Review Article Open Access
Synthetic Biodegradable Polymers Used in Controlled Drug Delivery System: An Overview
| Amit Jagannath Gavasane1 and Harshal Ashok Pawar2* | |
| 1Research Scholar (M.Pharm.), Dr. L.H. Hiranandani College of Pharmacy, Ulhasnagar, Maharashtra, India | |
| 2Assistant Professor and HOD (Quality Assurance), Dr. L.H.Hiranandani College of Pharmacy, Ulhasnagar, Maharashtra, India | |
| Corresponding Author : | Harshal Ashok Pawar Assistant Professor and Head of Department (Quality Assurance) Dr.L.H.Hiranandani College of Pharmacy Smt. CHM Campus, Opp. Ulhasnagar Railway Station Ulhasnagar-421003, Maharashtra, India Tel: +91-8097148638 E-mail: hapkmk@rediffmail.com |
| Received September 08, 2014; Accepted September 18, 2014; Published September 22, 2014 | |
| Citation: Gavasane AJ, Pawar HA (2014) Synthetic Biodegradable Polymers Used in Controlled Drug Delivery System: An Overview. Clin Pharmacol Biopharm 3:121.doi:10.4172/2167-065X.1000121 | |
| Copyright: 2014 Gavasane AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Polymers are becoming increasingly important in the field of drug delivery. The pharmaceutical applications of polymers range from their use as binders in tablets to viscosity and flow controlling agents in liquids, suspensions and emulsions. Use of polymer is now extended to controlled release and targeting drug delivery system. Polymers are obtained from natural source as well as synthesized chemically. Polymers are classified as biodegradable and nonbiodegradable. Biodegradable polymers have been widely used in biomedical applications because of their known biocompatibility and biodegradability. The present review gives an overview of the different biodegradable polymers that are currently being used in the development of controlled drug delivery system.
| Keywords |
| Synthetic polymer; Drug delivery; Biodegradable polymer |
| Introduction |
| Drug delivery is the method or process of administering pharmaceutical compound to achieve a therapeutic effect in humans or animals [1,2]. Drug delivery technologies modify drug release profile, absorption, distribution and elimination for the benefit of improving product efficacy, safety, as well as patient compliance and convenience [3]. Controlled drug delivery technology represents one of the most rapidly advancing areas of science in which chemists and chemical engineers are contributing to human health care. Such delivery systems offer numerous advantages compared to conventional dosage forms including improved efficacy, reduced toxicity, and improved patient compliance and convenience. Such systems often use synthetic polymers as carriers for the drugs [4]. The objective of the present review was to compile information about various biodegradable polymers that are currently used in the development of controlled drug delivery system. |
| Controlled drug delivery system (CDDS) |
| Controlled release dosage forms cover a wide range of prolonged action formulations which provide continuous release of their active ingredients at a predetermined rate and for a predetermined time. The majority of these formulations are designed for oral administration; however recently such devices have also been introduced for parenteral administration, ocular insertion and for transdermal application. The most important objective for the development of this system is to furnish an extended duration of action and thus assure greater patient compliance [5]. |
| Advantages of controlled drug delivery |
| Controlled drug delivery system has various advantages over conventional drug delivery as discussed below [5]: |
| • Decreased occurrence and intensity of adverse effects and toxicity. |
| • Better drug utilisation and reduced dosing frequency. |
| • Controlled rate and site of release. |
| • More uniform drug concentration in systemic circulation |
| • Improved patient compliance. |
| • More reliable and prolonged therapeutic effect. |
| • A greater selectivity of pharmacological action. |
| Classification of Polymer |
| Polymers are macromolecules having very large chains contain a variety of functional groups, can be blended with low and high molecular weight materials. Polymers are becoming increasingly important in the field of drug delivery. Advances in polymer science have led to the development of several novel drug delivery systems [3]. Polymers are classified into the two major types [6-10] as mentioned below. |
| Natural polymer |
| 1. Protien-based polymer: Collagen, Albumin, Gelatin |
| 2. Polysaccharides: Alginate, Cyclodextrin, Chitosan, Dextran, Agarose, Hyaluronic acid, Starch, Cellulose |
| Synthetic polymers |
| Biodegradable polymer |
| a) Polyester: Poly lactic acid, Poly glycolic acid |
| Poly hydroxyl butyrate, Polyester, Polycaprolactone |
| Poly lactide-co-glycolide (PLGA), Poly diaxonone |
| b) Polyanhydride: Poly adepic acid, Poly sebacic acid |
| Poly terpthalic acid, |
| c) Polyamides: Poly amino acid, Poly imino carbonate |
| d) Phosphorous based polymer: Polyphosphates, Poly phosphonates, Poly Phosphazenes |
| e) Others: Poly cyanoacrylates, Poly urethanes, Poly ortho ester, Polyacetals etc. |
| Non-Biodegradable polymers |
| a) Cellulose derivative: Carboxy methyl cellulose, Ethyl cellulose |
| Cellulose acetate hydroxyl propyl methyl cellulose |
| b) Silicons: Polydimethyl siloxane, Colloidal silica, Polymethacrylate, Polymethyl methacrylate |
| c) Others: Poly vinyl pyrolidine, Ethyl vinyl acetate, Poloxamine etc. |
| Why synthetic polymers preferred over natural polymers? |
| Natural polymers suffer from some disadvantages as summarized below: |
| Microbial contamination-The moisture present in the gums and mucilages is normally 10% or more and, structurally, they are carbohydrates and, during production, they are exposed to the external environment and, so there is a chance of microbial contamination. |
| Batch to batch variation-Synthetic manufacturing is a controlled procedure with fixed quantities of ingredients, while the production of gums and mucilages is dependent on environmental and seasonal factors [11]. |
| Uncontrolled rate of hydration-Due to differences in the collection of natural materials at different times as well as differences in region, species, and climate conditions the percentage of chemical constituents present in a given material may vary. There is a need to develop suitable monographs on available gums and mucilages. Reduced viscosity on storage normally, when gums and mucilages come into contact with water there is an increase in the viscosity of the formulations. Due to the complex nature of gums and mucilages (monosaccharides to polysaccharides and their derivatives), it has been found that after storage there is reduction in viscosity [12]. |
| The use of synthetic polymer overcomes above disadvantages and hence their use in formulation is preferred. |
| Considerations for Selection of Polymers |
| The selection of a polymer is a challenging task for controlled drug delivery system because of the inherent diversity of structures and thus it requires a thorough understanding of the surface and bulk properties of the polymer that can give the desired chemical, interfacial, mechanical and biological functions. The choice of polymer, in addition to its physico-chemical properties, is dependent on the need for extensive biochemical characterization and specific preclinical tests to prove its safety. Surface properties such as hydrophilicity, lubricity, smoothness and surface energy govern the biocompatibility with tissues and blood, in addition to influencing physical properties such as durability, permeability and degradability [13]. The surface properties also determine the water sorption capacity of the polymers, which undergo hydrolytic degradation and swelling (hydrogels) [14]. Bulk properties that need to be considered for controlled delivery systems include molecular weight, adhesion, solubility based on the release mechanism (diffusion or dissolution controlled), and its site of action [15]. Structural properties of the matrix, its micromorphology and pore size are important with respect to mass transport (of water) into and (of drug) out of the polymer. For non-biodegradable matrices, drug release in most cases is diffusion-controlled and peptide drugs with low permeability can only be released through the pores and channels created by the dissolved drug phase [16]. Polymer should have some characteristics so that specific polymer can be select for the drug delivery system. |
| Following characteristics needs to be considered while selecting polymer for CDDS. |
| � It should be versatile. |
| � It should possess a wide range of mechanical, physical and chemical properties. |
| � It should be non�toxic and have good mechanical strength |
| � It should be inexpensive and easy to construct. |
| � It should be inert to host tissue and compatible with environment. |
| Synthetic Biodegradable Polymers |
| There are various synthetic biodegradable polymers currently being investigated as drug delivery systems or as scaffolds for tissue engineering [17]. Biodegradable polymers are mainly used where the transient existence of materials is required and they find applications as sutures, scaffolds for tissue regeneration, tissue adhesives, haemostats, and transient barriers for tissue adhesion, as well as drug delivery systems. Each of these applications demands materials with unique physical, chemical, biological, and biomechanical properties to provide efficient therapy. Consequently, a wide range of degradable polymers, both natural and synthetic, have been investigated for these applications. However, natural polymer composition varying from source to source |
| Advantages of Biodegradable Polymers as Drug Carriers |
| The five most important advantages of Biodegradable polymers as drug carriers include: localized delivery of drug, sustained delivery of drug, stabilization of the drug, release rate which is less dependent on the drug properties and steady release rate with time [18]. |
| Drug release mechanisms for Controlled drug delivery |
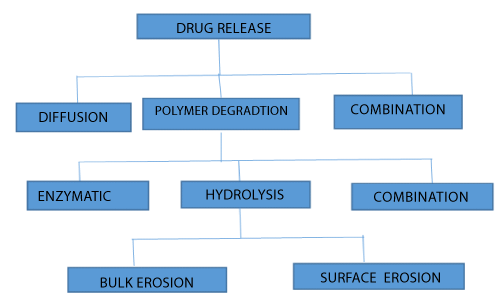
| The possible drug release mechanisms for polymeric drug delivery are depicted in Figure 1. |
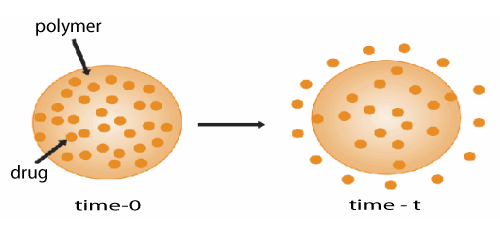
| Controlled-release approaches can be classified on the basis of the mechanism that controls the release of the pharmaceutically active agent from the delivery system by diffusion, osmosis, or polymer erosion. In some cases, the term ‘biodegradation’ is limited to the explanation of chemical processes, while ‘bio erosion’ may be limited to refer to physical processes that result in weight loss of a polymer device. General mechanism for controlled drug delivery system is shown in Figure 2. |
| The degradation is primarily the process of chain cleavage leading to a reduction in molecular weight. On the other hand, erosion is some all of the processes leading to the loss of mass from matrix of polymer [19,20]. |
| Degradation by erosion normally takes place in devices that are prepared from soluble polymers. In such cases, the device erodes as water is absorbed into the systems causing the polymer chains to hydrate, swell, disentangle, and finally dissolved away from the dosage form. Alternatively, degradation can also result from chemical changes to the polymer including cleavage of covalent bonds, ionization and protonation of polymer backbone or side chains. The erosion mechanism of polymers can be described both physically and chemically. |
| Chemical erosion: There are three general chemical mechanisms that cause bio erosion. |
| Type I erosion: This type of erosion is evident with water soluble polymers cross-linked to form three-dimensional network. As long as crosslinks remain intact, the network is intact and is insoluble. When it is placed in aqueous environment, it swells only to the extent permitted by its cross-link density [20]. Generally, degradation of type IA polymers provide high molecular weight, water-soluble fragments, while degradation of type IB polymers provide low molecular weight, water soluble oligomers and monomers. |
| Type II erosion: This type of erosion occurs with polymer that were earlier water insoluble but converted to water soluble forms by ionization, protonation or hydrolysis of the pendant group [20]. With this mechanism, the polymer does not degrade and its molecular weight remains essentially unchanged. Materials like cellulose acetate derivatives and partially esterified copolymers of maleic anhydride are displaying type II erosion. These polymers showing type II erosion and become soluble by ionization of carboxylic group [18]. |
| Type III erosion: Degradation of insoluble polymers with labile bonds Hydrolysis of labile bonds causes scission of the polymer backbone, thereby forming low molecular weight, water-soluble molecules. Polymers like poly (lactic acid), poly (glycolic acid) and their copolymers, poly (ortho esters), undergoes type III erosion. The three mechanisms described are not mutually exclusive; combinations of them can occur [18]. |
| Physical erosion: The physical erosion mechanisms can be characterized as heterogeneous or homogeneous type. In heterogeneous erosion, also called as surface erosion, the polymer erodes only at the surface, and sustains its physical integrity as it degrades. Crystalline regions eliminate water. Therefore, highly crystalline polymers tend to undergo heterogeneous erosion. Most polymers undergo homogeneous erosion, means the hydrolysis occurs at uniform rate throughout the polymeric matrix. Generally these polymers tend to be more hydrophilic than those showing surface erosion. As a result, water penetrates the polymeric matrix and increases the rate of diffusion. In homogeneous erosion, there is loss of integrity of the polymer matrix [18]. Hydrophilic excipients can accelerate the release of drugs, though they may also increase the initial burst effect [21]. |
| Factor affecting Biodegradation |
| Following are the factors which affect the biodegradation process of Polymer [22,23]. |
| Synthetic Biodegradable Polymers for CDDS |
| Polylactic acid (PLA) |
| PLA is thermoplastic biodegradable polymer produced synthetically by polymerization of lactic acid monomers or cyclic lactide dimmers (Figure 3). Lactic acid is produced by fermentation of natural carbohydrates for example, maize or wheat or waste products from the agricultural or food industry. PLA has number of biomedical applications, such as sutures, stents, dialysis media and drug delivery devices. |
| Aliphatic polyester undergoes bio-degradation by bulk erosion. The lactide/glycolide chains are cleaved by hydrolysis to the acids and are eliminated from the body through Krebs cycle, primarily as carbon dioxide and in urine [24]. Slow release drug delivery system with Polylactic acid hydrogels was developed for Mitomycin C and Dexamethasone sodium phosphate for prevention of tracheal wall fibroplasias [25]. |
| some examples of the drugs with which PLA used for controlled drug delivery system are shown in Table 1 [20]. |
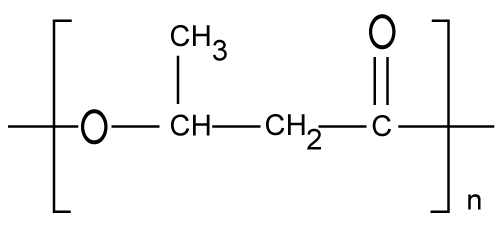
| Polyglycolic acid (PGA) |
| PGA (Figure 4) is commonly obtained by ring-opening polymerization of the cyclic diester of glycolic acid, glycolide [26,27]. PGA is a hard, tough, crystalline polymer with a melting temperature of 225°C and a glass transition temperature, TG, of 36°C [26]. Unlike closely related polyesters such as PLA, PGA is insoluble in most common polymer solvents. PGA has excellent fiber-forming properties and was commercially introduced in 1970 as the first synthetic absorbable suture under the trade name Dexon™ [26]. The low solubility and high melting point of PGA limits its use for drug delivery applications, since it cannot be made into films, rods, capsules, or microspheres using solvent or melt techniques. Lactide/glycolide polymers, show wide range of hydrophilicity which makes them versatile in designing controlled release system [24]. |
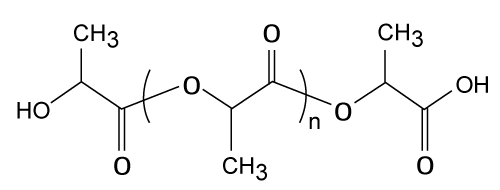
| Poly (lactide-co-glycolide), PLGA |
| Both L- and DL-lactides have been used for co polymerization. The ratio of glycolide to lactide at different compositions permits control of the degree of crystallinity of the polymers [28]. When the crystalline PGA is co-polymerized with PLA, the degree of crystallinity is decreased and as a result this leads to increases in rates of hydration and hydrolysis. It can therefore be concluded that the degradation time of the copolymer is related to the ratio of monomers used in production. In general, the higher the content of glycolide the quicker the rate of degradation. However, an exception to this rule is the 50:50 ratio of PGA: PLA, which shows the fastest degradation [29,30]. PLGA (Figure 5) is used in various drug delivery applications. Studies have been performed on PLGA for delivering anticancer agent having low water solubility [31]. |
| Non-steroidal anti-inflammatory drugs, e.g., diflunisal [32] and diclofenac sodium [33,34], have been incorporated into PLGA microspheres and investigated for the treatment of rheumatoid arthritis, osteoarthritis, and related diseases. Also in the Implants of Trypsin inhibitor, PLG 50:50 is used as polymer. |
| Some examples of PLGA with their biodegradation time are shown in following Table 2 [24]. |
| Polyhydroxybutyrate (PHB) |
| PHB (Figure 6) is a biopolymer, which is present in all living organisms. Many bacteria produce PHB in large quantities as storage material. It is not toxic and is totally biodegradable. The polymer is primarily a product of carbon assimilation (from glucose or starch) and is employed by microorganisms as a form of energy storage molecule to be metabolized when other common energy sources are not available. PHB and its copolymers have attracted much attention because they are produced biosynthetically from renewable resources. Microcapsules from PHB has been prepared by various techniques and investigated for the release of bovine serum albumin [35]. PHB matrix was used for development of controlled delivery system of Nanogels of lithium neutralized polyacrylic acid for bone regeneration [36]. |
| Poly (e-caprolactone), PCL |
| PCL (Figure 7) is obtained by ring-opening polymerization of the 6-membered lactone, e-caprolactone (e-CL). Anionic, cationic, coordination, or radical polymerization routes are all applicable [34]. Recently, enzymatic catalyzed polymerization of e-CL has been reported [37,38]. PCL crystallizes readily due to the regular structure and has a melting temperature of 61°C. It is tough and flexible [34]. The Tg of PCL is low (–60°C). Thus, PCL is in the rubbery state and exhibits high permeability to low molecular species at body temperature. These properties, combined with documented biocompatibility, make PCL a promising candidate for controlled release applications [39]. |
| PCL degradation proceeds through hydrolysis of backbone ester bonds as well as by enzymatic attack. Hence Hydrolysis of PCL yields 6-hydroxycaproic acid, an intermediate of the w-oxidation, which enters the citric acid cycle and is completely metabolized. Hydrolysis, however, proceeds by homogeneous erosion at a much slower rate than PLA and PLGA [40]. Hydrolysis of PCL is faster at basic pH and higher temperatures [34]. PCL hydrolyzes slowly compared to PLA and PLGA; it is most suitable for long term drug delivery. PCL is show long term delivery system for a period of more than one year. PCL and its derivative have been assessed to be well suited for controlled drug delivery due to high permeability to many drugs freedom from toxicity [24]. Biodegradable In Situ gel-forming Controlled drug delivery system based on thermosensitive Poly (e-caprolactone)- Poly(ethylene glycol)-Poly(e-caprolactone) hydrogel has been reported in literature (Table 3) [41]. |
| Polydioxanone (PDS) |
| Polydioxanone (Figure 8) is prepared by a ring-opening polymerization of the pdioxanone monomer. It is characterized by a degree of crystallinity of about 55%. Constituents prepared with PDS show improved flexibility due to the presence of ether oxygen within the backbone of the polymer chain. When used in vivo, it degrades into monomers with low toxicity and also has a lower modulus than PLA or PGA. Poly-dioxanone on implantation does not display any severe or toxic effects [42]. Recently PDS has been used for nanofibrous drug delivery system of metronidazole and ciprofloxacin [43]. |
| Polyanhydrides |
| Poly anhydride (Figure 9) is class of biodegradable polymer characterized by anhydride bonds that connect repeat unit of polymers backbone chain. The majority of polies (anhydrides) are prepared by melt-condensation polymerization. To obtain a device that erodes heterogeneously, the polymer should be hydrophobic yet contain water sensitive linkages. One type of polymer system that meets this requirement is the poly (anhydrides). Poly- (anhydrides) undergoes hydrolytic bond cleavage to form water-soluble degradation products that can dissolve in an aqueous environment, thus resulting in polymer erosion. Polies (anhydrides) are believed to undergo predominantly surface erosion due to the high water liability of the anhydride bonds on the surface and the hydrophobicity which prevents water penetration into the bulk [44]. |
| Polyamide |
| The synthetic aliphatic polyamides are polymeric compounds frequently referred to as Nylons which form an important group of poly condensation polymers. They are linear molecules (i.e. aliphatic) that are semi-crystalline and thermoplastic in nature. A typical polyamide chain consists of amide groups separated by alkane segments and the number of carbon atoms separating the nitrogen atoms which defines the particular polyamide type (Figure 10). The aliphatic polyamides are very useful and versatile material that are Polyglutamic acid [20]. In 2012 US patent has been published on ‘Polyamide rate-modulated monolithic drug delivery system’ [20]. |
| Phosphorous based derivatives |
| Polyphosphazenes |
| It consists of phosphorous atoms attached to either carbon or oxygen. Polyphosphazenes (Figure 11), another new class of polymer are being investigated for delivery of proteins. The uniqueness of this class of polymer lies in the chemical reactivity of phosphorous which enables a wide range of side chains to be attached for manipulating the biodegradation rates and the molecular weight of polymer [16]. Phosphazenes undergo facile hydrolysis to Phosphate and ammonia which are easily undergo metabolised and excreted respectively [20]. Studies were reported in literature on drug and gene delivery using polyphosphazenes and its chemically modified form [46]. |
| Poly (phosphoester) s (PPE) |
| General structure of Poly (phosphoester) s (PPE) is shown in Figure 12. These polymers are generally referred to as Phophonates (P-O-C), polyphosphonates (P-C) orpolyphosphites depending upon the nature of the side chain attached to the phosphorus. This undergoes hydrolysis to alcohol and phosphates which easily undergo excretion and metabolism respectively. |
| Co-polymer of PPE:- |
| 1) Poly (ethylene Terephthalate) based PPEs [BHET-EOP] |
| 2) PPEs based on cyclohexane-1, 4-dimethyl (CHDM) phosphate backbone |
| A controlled gene delivery system with PPE has been developed in 2008 and has been patented (Table 4) [47]. |
| Polyorthoesters |
| Poly (orthoester) s (POE) (Figure 13) is another family of polymers identified as degradable polymers suitable for orthopaedic applications. With the addition of lactide segments as part of the polymer structure, tuneable degradation times ranging from 15 to hundreds of days can be achieved. The degradation of the lactide segments produces carboxylic acids, which catalyze the degradation of the orthoester. |
| POE-based norethindrone implants were prepared along with water soluble excipients. These water soluble osmogens attract water into the otherwise hydrophobic polymer and there is subsequent swelling and release of incorporated drug. |
| Even low Water-soluble acidic salt calcium lactate induces both bulk erosion and surface erosion. |
| Long term erosion controlled of levonorgestrel was achieved by stabilising the device interior with Mg (OH) 2 [20] |
| Poly (amino acids) |
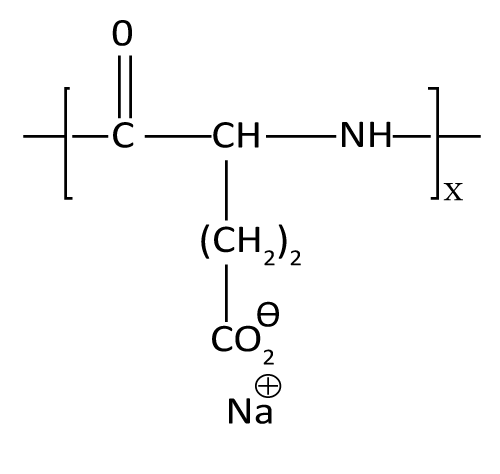
| Polyglutamic acid: General structure of Poly-L-glutamic acid is shown in Figure 14. Poly-L-glutamic acids due to its biodegradability, high water solubility, presence of multiple carboxyl groups are amenable for chemical modification, low immunogenicity and low toxicity. Its dielectric charge is also favourable for controlling in vivo disposition characteristic of anti-tumour agent Poly-L-glutamic acid has been glycosylated to facilitate liver-specific targeting [20]. Nanoscaled Poly (l-glutamic acid)/Doxorubicin-Amphiphile Complex as pH-responsive Controlled drug delivery has been reported in literature for effective treatment of Nonsmall Cell Lung Cancer in Non-small cell lung cancer (NSCLC) [48]. |
| Poly (iminocarbonates): Poly (amino acids) are highly insoluble, nonprocessible, and antigenic when the polymers contain three or more amino acids [49]. To circumvent these problems, “pseudo”-poly (amino acids) synthesized from tyrosine dipeptide were investigated [50]. These degradable polymers are derived from the polymerization of desaminotyrosyl tyrosine alkyl esters. Tyrosine-derived polies (carbonates) are readily processible polymers that support the growth and attachment of cells and have also shown a high degree of tissue compatibility [51]. Tyrosine-derived poly (carbonates) is characterized by their relatively high strength and stiffness exceeding poly (esters) such as poly (ortho esters) but not poly (lactic acid) or poly- (glycolic acid) [52,53]. The postulated mechanism of in vitro degradation involves hydrolysis of the pendent ester bonds and the imino-carbonate bonds of the backbone [54]. Degradation rates are comparable to the degradation rate of poly (L-lactic acid), occurring over a period of months. Polies (iminocarbonates) are currently being investigated for use in small bone fixation devices as bone screws and pins [54]. |
| Conclusion |
| Polymers possess a unique strength in their application towards drug delivery systems which enables the new advancement in the formulation of new drug delivery systems which improves the therapy and treatment. Biodegradable polymers have proven their potential for the development of new, advanced and efficient drug delivery system. They are capable of delivering a wide range of bioactive materials. From a polymer chemistry perspective, it is important to appreciate that the mechanisms of controlled-release require polymers with a variety of physico-chemical properties. Several types of polymers have been investigated as potential drug delivery systems, including Nano and micro-particles, dendrimers, Nano and micro-spheres, capsosomes and micelles. In these systems, drugs can be encapsulated or conjugated into polymer matrices to control the drug release. |
| Acknowledgements |
| Authors are very much thankful to Dr. P. S. Gide, Principal, Hyderabad (Sindh) National Collegiate Board’s Dr. L.H.Hiranandani college of pharmacy, Ulhasnagar for his continuous support, guidance and encouragement. |
| Conflict of Interest |
| The author(s) declare(s) that there is no conflict of interests regarding the publication of this article. |
| References |
References
- Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, et al. (2012) Drug delivery systems: An updated review. Int J Pharm Investig 2: 2-11.
- Gupta S, Kumar P (2012) Drug Delivery Using Nanocarriers: Indian Perspective. Proc Natl Acad Sci, India Sect B: Biol Sci 82: 167-206.
- Pillai O, Panchagnula R (2001) Polymers in drug delivery. Curr Opin Chem Biol 5: 447-451.
- Langer R (1998) Drug delivery and targeting. Nature 392: 5-10.
- Shaik MR, Korsapati M, Panati D (2012) Polymers in Controlled Drug Delivery Systems. International Journal of Pharma Sciences 2: 112-116.
- Reis RL, Cunha AM, Allan PS, Bevis MJ (1996) Mechanical behaviour of injection- molded starch based polymers. Polym Adv Technol 7: 784-790.
- Seal BL, Otero TC, Panitch A (2001) Polymeric biomaterials for tissue and organ regeneration. Mater Sci Eng Rep 3: 147-230.
- Di Martino A, Sittinger M, Risbud MV (2005) Chitosan: a versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 26: 5983-5990.
- Lee SB, Kim YH, Chong MS, Hong SH, Lee YM (2005) Study of gelatin-containing artificial skin V: fabrication of gelatin scaffolds using a salt-leaching method. Biomaterials 26: 1961-1968.
- Mohanty AK, Misra M, Hinrichsen G (2000) Biodegradable polymers and biocomposites: An overview. Macromol Mater Eng 277: 1-24.
- Kottke MJ, Edward MR (2002) Tablet Dosage Forms. In: Banker GS, Rhodes CT Modern Pharmaceutics. Marcel Dekker, Inc, New York 287-333.
- Alam AS, Parrott EL (1971) Effect of aging on some physical properties of hydrochlorothiazide tablets. J Pharm Sci 60: 263-266.
- Angelova N, Hunkeler D (1999) Rationalizing the design of polymeric biomaterials. Trends Biotechnol 17: 409-421.
- Brocchini S, Duncan R (1999) Pendant drugs release from polymers. In Encyclopaedia of Controlled Drug Delivery, John Wiley and Sons, New York, USA 786-816.
- Colthurst MJ, Williams RL, Hiscott PS, Grierson I (2000) Biomaterials used in the posterior segment of the eye. Biomaterials 21: 649-665.
- Mao HQ, Kdaiyala I, Leong KW, Zhao Z, Dang W (1999) Biodegradable Polymers: poly (phosphoester). In: Encyclopaedia of Controlled Drug Delivery, John Wiley and Sons, New York, USA 45-60.
- Nair LS, Laurencin CT (2006) Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. Adv Biochem Eng Biotechnol 102: 47-90.
- Kotwal VB, Saifee M, Inamdar N, Bhise K (2007) Biodegradable polymers: Which, when and why? 16-625.
- Manthina M, Kalepu S, Padavala V (2013) Oral lipid-based drug delivery systems – an overview. 3: 361–372.
- Vyas SP, Khar RK (2010) Controlled Drug Delivery – Concepts and Advances. First Edition, Vallabh Prakashan 97-154
- Nair Lakshmi S, Laurencin Cato T (2006) Polymers as Biomaterials for Tissue Engineering and Controlled Drug Delivery. Tissue Engineering I, Springer Berlin, Heidelberg 203-210.
- Vert M, Makromol A (1992) Synthesis of Degradable Polyesters. Polymer Journal 24: 1109-1117.
- Vainionpaa S, Rokkanen P, Tormala P (1989) Surgical application of biodegradable polymers in human tissue. Prog Polym Sci 14:679-716.
- Jain NK Controlled and novel drug delivery system. First edition, CBS publication 27-51.
- Li J, Peng L, Sun J, Guo H, Guo K, et al. (2012) Slow-Release Drug Delivery System with Polylactic Acid Hydrogels in Prevention of Tracheal Wall Fibroplasia. Arch Clin Exp Surg 1: 1-7
- Frazza EJ, Schmitt EE (1971) A new absorbable suture. J Biomed Mater Res 5: 43-58.
- Benicewicz BC, Hopper PK (1990) Biodegradable Poly (Lactic Acid): Synthesis, Modification, and processing. J Bioact Compat Polym 5:453.
- Dinarvand R, Moghadam SH, Mohammadyari-Fard L, Atyabi F (2003) Preparation of biodegradable microspheres and matrix devices containing naltrexone. AAPS Pharm Sci Tech 4: E34.
- Park TG, Lu WQ, Crotts GJ (1995) Evaluation of controlled release products in vitro. 33: 211–222.
- Miller RA, Brady JM, Cutright DE (1977) Degradation rates of oral resorbable implants (polylactates and polyglycolates): rate modification with changes in PLA/PGA copolymer ratios. J Biomed Mater Res 11: 711-719.
- Dinarvand R, Sepehri N, Manoochehri S, Rouhani H, Atyabi F (2011) Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int J Nanomedicine 6: 877-895.
- Castelli F, Giunchedi P, LaCamera O, Conte U (2000) A calorimetric study on diflunisal release from poly (lactide-co-glycolide) microspheres by monitoring the drug effect on dipalmitoylphosphatidylcholine liposomes: temperature and drug loading influence. Drug Deliv 1:45-53.
- Aggarwal S, Goel A, Singla S (2011) Drug delivery - Special emphasis given on biodegradable polymers, Universal Research Publications, 2.
- Pitt CG (1990) Poly-e-caprolactone and its copolymers. In: Chapter 3, Chasin M, Langer R (Eds), Biodegradable polymers as drug delivery systems. Marcel Dekker, New York, 71.
- Atkins TW, Peacock SJ (1997) In vitro biodegradation of polyhydroxybutyrate-hydroxyvalerate microcapsules exposed to Hank's buffer, newborn calf serum, pancreatin and synthetic gastric juice. J Microencapsul 14: 35-49.
- Larsson M, Bergstrand A, Mesiah L, Van Vooren C, Larsson A (2014) Nanocomposites of Polyacrylic Acid Nanogels and Biodegradable Polyhydroxybutyrate for Bone Regeneration and Drug Delivery. Journal of Nanomaterials 2014
- Dong H, Cao SG, Li ZQ, Han SP, You DL, et al. (1999) Study on the enzymatic polymerization mechanism of lactone and the strategy for improving the degree of polymerization. J PolymSci A PolymChem 37:1265-1275
- Henderson LA, Svirkin YY, Gross RA, Kaplan DL, Swift G (1996) Enzyme-Catalyzed Polymerizations of e-Caprolactone: Effects of Initiator on Product Structure, Propagation Kinetics, and Mechanism. Macromolecules 29: 7759-7766.
- Dubernet C, Benoit JP, Couarraze G, Duchêne D (1987) Promising polymers for controlled release drug delivery. Int J Pharm 35:145.
- Merced JE, Martinez-Rosales, Martinez-Richa A (2003) Ring-opening polymerization of lactones catalyzed by decamolybdate anion Polymer. 44: 6767–6772.
- Khodaverdi E, Golmohammadian A, Mohajeri SA, Zohuri G, Tekie FSM, et al. (2012) Biodegradable In Situ Gel-Forming Controlled Drug Delivery System Based on Thermosensitive Poly( -caprolactone)-Poly(ethylene glycol)-Poly( -caprolactone) Hydrogel. ISRN Pharmaceutics.
- Krukowski ZH, Cusick EL, Engeset J, Matheson NA (1987) RESOMER® Biodegradable Polymers for Medical Device Applications. Br J Surg 74: 828.
- Bottino MC, Arthur RA, Waeiss RA, Kamocki K, Gregson KS, et al. (2014) Biodegradable nanofibrous drug delivery systems: effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin Oral Investig .
- Leong KW, Brott BC, Langer R (1985) Bioerodible polyanhydrides as drug-carrier matrices. I: Characterization, degradation, and release characteristics. J Biomed Mater Res 19: 941-955.
- Kolawole OA, Pillay V, Choonara YE (2012) Polyamide rate-modulated monolithic drug delivery system, US 8277841 B2.
- Teasdale I, Brüggemann O (2013) Polyphosphazenes: Multifunctional, Biodegradable Vehicles for Drug and Gene Delivery. Polymers (Basel) 5: 161-187.
- Wang J, Mao HQ, Leong KW (2008) Biodegradable polyphosphates for controlled release of bioactive substances. US 7345138 B2.
- Li M, Song W, Tang Z, Lv S, Lin L, et al. (2013) Nanoscaled poly(L-glutamic acid)/doxorubicin-amphiphile complex as pH-responsive drug delivery system for effective treatment of nonsmall cell lung cancer. ACS Appl Mater Interfaces 5: 1781-1792.
- Anderson J, Spilizewski K, Hiltner A (1985) In: Biocompatibility of Tissue Analogs, CRC Press, Boca Raton, Florida, USA.
- Kohn J, Langer R.J (1987) Biodegradable polymers. Am Chem Soc 109-817.
- Silver FH, Marks M, Kato YP, Li C, Pulapura S, et al. (1992) Tissue compatibility of tyrosine-derived polycarbonates and polyiminocarbonates: an initial evaluation. J Long Term Eff Med Implants 1: 329-346.
- Engelberg I, Kohn J (1991) Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials 12: 292-304.
- Daniels A, Chang M, Andriano K, Heller JJ (1990) Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. Appl Biomater 1: 57-78.
- Ertel SI, Kohn J, Zimmerman MC, Parsons JR (1995) Evaluation of poly (DTH carbonate), a tyrosine- derived degradable polymer, for orthopedic application. J Biomed Mater Res 29: 1337-1348
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
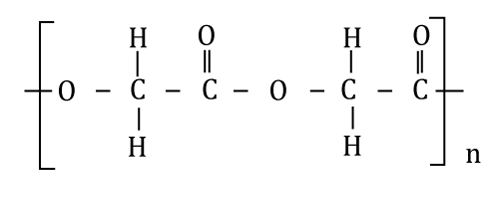 |
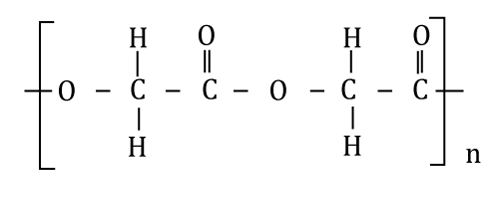 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
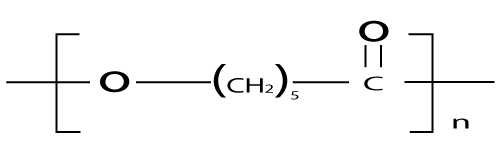 |
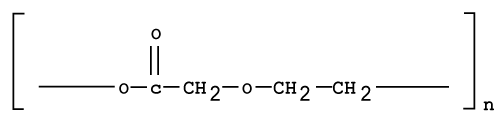 |
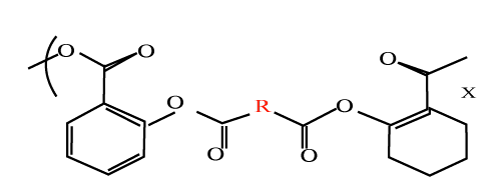 |
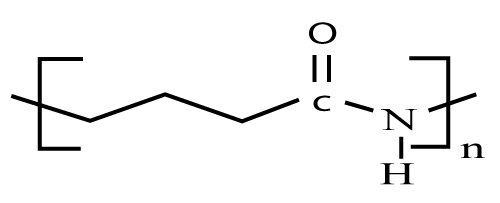 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
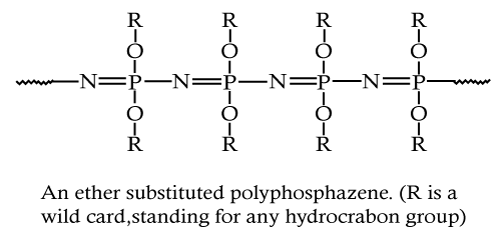 |
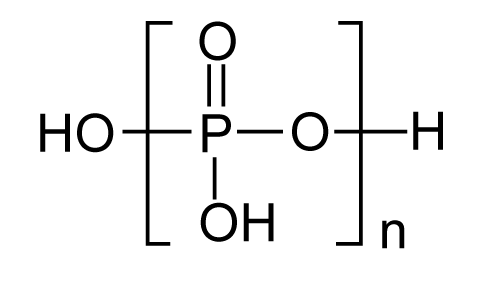 |
 |
 |
| Figure 11 | Figure 12 | Figure 13 | Figure 14 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 27281
- [From(publication date):
November-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 21510
- PDF downloads : 5771
