. The publication is based on a sub-group analysis of a large non-interventional study conducted in Germany in patients with chronic pain due to different non-malignant and malignant origins. As a substantial number of patients with malignant pain were included, a post-hoc sub-group analysis on patients with malignant pain was done. Only those parameters that are captured in clinical routine in treating non-malignant pain were documented. Therefore, the influence of other factors, such as anti-cancer pain treatment on pain severity cannot be ruled out.
The majority of tumor-pain patients (85%) were categorized as having mixed pain; the duration of pain was shorter than in the total patient cohort from the non-interventional study, in which the pain was predominately severe, chronic, and non-tumor related. This may be explained by the life-limiting underlying illnesses of patients with tumor pain. Almost all patients (96.5%) received a long-term analgesic therapy before the start of treatment with tapentadol PR, 49% of which were strong opioids. Despite strong prior pain medication, pain intensity was assessed by many patients as ‘severe’ at the baseline visit and many patients complained of sometimes substantial impairment of their sleep and quality of life. Thus, the main reasons for switching to tapentadol PR therapy were insufficient analgesic efficacy of previous therapy and patients’ reduced quality of life.
In two-thirds of the tumor-pain patients (67%), a three-month treatment with tapentadol PR led to clinically relevant pain relief with at least 50% pain reduction. In the group of patients with WHO III classified pretreatment, this percentage was slightly higher (68%). At the end of the observation period, mean pain reduction was on average only 0.4 points over the 2.9 target at the start of therapy and more than half of the patients (61%) achieved the pain reduction. The pain-free periods increased significantly in patients with persistent pain.
Sleep and quality of life, social activities, independence and libido at baseline visit were more strongly impaired among the tumor-pain patients than among the patients of the total cohort in the non-interventional study.
Despite a worse initial condition, the tumor-pain patients had achieved good results comparable to the total cohort at end of the observation period. Modern pain treatment guidelines now consider the restoration or preservation of individual quality of life of patients with chronic pain as a primary treatment goal. Therefore, the improvement shown in the quality of life and the general condition of these seriously ill patients following treatment with tapentadol PR indicates that there may be benefits beyond pain relief, which should be investigated in future studies.
The additional analgesic long-term therapy these patients required could be significantly reduced with tapentadol treatment. Similar to the total cohort (35%), 32% of the patients were able to completely discontinue additional analgesics by the end of the observation period. The most common tapentadol dosages at the end of observation were 2 × 50 mg to 2 × 150 mg/day and led to strong pain relief, improvement in quality of life, a reduction in additional analgesic long-term therapy. Both dosages were well-tolerated. Given the progressive course of their disease, treatment success in tumor patients may be further improved by using tapentadol PR up to the maximum recommended dose of 500 mg/day, which may offer additional pain-relief.
The majority of tumor-pain patients (85%) suffered with both nociceptive and neuropathic pain. Tapentadol PR’s potent efficacy has been shown for various chronic pain conditions in clinical studies and documented in routine practice [10-16]. Compared with opioids, such as morphine, which primarily act only on the μ-components [6], the synergistic μ-opioid receptor agonism - norepinephrine reuptake inhibition mechanism of action of tapentadol PR could prove to be an advantage for the treatment of pain in tumor patients.
Cancer pain can be characterized as tumor-related, tumor therapy-related and tumor-independent [18]. A corresponding differentiation of cancer pain was not performed in this noninterventional study. Therefore, in this subgroup analysis the efficacy and tolerability of tapentadol PR can only be characterized for strong chronic tumor pain. Information on tumor staging and progression in tumor diseases were not recorded. Therefore, in this subgroup analysis it could not be determined whether the patients were in a palliative stage or in a stage of tumor regression.
Conclusions
Despite the limitations of the subgroup analysis from a routine clinical practice non-interventional study, the results indicate that tapentadol may be efficacious for treatment of severe tumor pain. The results of this sub-group analysis are consistent with those in non-tumor related severe chronic pain. Treatment with tapentadol PR for severe chronic tumor pain resulted in effective and well-tolerated pain relief associated with an improvement in pain-related impairment of daily life and quality of life and was positively assessed by both physicians and patients. This indicates that tapentadol PR, an innovative potent analgesic, may also be successfully used in tumor pain therapy.
Disclosures
Dr. Agbalaka is a consultant for Grünenthal GmbH, Aachen. He participated as a study physician and received an expense allowance for the complete documentation of the patients. Dr. Litzenburger and Dr. Schwenke were employees of Grünenthal GmbH, Aachen at the time the work was conducted. Dr. Litzenburger can now be contacted at the University Of Texas M.D. Anderson Cancer Center, Houston, Texas.
Trial Registration
Clinicaltrials.gov identifier, NCT00472303.
Study Funding
This study was sponsored by Grunenthal GmbH, Aachen.
Acknowledgments
This study was sponsored by Grünenthal GmbH, Aachen. The authors would like to thank all the physicians and patients who participated in the study, as well as Elke Grosselindemann and Birgit Brett, both of whom are independent consultants, for the editing of the manuscript. All costs associated with the preparation of this manuscript were assumed by Grünenthal GmbH. Aachen. Akhilesh Singh, PhD (SIRO Clinpharm Pvt. Ltd.) and Wendy P. Battisti PhD (Janssen Research & Development, LLC) provided reformatting and minor editorial support of this translated version.
References
- Robert Koch institute and the society of population-based cancer registries in Germany cancer in Germany 2007/2008. 8th edition: Berlin (2012).
- Portenoy RK, Lesage P (1999) Management of cancer pain.Lancet 353: 1695-1700.
- Jost L1, Roila F; ESMO Guidelines Working Group (2010) Management of cancer pain: ESMO Clinical Practice Guidelines.Ann Oncol 21 Suppl 5: v257-260.
- Deandrea S, Montanari M, Moja L, Apolone G (2008) Prevalence of undertreatment in cancer pain. A review of published literature.Ann Oncol 19: 1985-1991.
- Bekkering GE, Soares-Weiser K, Reid K, Kessels AG, Dahan A, et al. (2011) Can morphine still be considered to be the standard for treating chronic pain? A systematic review including pair-wise and network meta-analyses.Curr Med Res Opin 27: 1477-1491.
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, et al. (2012) Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC.Lancet Oncol 13: e58-68.
- Caraceni A, Pigni A, Brunelli C (2011) Is oral morphine still the first choice opioid for moderate to severe cancer pain? A systematic review within the european palliative care research collaborative guidelines project. Palliat Med 25(5):402-409.
- Kalso E (2011) The Vicious Circle in chronic pain management: balancing efficacy and adverse effects.Curr Med Res Opin 27: 2069-2071.
- Tzschentke TM, Christoph T, Schröder W, Englberger W, De Vry J, et al. (2011) [Tapentadol: with two mechanisms of action in one molecule effective against nociceptive and neuropathic pain. Preclinical overview].Schmerz 25: 19-25.
- Lange B, Kuperwasser B, Okamoto A, Steup A, Häufel T, et al. (2010) Efficacy and safety of tapentadol prolonged release for chronic osteoarthritis pain and low back pain.AdvTher 27: 381-399.
- Merker M, Dinges G, Koch T, Kranke P, Morin AM (2012) [Undesired side effects of tapentadol in comparison to oxycodone. A meta-analysis of randomized controlled comparative studies].Schmerz 26: 16-26.
- Schwartz S, Etropolski M, Shapiro DY, Okamoto A, Lange R, et al. (2011) Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial.Curr Med Res Opin 27: 151-162.
- Wild JE, Grond S, Kuperwasser B, Gilbert J, McCann B, et al. (2010) Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain.Pain Pract 10: 416-427.
- Imanaka K, Tominaga Y, Etropolski M, van Hove I, Ohsaka M, et al. (2013) Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain.Curr Med Res Opin 29: 1399-1409.
- Kress HG, Koch ED, Kosturski H, Steup A, Karcher K, et al. (2014) Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain.Pain Physician 17: 329-343.
- Schwittay A, Schumann C, Litzenburger BC, Schwenke K (2012) [Tapentadol prolonged release for severe chronic pain. Results of a non-interventional study involving general practitioners and internists].MMW Fortschr Med 154 Suppl 3: 85-93.
- GrA¼nenthal gmbh. Specialized information PalexiaA® retard. Accessed on 06August 2014.
- Drug commission of the German medical association. Drug prescription in practice. In: 34, As of 2012.
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: Open Access
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 14225
- [From(publication date):
March-2015 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9679
- PDF downloads : 4546