Commentary Open Access
T Cell Subpopulations, CCR5 and CXCR4 in Discordant Couples when Compared with Concordant and Healthy Control Subjects
Yohannis M Hambissa1*, Yohannes Mengistu2, Dawit Wolday3, Aster Tsegaye4, Rawley C Howe5, Nick Anderson6, Ermias Hailu7 and Tsehaynesh Messele81Kotebe University College, Addis Ababa, Ethiopia
2Mauricio’s, Botswana, South Africa
3Medical Biotech Laboratory, Addis Ababa, Ethiopia
4Department of Medical Laboratory Science, Addis Ababa University, Addis Ababa, Ethiopia
5Armour Hansen Research Institute (AHRI), Addis Ababa, Ethiopia
7Ethiopian Health and Nutrition Research Institute (EHNRI), Addis Ababa, Ethiopia
8African Society for Laboratory Medicine (ASLM), Bole Road, Behind Friendship Building, Bole sub- city, Kebele 02, Addis Ababa, Ethiopia
- *Corresponding Author:
- Yohannis Meseret Hambissa
Kotebe University College, Addis Ababa, Ethiopia
Tel: 251911627786
E-mail: yohannis_meseret@yahoo.com
Received date: February 02, 2017; Accepted date: February 13, 2017; Published date: February 17, 2017
Citation: Hambissa YM, Mengistu Y, Wolday D, Tsegaye A, Howe RC, et al. (2017) T Cell Subpopulations, CCR5 and CXCR4 in Discordant Couples when Compared with Concordant and Healthy Control Subjects. J Mol Immunol 2:107.
Copyright: © 2017 Hambissa YM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Molecular Immunology
Abstract
Analysis of T cell subpopulations in discordant couples showed no activation of a specific marker between discordant positives and negatives and the expression of T cell subpopulations was comparable. The expression of activation markers was in significantly (P<0.05) higher in concordant couples compared with discordant positives and apparently healthy control, indicating lower immune activation in discordant couples. Moreover, we observed in discordant positives, certain markers (CD4+CD45RA-CD27-) in a high proportion (>30%), similar to non-progressors. CCR5 co receptors were significantly (P<0.05) lower in discordant couples when compared with concordant couples and healthy control. In discordant negatives CD4 and CD8 cells were higher similar with healthy negative controls. Unlike the concordant couples, immune activation markers were significantly low and expressed significantly lower CCR5 receptors. However, high memory T-cells was observed.
Introduction
Different T cell subpopulations may be selectively activated or suppressed in different infections. This is very common in HIV infection in which the collapse of a specific T cell population results in immunodeficiency. The expression of co receptors on T cells may be an important factor in controlling pathogenic events [1]. It is known that chemokine receptors, for example, are down or up regulated and this may increase or decrease susceptibility to HIV infection [2]. HIV uses CCR5 or CXCR4 or both of them to enter the host cell [3-7]. Depending up on the expression and abundance of the chemokine receptors the fate of HIV infection can be determined.
Immune activation is one the factors contributing to susceptibility to HIV infection. Chronic activation has an important role in further driving T cells differentiation [8], which prepares raw materials for HIV infection and fuels its replication. HLA-DR and CD38 are surface phenotypes which are expressed during immune activation [9]. Due to this, their status indicates whether there is immune activation or not. Measurement of nuclear antigen Ki-67 can also indicate the rate of proliferation of T cells [10]. Upon stimulation, T cells can also switch from one form to another [11] and the proportion of the various pools change dramatically and differentially with age [12].T cell differentiation or post-thymic developments, which are similar to both CD4 and CD8 T cell subpopulations, involves sequential down regulation or up regulation of cell surface molecules, which is exploited very well by HIV. T cells are less differentiated [13], while in HIVinfected long-term asymptomatic individuals; highly differentiated memory effector cells are very common.
In Ethiopia healthy population, both proportions and absolute numbers of the effector CD8+ and CD4+ T cells are increased [14,15] and decreased naïve CD8+ T cells and an increase of memory CD8+ T cells in AIDS patients. These results suggest a generally activated immune system among HIV-Ethiopians. These immune alterations observed in adult healthy Ethiopians is not due to genetic differences rather than could be environmental factors [16]. The distribution of T cell subpopulations, CCR5 and CXCR4 among HIV discordant and concordant couples may have a different pattern. Thus, this study, attempts to see the difference in T cell subpopulations among discordant couples compared with concordat couples.
Material and Method
Study area
The study was carried out on HIV discordant, concordant and HIV-seronegative (as a control) couples from January 2009-January 2011 in four administrative regions and Addis Ababa, the capital city of Ethiopia. The subjects were all on follow up for a long time (7-9 years) in their respective health centers and were discordant in their HIV sero-status for a long time.
Study design
The study design was a single spot prospective cross sectional study involving comparisons of T cell subpopulations, their developmental and activation states, and proliferation and effector functions contributing to resistance and/or susceptibility to HIV infection in discordant couples.
Study population and sample size
A total of 27 discordant couples (one HIV+ve and the other HIV-ve Couples), 10 concordant couples (both HIV+ve couples) and 4 low risk seronegative couples (both HIV-ve couples) were investigated for the study. These study subjects (discordant, concordant and seronegative couples) were in a permanent marriage relationship for more than one year and were consistently seronegative or seropositive for a long time and were treatment (Antiretroviral treatment) naïve.
Sample collection, transportation and analysis
20 ml whole blood was collected from each study subject in vacationer tubes in EDTA and transported to the laboratory on the same day it was collected for analysis. Blood samples were always collected at the same time starting early in the mornings from 8:00 AM to 11:30 AM and was analysed within 24 h.
Methods
HIV-testing
HIV testing was performed by using a combination of HIV rapid assays (according to the National HIV testing algorithm) using Determine (Abbott, Japan), Capillus (Biotech, Ireland) and Uni-gold (Biotech, Ireland) and enzyme-linked immunosorbent assay (Vironostica, HIV Uni form Ag/Ab, Boxtel, The Netherlands). The testing involved serial testing algorithm and this was done to re-test subjects which were already tested in their respective health institutions to prove whether the subjects were truly HIV positive or not and hence truly discordant or concordant couples. The enzyme-linked immunosorbent assay was carried out first and samples which were both positive and negative were re-tested by serial testing algorithm and categorized as positive and negative after the completion of serial testing algorithm. Results were interpreted as positive when the test was positive by ELISA and by two successive tests of serial algorithm and negative when it was negative by ELISA and the two successive serial algorithm tests.
Peripheral blood mononuclear cell isolation
Venous blood was collected from the study subjects in EDTA vacutainer tubes, Plasma and blood cells were separated by centrifugation. The plasma was separated and stored at -80ºC until further analysis was carried out. The remaining blood cells were diluted with PBS and layered over Ficoll-Hypaque. After density gradient centrifugation on Ficoll-Hypaque, PBMC was collected and viable frozen in liquid nitrogen until further analysis was carried out.
Cell surface and intracellular staining and analysis
Surface and intracellular staining and analysis were performed using standard flow cytometry procedure by FACSCalibur (BD, San Jose,) as described previously (submitted for publication). Briefly, all staining were carried out by monoclonal antibody (mAb) to which four different kinds of florochromes were conjugated: Allophycocyanine (APC), Peridinin chlorophyll protein (PerCP), Flourescein Isothiocyanate (FITC) and Phychoerythrin (PE) (all from BD, San Jose, CA). Four colour surface staining was carried out by staining with four florochrome conjugated monoclonal antibody as follows: CD8PerCP-CCR5PE-CXCR4FITC-CD4APC, CD8PerCP-HLADRPE-CD38FITC- CD4APC, CD4PerCP-CD45RAPE-CD27APC-Ki67FITC, and CD8PerCP-CD45RAPE-CD27APC-Ki67FITC. PBMC were thawed (RPMI with 10% fetal calf serum, FCS), washed with PBA (phosphate Buffer Saline with 0.05 Bovine Serum Albumin (BSA) and stained for CD4, CD8, CD45RA, CD38, CCR5, CXCR4, CD27, HLADR monoclonal antibodies in dark for 20 min. After a second washing step with PBS, the cells were fixed and permeablized by incubating cells with permeabilization buffer and FACS lysing solution (BD) for 10 min at room temperature. Cells were then washed and stained with Ki-67 FITC for intracellular staining for 20 min at 4ºC in the dark. After a final washing step with permeabilization buffer, analysis was performed using three colors or four colors FACSCalibur (cellquest software, BD). In the lymphocyte gate, 50,000 to 100,000 events were acquired and results were expressed in terms of absolute number (three-color analysis) and as percentage if cell surface markers in four-color analysis. In all cases a control was set up using IgGγ APC and TriTEST (IgGγ1FITC/γ2PE/CD45PerCP. The FASCan/ FACSCalibur was calibrated with CaliBRITE fluorescent beads on weekly basis.
Ethical clearance
The study was conducted after obtaining the national ethical clearance from the Ethiopian science and technology minister and the institutional clearance from Ethiopian Health and Nutrition Research Institute (EHNRI) and Addis Ababa University (AAU). Participation in the study was voluntary. Only patients who gave informed consent were included in the study. The consent form was completed only after the patient had understood the points enumerated in the information sheet.
Data analysis
The collected data was entered and analysed using SPSS version 13 software. Mean, median, mode and standard deviation were collected for many parameters in the study. Results were compared in discordant, concordant and negative control. When the comparisons involved two groups, non-parametric (Mann-Whitney U-test) method was used. But when comparisons were made between three groups or more groups, the level of significance (α) was adjusted using Boferroni corrections (α=0.033). This association between several parameters was determined using a multivariate regression analysis. Correlation coefficients were calculated by the Spearman’s test.
Result
To investigate the proportions of naive, memory and effector T cell subsets, T cell subsets were measured using a combination of CD27 and CD45RA monoclonal antibodies. Activated and resting T cell subsets were measured using a combination of HLA-DR and CD38 monoclonal antibodies and the expression of CCR5 and CXCR4 was measured using a combination of CCR5 and CXCR4 monoclonal antibodies as indicated under material and method. In a similar way, recently proliferating T cell subpopulations of memory, naive and effector T cells were measured by nuclear staining of Ki-67 antigen using Ki-67monoclonal antibodies.
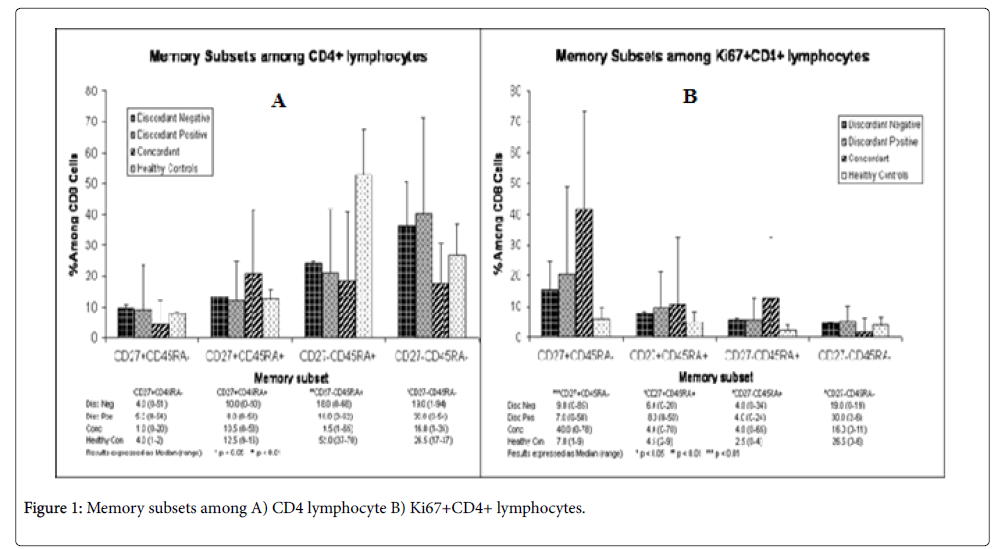
Comparisons between T cell subpopulations were not significantly different in most cases between discordant negatives and discordant positives and the results were also closer to the negative control with some exceptions (Figures 1A and 1B). But the results of concordant couples were in many cases different from both discordant couples and the negative control (Figures 1A and 1B).
CD4+ memory T cells (CD4+CD27+CD45RA-) were almost equal with the negative control in discordant positives and discordant negative and was significantly (p<.05) different from concordant partners (Figure 1A). The difference in the proportion of memory T cells between discordant couples was almost equal. The proportions of naive CD4+(CD4+CD27+CD45RA+) T cell subsets were comparable between all groups although it was lower than the negative control in all cases but was not significant(p>0.05). The proportion of naive and memory T cell subset was almost 1:11 in concordant couples. The ratio of memory to naive T cells (0.5) was highest for discordant positives when compared with discordant negative(0.4) and the negative control(0.32).Thus, there were more memory T cells in discordant negative and positive partners( although naive T cells were greater than memory T cells).
There was no difference on effector T cell subpopulation (CD4+CD27-CD45RA+) among discordant partners and concordant couples (Median IQR, xx+xx versus xx+xx; P=0.). However, these cells were 2-fold greater in the negative controls when compared with discordant and concordant partners and the difference was statically significant (P<0.01).
Memory/effector T cell subpopulation was highest in discordant positives (30%) (Figure 1A), followed by discordant negatives (19%) and was lowest (16%) in concordant couples. The median percentage of memory/effector T cell subpopulation in discordant couples was also greater than the negative control and the difference between discordant and concordant couples was significant (p<0.05). In all groups’ memory/effector T cell subpopulation was highest when compared to naive, memory or effector T cell subpopulations independently.
Rate of T cell subpopulation proliferation was highest (40%) in concordant couples and these were memory T cells. In the remaining subpopulations rate of recent proliferation ranged from 0-11%, indicating slow rate of recent proliferation (Figure 1B). The rate of proliferation was very similar between discordant partners, showing that their rate of proliferation was not different. Overall, currently proliferating T cell subpopulations were highest in concordant couples than in others. For the remaining groups of T cell subpopulations recently proliferating cells were less than 10% but in all the proportion of recently proliferating T cell subpopulations were greater than the negative control.
The ratio of CD27+:CD27- was lowest for concordant couples (0.36) highest in discordant positives followed by discordant negatives (0.43, 0.40), respectively. In discordant positives, the ratio was greater than the negative control and in discordant negatives it was equal to the negative control.
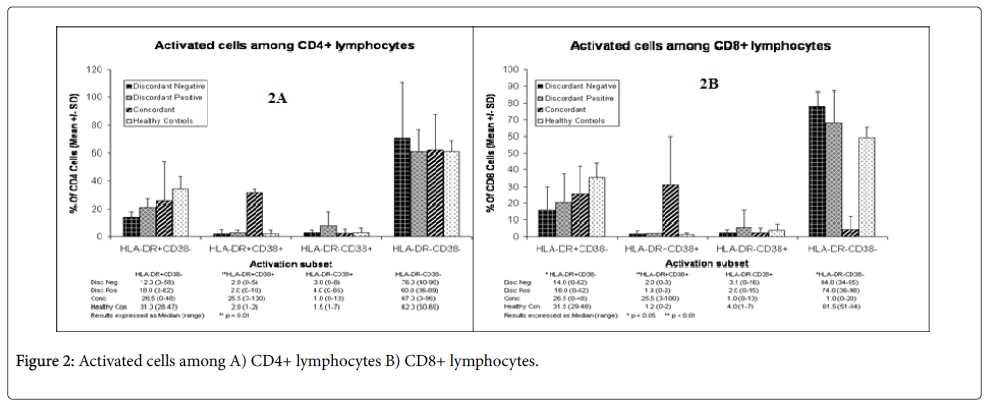
The majority (60-78) of the T cell subpopulations were resting T cells (Figures 2A and 2B), as assessed by the measurement of CD4+HLA-DR-CD38- expression. The remaining subpopulations were activated, although T cells from the negative control were most activated when compared with discordant and concordant couples. Most of the activated T cells expressed CD4+HLA-DR+CD38- markers (Figures 2A and 2B). But in concordant couples equal amount of CD4+HLA-DR+CD38- and CD4+HLA-DR+CD38+ (26.5% vs 25.5%, almost 1:1) (Figure 2A) markers were expressed. T cell subpopulations from discordant positive (18%) were more activated than T cell subpopulations from discordant negatives (12%) but the difference was not statistically significant (p>0.05). But this was highest in concordant couples and the negative control, showing that concordant couples and the negative control subjects were significantly more activated than discordant couples.
Like CD4+ T cells, CD8 T cells from discordant positives and discordant negatives were largely resting T cells (74-84%) (Figure 2B) and this was also similar to the negative control (61.5%). But here the negative control is less than discordant partners. The proportion of resting T cells in concordant couples was much lower (1%) than discordant partners and the negative control and this was highly significant (p<0.01). There were many activated CD8+T cells in concordant couples than in discordant partners (Figure 2B). Like activated CD4+ T cells, the healthy negative controls had also more activated CD8+ T cells than all of them. The difference between the negative control, discordant negatives and concordant couples was also significant (p<0.05). Overall, discordant negatives were the least activated, which were followed by discordant positives. The activated T cells in both CD4+ and CD8+ expressed more HLA-DR+CD38- markers than HLA-DR-CD38+ subpopulations (Figures 2A and 2B).
The majority of T cell subpopulations (80-90%) didn’t express either CCR5 or CXCR4 receptors except in discordant couples (Table 1). CCR5 expression was higher in concordant couples (8%) when compared with discordant positives (4%) and discordant negatives (6%). The negative controls expressed more CCR5 (9.5%). In all CXCR4 expression was lowest (1-2%) including the negative control. The double positives were also lowest in all except in concordant couples (6%); overall the expression of both receptors was comparable between discordant positives and negatives and could not account for sero-discordance.
Discussion
As can be deduced from the median percentile (Figures 1 and 2) of CCR5 and CXCR4, both CCR5 and CXCR4 were expressed in lower amount in both discordant positives and discordant negatives and these were not significant to account for their differences. This is also in agreement with previous studies (both domestic and African) which showed that CCR5 and CXCR4 are lower than other parts of the world [14,17]. In all groups, including the negative control, the double negatives were the majority (85-90%); showing that these receptors were not expressed or expressed in lower amount in the study subjects. Relatively CCR5 receptors were expressed more in concordant couples and the negative control (8-10%).
The reason why these receptors were expressed more (although not significant) in concordant couples and the negative control was not clear from this study. Although it is known that memory cells express these receptors [18], memory T cells were also very small in the group. But cells expressing HLA-DR were found in higher number in concordant couples and the negative control. For concordant couples HLA-DR expression could account for CCR5 expression, since CCR5 expression is associated with increased HLA-DR expression and disease progression [19].
CD4 and CD8 count as well as CD4/CD8 ratio and other immunological profiles, all indicated that concordant couples were at AIDS stage and this may support the idea that increased CCR5 expression may be due to increased expression of HLA-DR. Moreover, there was also a week positive association (r=0.5, p>0.05) (Data not shown) between CCR5 and HLA-DR expression. In general, the difference in the expression of CCR5 and CXCR4 receptors was not significant between discordant partners and concordant couples.
There was no significant difference between discordant couples in the percentage of CD4 HLA-DR+, CD38+, and HLA-DR+ CD38+ markers, which are activation markers, showing that differences in activation markers could not account for the difference between discordant negatives and discordant positives. These subpopulations were almost comparable. The majority of these subpopulations (60-70%) were resting T cells. But the percentage of resting T cells of discordant negative partners was significantly (p<0.05) greater than concordant couples, showing less antigenic challenge in discordant negatives [11].
The proportions of resting CD4+ T cells were comparable between discordant positives and concordant couples and were not significant (p>0.05). The negative control was also similar with the HIV positives. The reason for this is unknown, although it known that there may be antigenic challenge by other infectious agent which may be viral or other pathogenic agent with similar immune profiles.
T cells from Concordant couples were more activated than either discordant positives or discordant negatives and the difference was significant (p<0.05) as can be deduced from the expression of activation markers. This showed that both discordant positives and negatives were less activated than concordant couples. It is known that immune activation increases rate of HIV infection and it is the major cause of intensified HIV infection in Ethiopia [14]. It is possible that particularly discordant negatives were less immune activated, probably due to genetic or other factors and could avoid or abort HIV infection, if any infection had ever occurred. For example, genetic factors which may reduce CCR5 expression can make immune cells less susceptible to HIV infection [18].
Host and environmental factors (like less exposure to environmental pathogen or resistance to infection to environmental pathogens) may also decrease immune activation [12]. Similar mechanism may also operate in discordant positives, enabling them to fight HIV by reducing activation markers and keeping them in check, as activation markers were not significantly different from discordant negatives and the negative controls.
Concordant couples showed typical features of people with AIDS (increased immune activation markers and lower resting T cells) and were completely different from discordant couples. The negative controls also showed more activation markers than discordant negatives, giving evidence for the differently functioning of the immune system of discordant couples. Discordant negatives in particular may be resistant to HIV due to their lower activation markers and discordant positives might have kept HIV at lower level by being less immune activated. This was observed only in our study as no comparisons were made between discordant positive and concordant couples up to now.
The number of activated CD8+ T cells was again significantly higher in concordant couples when compared with discordant couples. The increase of activation markers in CD8+ T cells could also be associated with immune activation and susceptibility of T cells to HIV in concordant couples, as activated CD8+ T cells in AIDS stage are known even to destroy the would be fighter immune cells and cause more damage than help [20]. Thus, the presence of less activated T cells might have benefited discordant couples by reducing immune activation and strengthened the immune system to fight HIV. Although immune activation is prominent in this country [14], these subjects might have less immune activated T cells due to host or environmental factors or, co-infection by other infectious diseases.
Differences in the proportion of naive, memory and effector T cell subpopulations were not significant (p>0.05) between discordant positives and negatives and was very similar to the negative control. As a result, differences in these subpopulations could not account for the difference in susceptibility/resistance to HIV. Memory T cells expressing CD4+CD27+CD45RA- were depleted in concordant couples but were almost equivalent to the negative control in discordant couples. Naive T cell subpopulations were comparable in all groups, including the negative control. The ratio of CD27- to CD27+ was relatively higher in discordant positives than in concordant couples, showing unimpaired and actively differentiating virus-specific T cells inhibiting or retarding disease progression [13]. Thus, this is additional evidence that discordant positives showed long- term- non-progressor’s profile and this in agreement with previous features of long-term-non-progressors.
A relatively higher proportion of CD4+CD27-CD45RA- memory/ effector T cell subpopulation, which were significantly different from both discordant negatives and concordant couples, was observed in discordant positives, a scenario which is frequently observed in long-term –non-progressers.. This T cell subpopulation is known in delaying disease progression in HIV positives but with no progressing disease. The presence of CD27-effector/memory T cell subsets in higher proportion in discordant couples (in particular discordant positives), showed repeated antigenic challenge and hence efficient immune system capable of keeping the viral load to a minimum, preventing further depletion of CD4 and disease progression. The observation that proliferation increased in CD4+CD27+CD45RA-memory T cells might be a homeostatic mechanism to replace the depleted memory T cells in an effort to maintain the immune system in concordant couples, although the final fate of these cells would also not be different. Therefore, one could suggest that this was repeated division in an attempt to compensate for the loss of depleted memory T cell but would finally lead to T cell wastage.
The reason why effector T cells were higher in the negative control was not clear from this study. But since immune activation is the characteristics of healthy Ethiopians, this may be the reason for the elevated number of effector T cells [14]. Repeated antigenic challenge from the environment may be the underlying reason for this [16]. This also showed that discordant couple’s immunity is different from healthy individuals.
In general, healthy T cell subpopulations similar to the negative control (even sometimes better), reduced activation markers, and hence reduced immune activation, and others might have enabled the discordant negatives to eradicate HIV and develop resistance. Discordant positives might have controlled viral load by reducing the activation markers and hence immune activation and using their immune system to fight HIV. The presence of CD4+ CD45RA-CD27- memory/effector T cells in higher proportion (30%) might have helped in delaying disease progression and further decline of CD4 T cells [13].
Higher CD27- to CD27+ ratio also indicated a relatively efficient immunity in discordant positives. But these factors alone could not be responsible for the resistance/susceptibility to HIV, as HIV/AIDS is more complicated than this. But these might be a share contributed by the immune system, helping other known or unknown host and genetic factors responsible for resistance and susceptibility to HIV. HIV resistance/susceptibility could not be caused by a single factor but a concerted activity of host, immunological, virological and genetic factors. Hence, further characterization and extended studies might elucidate how these complicated factors might prevent HIV.
Recent observation indicated that within the latent reservoir of some long-term-treated individuals, there was a population of proviral molecules, which contain a high level of DNA methylation presenting at the HIV-1 5' LTR sequences [21]. Since these methylation marks located at 5' LTR are able to restrict HIV-1 reactivation [22], understanding the mechanisms that associated with establishment and maintenance of these marks might shed light on fully activation of the latent virus and eventually eliminate them. As DNA methyltransferases (DNMTs) are the main enzymes that play a role in the establishment and maintenance of DNA methylation [23], it is worth to investigate the levels of DNMTs in the infected lymphocytes in patients. Interestingly, since H3K9me3 modifier Suv39h1 is responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency [24], and H3K9me2 modifiers G9a and GLP are also involved in the maintenance of DNA methylation [25], affect the levels of H3K9 methylation and histone modifiers associated DNA methylation might also create a novel direction for the treatment of latent virus in patients.
Conclusion
Analysis of T cell subpopulations in discordant couples showed no activation of a specific marker between discordant positives and negatives and the expression of T cell subpopulations was comparable. In discordant positives the immune system was perfectly healthy and there was no indication of any abnormality. Rather, activation markers, which are indicators of immune activation, were significantly lower than concordant couples. These less activated immune cells, with others, might have enabled them to fight HIV. In discordant positives, in addition to decreased number of activation markers there were also expression of certain markers (CD4+CD45RA-CD27-) in higher proportion, which were common in long-term-non-progressors. The presence of this marker in higher proportion (30%), with others, might have enabled them to fight HIV and prevented further spread, but might have not been able to clear HIV completely due to some unknown factors.
References
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW (1999) Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte Subjects, dendritic cells, and differentially conditioned monocyte derived macrophages. Proc Natl Acad Sci USA 96: 5215-5220.
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A (1999) Two subsets of memory T lymphocytes with distincthoming potentials and effector functions. Nature 401: 708-712.
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, et al. (1996) The Lymphocyte Chemoatractant SDF-1 is a ligendfor LESTR/fusin and blocks HIV-1entry. Nature 382: 829-833.
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, et al. (1996) HIV-1 entry CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381: 667-673.
- Grivel JC, Margolis LB (1991) CCRS and CXCR4 tropic HIV-1 are equally Cytopthic for their T cell targets in human lymphoid tissue. Nat Med 5: 344-346.
- Baggiolini M, Dewald B, Moster B (1997) Human Chemokinis; an update. Annu Rev Immunol 15: 675-705.
- Barker E, Mackewicz CE, Reyes-Terán G, Sato S, Stranford SA, et al. (1998) Virological and Immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to AIDS. Blood 92: 3105-3114.
- Appay V, Rowland-Jones SL (2002) Premature ageing of the immune system: the cause of AIDS?. Trend Immunol 23: 580-586.
- Kerstens L, Vanham G, Gigase P, Bach BA (1992) Expression of activation antigens HLA-DR and CD38 on CD8+ T lymphocytes during HIV-1 infection. Clin Exp Immunol 6:793-797.
- Clark DR, de Boer RJ, Wolthers KC, Miedema F (2000) T cell Dynamics in HIV-1 Infection. Adv Immunol 73:301-325.
- De Rosa SC (2001) II-Color, 13 Parameter flow Cytometry: identification of human naïve T- Cell receptor diversity. Nat Med 7: 245-248.
- Douek DC, Picker LJ, Koup RA (2003) T cell Dynamics in HIV-1Infection. Annu Rev Immunol 21: 265-304.
- Hentzen R (1993) Regulation of CD27 expression on subsets of mature T lymphocytes. J Immunol 151: 2426-2435.
- Messele T, Abdulkadir M, Fontanet AL, Petros B, Hamann D, et al. (1999) Reduced naive and increased activated CD4 and CD8 Cells in healthy adult Ethiopians compared with their Dutch Counterparts. Clin Exp Immunol 115: 443-450.
- Kassu A, Tsegaye A, Petros B, Wolday D, Hailu E, et al. (2001) Distribution of Lymphocyte subsets subjects in healthy human immunodeficiency virus negative adult Ethiopians from two Geographical Locales. Clin Diagn Lab Immunol 8: 1171-1176.
- Tsegaye A (2004) T cell dynamics and HIV specific CTL responses in Ethiopians; HLA class I frequencies in healthy and HIV infected Ethiopians, Ethio-Netherland AIDS Research Project (ENARP). Pp57-71.
- M. Fort, de Stefano GF, Cambon-Thomsen A, Giraldo-Alvarez P, Dugoujon JM, et al. (1998) HLA class 11 allele and Haplotype frequencies in Ethiopian Amhara and Oromo populations. Antigens 51: 327-336.
- Baggiolini M, Dewald B, Moster B (1997) Human Chemokines; an update. Annu Rev Immunol 15: 675-705.
- Gupta k, Klasse RJ (2006) How do viral host factors modulate the Sexual Transmission of HIV. PLOS Medicine 3: 181-185.
- Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM (2002) CD4 T cell depletion is linked toimmune activation in the pathogenesis of HIV-1 and HIV-2 but only in directs to viral load. JImmunol 169:3400-3406.
- Katerina Trejbalova (2016) Development of 5‘ LTR DNA methylation of latent HIV-1 provirus in cell line models and in long-term-infected individuals. Clin Epigenetics 8:19.
- Kauder SE (2009) Epigenetic Regulation of HIV-1 Latency by Cytosine Methylation. Plos Pathogens 5.
- Chen T, Li E (2006) STAT3: A Target to Enhance Antitumor Immune Response. Cur Top Microbial Immunol 130: 179-201.
- du Chenel RTX Calcium Binding Motifs Are Intrinsically Disordered in the Absence of Calcium implication for protein secretion. (2007) EMBO J 26: 424-435.
- Zhang (2016) Retraction Notice to: Gut-Colonizing Bacteria Promote C. elegans Innate Immunity by Producing Nitric Oxide. Cell Rep 15: 77-85.
Relevant Topics
- Bacteriostatic antibiotics
- Cell signaling and activation
- Chemokines
- Class I MHC molecules
- Class II MHC molecule
- Colitis Antibiotics
- Immune response
- Immunochemistry
- Immunogenicity of biopharmaceuticals
- Immunogenomics
- Immunoglobulins
- Immunoglycomics
- Immunomodulatory xenobiotics
- Immunopharmacology
- Immunoproteomics
- Immunosenescence
- Immunotolerance
- Molecular Immunology
- Non classical MHC class I molecules
Recommended Journals
Article Tools
Article Usage
- Total views: 3102
- [From(publication date):
June-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 2263
- PDF downloads : 839