Research Article Open Access
Synthesis of Surface Molecularly Imprinting Polymers for Methylphenidate and its Application in Separating Methylphenidate
Mehdi Rajabnia Khansari1,2*, Sara Shahreza4, Azam Rezvanirad1, Amin Nikavar2, Shahrzad Bikloo3 and Bahareh Sadat Yousefsani5
1Faculty of Pharmacy, Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2School of Chemical Engineering, Research Center, Iran University of Science and Technology, Tehran, Iran
3Lorstan University of Medical Sciences, Research Center, Khoramabad, Iran
4Department of Nanobiotechnology, Tarbiat Modates University, Tehran, Iran
5Department of Pharmacodynamy and Toxicology, School of Pharmacy, Pharmaceutical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
- *Corresponding Author:
- Mehdi Rajabnia Khansari
Research Center, Faculty of Pharmacy
Shahid Beheshti University of Medical Sciences
Tehran, Iran
E-mail: rajabniamahdi@yahoo.com
Received date: February 11, 2017; Accepted date: February 23, 2017; Published date: February 28, 2017
Citation: Khansari MR, Shahreza S, Rezvanirad A, Nikavar A, Bikloo S, et al. (2017) Synthesis of Surface Molecularly Imprinting Polymers for Methylphenidate and its Application in Separating Methylphenidate. J Anal Bioanal Tech 8:351. doi: 10.4172/2155-9872.1000351
Copyright: © 2017 Khansari MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In this study, a novel approach is proposed for determination of methylphenidate in biological fluids. In this method molecularly imprinted solid-phase extraction (MISPE), as the sample extraction technique, combined with high-performance liquid chromatography (HPLC) is used. The water-compatible molecularly imprinted polymers (MIPs) were prepared using methacrylic acid as functional monomer, ethylene glycol dimethacrylate as cross-linker, Hexane as porogen and methylphenidate as template molecule. Extraction of methylphenidate from human serum was carried out using a novel imprinted polymer as the solid-phase extraction (SPE). Various parameters affecting the extraction efficiency of the polymer were evaluated. Also, the optimal conditions for the MIP cartridges were studied. The limit of detection (LOD) and limit of quantification (LOQ) for methylphenidate in serum samples were 1.3 and 10 ng mL-1, respectively. The recoveries for serum samples were higher than 92%.
Keywords
Molecularly imprinted polymer; Methylphenidate; Pharmaceutical analysis; Solid-phase extraction; Affinity assay; Template polymerization
Introduction
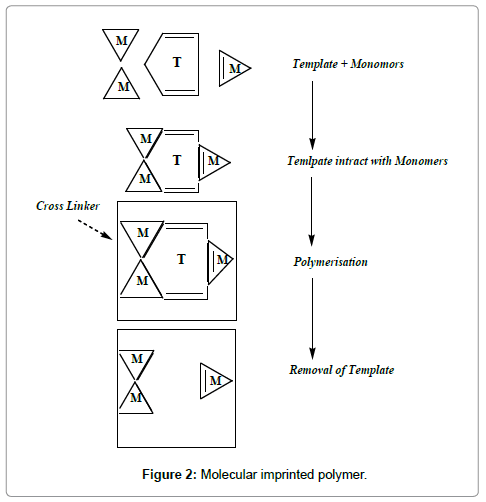
Recently, there have been an increasing interest in potential applications of highly selective molecularly imprinted polymers, MIPs. Especially their applications in analysis of drugs and other compounds in biological and environmental samples. Applicability of Imprinted polymers [1-4] are in various analytical techniques, including liquid chromatography [3,5], capillary electrophoresis, capillary electrochromatography [6], solid-phase extraction [7], and ‘immunoassay’ [8], have been investigated. An inhere advantage of molecular imprinting, which has extensively been testified by many examples above, is the possibility to synthesize sorbents with selectivity pre-determined for a particular analyte. The fundamental step in this technique is polymerization of functional and cross-linking monomers in the presence of a templating ligand, or imprint species. Subsequent removal of the imprint molecules leaves behind ‘memory sites’, or imprints, in a solid, highly cross-linked polymer network. The general belief holds that the functional monomers are spatially fixed in the polymer via their interaction with the imprint species during the polymerization reaction.
Attention deficit hyperactivity disorder (ADHD) is a common neurobehavioral disorder in childhood, which is estimated to strike up to 10% of the general population [9,10]. Methylphenidate (MPH) is a psychostimulant drug approved primarily for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy [11]. This drug fits to the piperidine class of compounds and increases the levels of dopamine and noradrenaline in the brain through reuptake inhibition of the monoamine transporters [11]. The main urinary metabolite is a de-esterified product, ritalinic acid (RA), which accounts for 80% of the dosage and has a half-life of about 8 h [11]. Reviews over pharmacy databases and treatment studies have shown that the incidences of medication discontinuation or non-adherence is between 13.2% and 64% [12]. The clinical laboratory has an important role in being able to detect MPH in serum and its metabolite RA in urine.
Various analytical methods such as immunoassay [13], HPLC with UV detection [14] and more recently liquid chromatography– electrospray ionization mass spectrometry [15-17], have been proposed for measuring MPH (in serum).
This study was meant to develop and validate a novel HPLC–SPE method with samples throughput for determinations of MPH in human serum. In these applications solid phase extraction (SPE) sample preparation procedures were used. The method outline with a selective chromatography in combination with specific UV detection fulfills the high quality standard required for accurate determinations in serum samples from human.
Experimental
Materials
MPH (Figure 1) was purchased from Cerillant (Texas, USA) as 1 mg/mL solutions in methanol and RA (Figure 1) from Sigma-Aldrich (Australia). HPLC grade acetonitrile was purchased from Thermo Fisher (Cambridge, UK), ammonium formate and dimethyloctylamine (DMOA) from Sigma-Aldrich (Germany), and HPLC grade methanol and reagent grade 89-91% pure formic acid from BDH (Poole, UK). Methacrylic acid (MAA), 4-vinyl pyridine (4VP) and ethylene glycol dimethacrylate (EDMA) were obtained from Sigma-Aldrich (Milwaukee, USA). 2,2′-Azobis-iso-butyronitrile (AIBN) was obtained from Acros (Geel, Belgium). All solvents used [acetonitrile (ACN), tetrahydrofuran (THF), hexane, acetone, methanol, acetic acid and Trifluoroacetic acid (TFA)] were of HPLC grade.
Instrumentation and analytical conditions
The HPLC system consisted of an isocratic HPLC pump (Model 590, Waters), an autoinjector (SIL-10A, Shimadzu) fitted with an injection loop of 200 μl and was directly connected to the chiral AGP column (150 × 4.0 mm id.). Detection of analyte was achieved using a variable wavelength UV detector (Model 481, Waters) set at 220 nm. A reversed-phase mode was used, and the mobile phase consisted of 0.4% acetic acid containing 0.1% DMOA, pH 3.4, which DMOA was applied as an organic modifier. The mobile phase was degassed before use. The flow-rate was 1 ml/min and an ambient column temperature was used. AGP columns are all stable for at least 3 months.
Standards
A standard stock solution of MPH was prepared by dissolving 1 mL of 1 mg/mL commercial standard in 50 mL methanol to give a final concentration of 20 mg/L.
The serum calibration curves for MPH were plotted by spiking drug-free human serum with standard solutions at concentrations of 0.5, 5, 10, 15, 20 μg/mL, giving a calibration range of 0.5-20 μg/mL for both MPH.
Synthesis of polymers
A non-covalent approach was used for preparation of MIPs. MPH as the template and MAA, 4VP as the functional monomer, were dissolved in 10 mL of organic solvent (hexane or acetone) in a screw-capped glass tube and kept at 4°C for 60 min. Then, EDMA as the cross-linker, and AIBN as the initiator, were added. The mixture was sparged, in an ice bath, with oxygen-free nitrogen for 20 min and heated at 60°C for 22 h to complete radical polymerization. The resultant bulk rigid polymers were crushed, grounded into powder and sieved through a 200-mesh stainless steel sieve (particle size less than 75 μm). The polymer particles were washed with a methanol-acetic acid (60:40, v/v) mixture, centrifuged at 4000 rpm for 5 min and supernatant was analyzed by HPLC. The washing was continued until no MPH or other compound was detected in supernatant. Blank non-imprinted polymers (NIPs) were prepared, in the absence of MPH, under the same condition described above. Imprinted (MIP) and non-imprinted (Blank) polymers prepared and examined in this study are presented in Table 1.
| Polymer | Template | Functional Monomer | Cross linker | Molar Ratio | Solvent |
|---|---|---|---|---|---|
| MPH | MAA | EDMA | MAA/MPH | Hexane | |
| Blank | - | 2.5 mmol | 10 mmol | - | 5 ml |
| MIP- A1 | 0.5 mmol | 2.5 mmol | 10 mmol | 5 | 5 ml |
| MIP- A2 | 0.25 mmol | 2.5 mmol | 10 mmol | 10 | 5 ml |
| 4-VP | 4-VP/MPH | Acetone | |||
| Blank | - | 2.5 mmol | 10 mmol | - | 5 ml |
| MIP- B1 | 0.5 mmol | 2.5 mmol | 10 mmol | 5 | 5 ml |
| MIP- B2 | 0.25 mmol | 2.5 mmol | 10 mmol | 10 | 5 ml |
Table 1: MIPs and Blank polymers preparation protocol.
Batch adsorption procedure
The recognition ability of both MIP and Blank polymer was examined by batch rebinding experiments. Dry polymer (10 mg) was incubated in 2 mL ACN with MPH and was shaken for 24 h at room temperature. The solution was centrifuged (3000 rpm for 10 min) and supernatant was analyzed by HPLC. The amount of bound MPH was calculated from the difference between initial and final concentrations in solution. Each test was carried out four times and Mean ± SD was reported.
The imprinting factor (IF) was calculated according to Eq. (1):
Eq. (1): 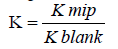 (1)
(1)
MISPE procedure
25 mg of polymer (MIP-A1 or NIP), in 3 mL ACN, was slurry packed into an empty polypropylene SPE cartridge. The column was washed and conditioned with 8 mL loading solution. MPH (10 μg) in 200 μL water was loaded into the column. Washing solvent was then passed through the column five times. Finally, 5 mL methanol–TFA (50:50, v/v) was applied to perform complete extraction of MPH. In order to find a solvent with maximum selectivity for MPH, Methanol and Acetic acid were evaluated as washing solvents. The loading, washing and eluting fractions were analyzed by HPLC.
Extraction of MPH from human serum samples
25 mg of MIP-A1 suspended in 3 mL ACN was packed into a polypropylene cartridge. The column was washed and conditioned by 5 mL of methanol-TFA (50:50, v/v) and 4 mL of CAN, respectively. 800 μL of ACN was added to 150 μL of serum in order to precipitate the serum proteins. After centrifugation (3000 rpm for 8 min), 1 mL deionized water was added to 0.5 mL supernatant and the mixture was loaded onto the column. Acetic acid (3 mL) was percolated through the column for selective washing and finally MPH was eluted with 8 mL methanol-TFA (50:50, v/v). The solvent was dried under a stream of nitrogen. Later, the residue was dissolved in 100 μL mobile phase and the concentration of MPH was determined by HPLC.
Results and Discussion
Choice of functional monomer and solvent
As molecular recognition of the template molecule by imprinted polymers is based on the intermolecular interactions between the template molecule and functional groups of the polymer [18], choosing a proper functional monomer is of great importance for creating a strong monomer-template comple [19]. The functional monomer used in this study was MAA, 4-VP. However, because of the amine groups in MPH structure, it easily binds to this acidic monomer. The ionic interaction between MAA and MPH makes the MIP suitable for MISPE procedure in aqueous conditions. Solvent has an important effect on conjuncture of functional monomers with the template. Before and during polymerization, the polarity of the solvent affects the extent of non-covalent pre-polymer complex. Less polar solvents such as chloroform or hexane increase complex formation, facilitating polar non-covalent interactions such as hydrogen bonding. On the other hand, more polar solvents tend to dissociate the non-covalent interactions in the pre-polymer complex, especially protic solvents which leads to a high degree of disruption to hydrogen bonds [20-22]. In this work, two organic solvents (hexane and acetic acid) were tested as the polymerization solvents for optimization of molecular imprinting procedure (Figure 2).
Batch adsorption measurements
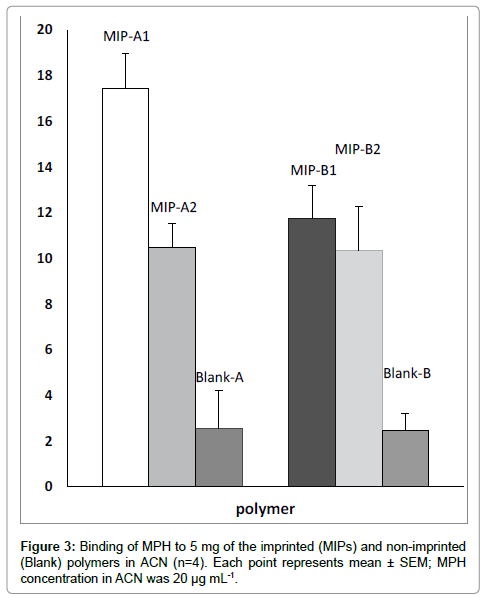
A best method for evaluating the binding sites in MIPs is batch adsorption test. Batch adsorption involves analysis of an MIP in a solution of substrate [19]. Therefore, in this research binding properties of polymers were studied in a conventional batch adsorption method [9,20]. Two MAA/MPH ratios (5 and 10) in two polymerization solvents (hexane and acetone) were used for optimization of MPH imprinting condition. The results of binding assay showed that MIP-A1 was the optimized imprinted polymer (Figure 3). The calculated imprinting factor (IF) values of MIPs are shown in Table 2. In comparison with other polymers, the highest affinity of MIP-A1 for MPH and its highest IF, indicates the superiority of binding properties of MIP-A1 for MPH. As the polarity of hexane is less than acetone, more MPH-MAA interaction is possible in hexane compared to acetone. Based on this fact, the optimized MIP was prepared in hexane. The optimized MAA/ MPH ratio was 5 and the best polymerization solvent was hexane. It has been proven, in other studies, that the best MIPs, with selective binding properties, have been prepared in less polar organic solvents with a template/monomer ratio of less than 1 [2,6]. Therefore, MIP-A1, as the optimized polymer, was used for Scatchard analysis and selective extraction of MPH from human serum.
| Imprinted polymer | IF=(KMIP/KNIP) |
|---|---|
| MIP-A1 | 60.3 |
| MIP-A2 | 18.6 |
| MIP-B1 | 23.8 |
| MIP-B2 | 9.1 |
Table 2: Imprinting factor (IF) of each imprinted polymer for methylphenidate (Ritalin).
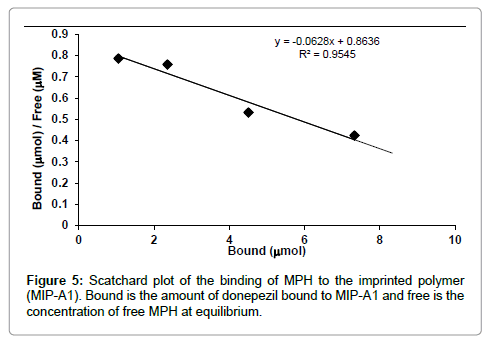
Scatchard analysis
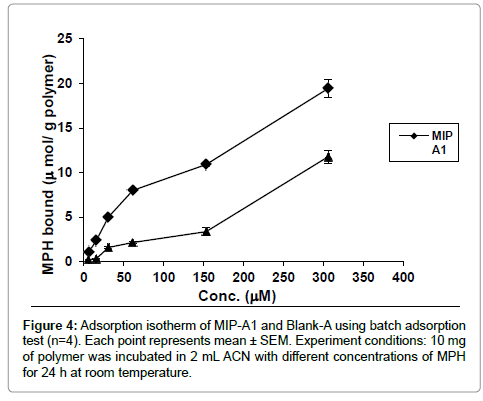
Binding of MPH to MIP-A1 and its blank polymer (Blank-A) was studied at different concentrations (Figure 4). The data showed that MPH binding to MIP-A1 was significantly more than Blank-A at all concentrations. From the Scatchard plot (Figure 5) one dissociation constants could be discerned, one was representing high affinity binding sites with a KD of 8 μM, and Bmax of 6.38 μmol g-1 polymers. The binding sites of MIPs prepared by non-covalent bulk polymerization are usually heterogeneous. The KD value in other studies ranged from μM to M (lamotrigine KD=16.2 mM [23], sulphametoxazole KD=18.8 mM [24], theophylline KD=1.5 M [25].
MISPE
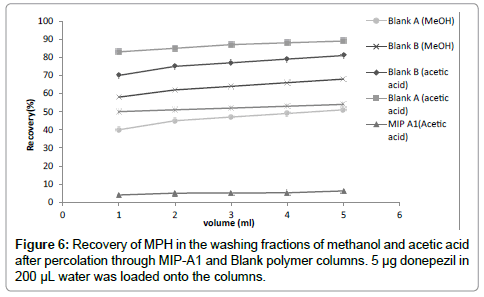
This study was aimed at determination of MPH in human serum.To fulfill this goal, water was selected as loading solvent in MISPE procedure [18]. The real challenge in MISPE is to exploit the selectivity of the MIP in aqueous media. The washing step is in fact the key factor for the development of specific interactions with the MIP. The aim of this step is finding a solvent which yields maximum selectivity and recovery of MPH. Two solvents (methanol and acetic acid) were applied in washing step. The percentage of washed MPH relative to the total loaded amount was calculated in each fraction and cumulative recovery was plotted against the volume of washing solvent (Figure 6). Although 5 mL acetic acid could disrupt 80% of non-specific bindings of MPH to Blank-B polymer, it could also wash 6.2% of drug from MIP-A1. Whereas 89% of MPH was removed from Blank-A column which is 14 times more than MIP-A1 polymer. Therefore acetic acid was selected as washing solvent. 3 mL acetic acid could be used to wash the polymer column after loading, without removing considerable amount of MPH from MIP-A1 cartridge. Acidic solutions are usually used for complete washing of template in elution step from the MIP column [23]. In this study, 8 mL of methanol–TFA (50:50, v/v) was used as eluting solvent.
Extraction of MPH from serum samples
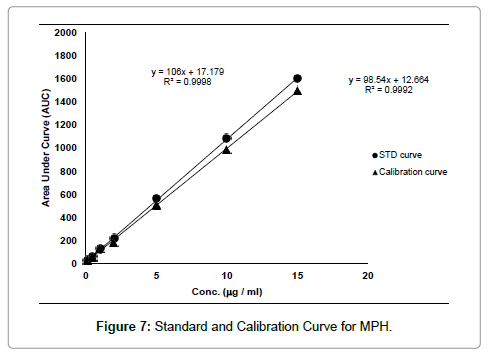
Serum samples with or without MPH was loaded onto the MIP-A1 column and MISPE procedure was carried out according to the method described in Section 2.6. The calibration curve of HPLC of spiked serum samples with known concentrations of MPH was established in the range of 0.5-20 μg mL-1 (y=98.54x+12.664, R2=0.9992). The standard curve of MPH was also plotted in the same range (y=106x+17.179, R2=0.9998) (Figure 7). The recovery of MPH in this MISPE process was calculated as 92%. The limit of detection (LOD) of assay (signal/noise ratio of 3) was found to be 1.3 ng mL-1. The limit of quantification (LOQ) of assay (signal/noise ratio of 20) was 10 ng.mL-1 which was much lower than the minimum therapeutic concentration of MPH in patient serum. LOD and LOQ values obtained in other studies (with similar MISPE method and HPLC assay carried out for other templates) ranged from ng mL-1 to μg mL-1 [14,21]. Also, LOD and LOQ values of HPLC assay of MPH in human serum, determined by other researchers, were less than 1 ng mL-1 and 50 ng mL-1, respectively [4]. The intra-day and interday precision values for MPH concentration of 0.8 μg mL-1 were 1.88 and 3.3%, respectively. In both cases the precision was calculated as the relative standard deviation of the spiked serum samples (n=4) in one day (intra-day precision) and four different days (inter-day precision).
Optimization of MISPE protocol
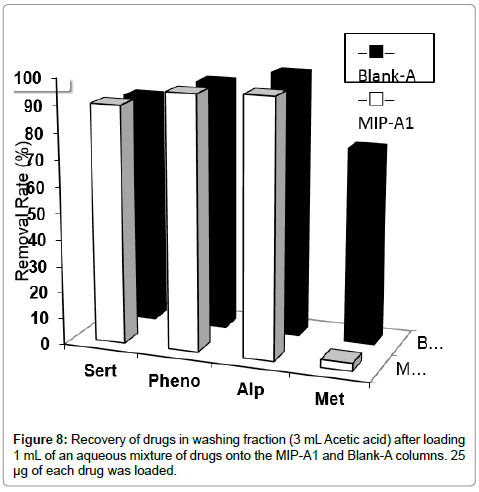
In the next step, the selectivity of MISPE, in washing step, for methylphenidate was evaluated in presence of other drugs in aqueous solution. After loading 1 mL of an aqueous mixture of sertraline, alprazolam, phenobarbital and methylphenidate onto the MIP-A1 or Blank-A column, the cartridge was washed with 3 mL ACN. The data showed that 90-100% of sertraline, alprazolam and phenobarbital were removed from MIP-A1 and Blank-A polymer. Also, 77% of methylphenidate was washed from Blank-A, while only about 4% of methylphenidate was removed from MIP-A1 column (Figure 8). This indicated a significantly higher affinity of MIP-A1 for methylphenidate in comparison with other drugs that could be present with methylphenidate, simultaneously, in serum of patients. We have also used ACN, in our previous studies, as washing solvent for selective extraction of donepezil in a MISPE method from their aqueous mixture [21]. Thus, optimized MISPE conditions were as follows: washing conditions, 3 mL acetic acid; elution conditions, 8 mL methanol-TFA (50:50, v/v).
Conclusion
In this paper for the first time, a novel MPH MIP is prepared by bulk polymerization. The MPH MIP shows higher molecular recognition than NIP on chromatographic evaluation. A SPE-HPLC method based on MIP is developed for the extraction of MPH from aqueous solutions. Furthermore, the MIP particles as new sorbents in SPE are successfully investigated for the extraction of human serum samples with an optimized procedure. This efficient method allows extracts with higher purity to be obtained and interfering peaks arising from complicated biologic samples to be suppressed. The method was applied to the trace MPH determination at three levels, and the recoveries for the spiked human serum samples were higher than 92%. It could be concluded that the proposed technique has a great potential in developing selective extraction method for other compounds too.
Acknowledgements
We gratefully acknowledge Research Center, School of Chemical Engineering, Iran University of Science and Technology and Research Center, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The work was financially supported by research grant from the Shahid Beheshti University of Medical Sciences, Deputy of Research.
References
- Olesen OV, Thomsen K, Jensen PN, Wulff CH, Rasmussen NA, et al. (1995) Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross-sectional study. Psychopharmacology (Berl). J Christensen and R Rosenberg 117: 371-378.
- Ramstrom O, Yu C, Mosbach K (1996) Chiral recognition in adrenergic receptor binding mimics prepared by molecular imprinting. J Mol Recognit 9: 691-696.
- Ansell RJO, Ramstrom R, Mosbach K (1996) Towards artificial antibodies prepared by molecular imprinting. Clin Chem 42: 1506-1512.
- Steinke JHG, Dunkin IR, Sherrington DC (1999) A simple polymerisable carboxylic acid receptor: 2-acrylamido pyridine. TrAC Trends in Analytical Chemistry 18: 159-164.
- Kempe M, Mosbach K (1995) Separation of amino acids, peptides and proteins on molecularly imprinted stationary phases. J Chromatogr A 691: 317-323.
- Schweitz L, Andersson LI, Nilsson S (2002) Molecularly imprinted CEC sorbents: investigations into polymer preparation and electrolyte composition. Analyst 127: 22-28.
- Olsen JP, Martin M, Wilson ID (1998) Molecular imprints as sorbents for solid phase extraction: potential and applications. Analytical Communications 35: 13H-14H.
- Andersson LI (2000) Molecular imprinting for drug bioanalysis: a review on the application of imprinted polymers to solid-phase extraction and binding assay. Journal of Chromatography B: Biomedical Sciences and Applications 739: 163-173.
- Cantwell DP (1996) Attention deficit disorder: a review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry 35: 978-987.
- Rösler M, Casas M, Konofal E, Buitelaar J (2010) Attention deficit hyperactivity disorder in adults. The World Journal of Biological Psychiatry 11: 684-698.
- Pae CU, Marks DM, Masand PS, Peindl K, Hooper-Wood C, et al. (2009) Methylphenidate extended release (OROS MPH) for the treatment of antidepressant-related sexual dysfunction in patients with treatment-resistant depression: results from a 4-week, double-blind, placebo-controlled trial. Clinical Neuropharmacology 32: 85-88.
- Adler LD, Nierenberg AA (2010) Review of medication adherence in children and adults with ADHD. Postgraduate Medicine 122: 184-191.
- Seçilir A, Schrier L, Bijleveld YA, Toersche JH, Jorjani S, et al. (2013) Determination of methylphenidate in plasma and saliva by liquid chromatography/tandem mass spectrometry. Journal of Chromatography B 923: 22-28.
- Zhang J, Deng Y, Fang J, McKay G (2003) Enantioselective analysis of ritalinic acids in biological samples by using a protein-based chiral stationary phase. Pharmaceutical Research 20: 1881-1884.
- Marchei EJ, Munoz Garcia-Algar O, Pellegrini M, Vall O, Zuccaro P, at al. (2008) Development and validation of a liquid chromatography–mass spectrometry assay for hair analysis of methylphenidate. Forensic Science International 176: 42-46.
- Marchei E, Farrè Pellegrini M, Rossi S, García-Algar Ó, Vall O, et al. (2009) Liquid chromatography–electrospray ionization mass spectrometry determination of methylphenidate and ritalinic acid in conventional and non-conventional biological matrices. Journal of Pharmaceutical and Biomedical Analysis 49: 434-439.
- Letzel MK, Weiss Schüssler W, Sengl M (2010) Occurrence and fate of the human pharmaceutical metabolite ritalinic acid in the aquatic system. Chemosphere 81: 1416-1422.
- Yang J, Hu Y, Cai JB, Zhu XL, Su QD, et al. (2007) Selective hair analysis of nicotine by molecular imprinted solid-phase extraction: an application for evaluating tobacco smoke exposure. Food Chem Toxicol 45: 896-903.
- Alizadeh T, Zare M, Ganjali MR, Norouzi P, Tavana B (2010) A new molecularly imprinted polymer (MIP)-based electrochemical sensor for monitoring 2, 4, 6-trinitrotoluene (TNT) in natural waters and soil samples. Biosensors and Bioelectronics 25: 1166-1172.
- Azodi-Deilami S, Abdouss M, Hasani SA (2010) Preparation and utilization of a molecularly imprinted polymer for solid phase extraction of tramadol. Central European Journal of Chemistry 8: 861-869.
- Khansari MR, Bikloo S, Shahreza S (2016) Determination of donepezil in serum samples using molecularly imprinted polymer nanoparticles followed by high-performance liquid chromatography with ultraviolet detection. Journal of Separation Science.
- Javidi JM, Esmaeilpour E, Khansari MR (2015) Synthesis, characterization and application of core-shell magnetic molecularly imprinted polymers for selective recognition of clozapine from human serum. RSC Advances 5: 73268-73278.
- Mohajeri SA, Ebrahimi SA (2008) Preparation and characterization of a lamotrigine imprinted polymer and its application for drug assay in human serum. J Sep Sci 31: 3595-3602.
- Huamin Q, Lulu F, Li X, Li L, Min S, et al. (2013) Determination sulfamethoxazole based chemiluminescence and chitosan/graphene oxide-molecularly imprinted polymers. Carbohydr Polym 92: 394-399.
- Sun HW, Qiao FX, Liu GY (2006) Characteristic of theophylline imprinted monolithic column and its application for determination of xanthine derivatives caffeine and theophylline in green tea. J Chromatogr A 1134: 194-200.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 5121
- [From(publication date):
April-2017 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 4371
- PDF downloads : 750