Make the best use of Scientific Research and information from our 700+ peer reviewed, Open Access Journals that operates with the help of 50,000+ Editorial Board Members and esteemed reviewers and 1000+ Scientific associations in Medical, Clinical, Pharmaceutical, Engineering, Technology and Management Fields.
Meet Inspiring Speakers and Experts at our 3000+ Global Conferenceseries Events with over 600+ Conferences, 1200+ Symposiums and 1200+ Workshops on Medical, Pharma, Engineering, Science, Technology and Business
Research Article Open Access
Synthesis of Dihydroimidazole Derivatives under Solvent Free Condition and Their Antibacterial Evaluation
| Mahmoud Abd El Aleem Ali Ali El-Remaily1*, Shaaban K Mohamed2, Ahmed MM Soliman1, and Hossam Abd el-Ghanya1 | |
| 1Department of Chemistry, Sohag University, Egypt | |
| 2School of Chemistry and Environmental Science, Manchester Metropolitan University, Manchester, England | |
| *Corresponding Author : | Abd El Aleem Ali Ali El-Remaily Department of Chemistry Sohag University, Sohag 82524, Egypt Tel: +20108036348 Fax: +2093601159 E-mail: msremaily@yahoo.com |
| Received July 03, 2014; Accepted July 25, 2014; Published August 01, 2014 | |
| Citation: Ali El-Remaily MAEAA, Mohamed SK, Soliman AMM, Abd el-Ghanya H (2014) Synthesis of Dihydroimidazole Derivatives under Solvent Free Condition and Their Antibacterial Evaluation . Biochem Physiol 3:139. doi:10.4172/2168-9652.1000139 | |
| Copyright: © 2014 Ali El-Remaily MAEAA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
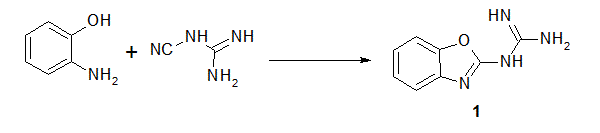
Reaction of 2-guanidinobenzoxazole with several halogenated active methylene compounds has revealed formation of the corresponding dihydroimidazole derivatives under solvent free conditions in excellent yield. The anti-bacterial evaluation of the newly synthesized products against different types of Gram-positive and Gramnegative bacteria was performed. Most of products showed high inhibitory effect. The structure of all compounds has been characterized by on IR, 1H-NMR, 13C-NMR, Mass spectrum and some X-ray diffractions.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
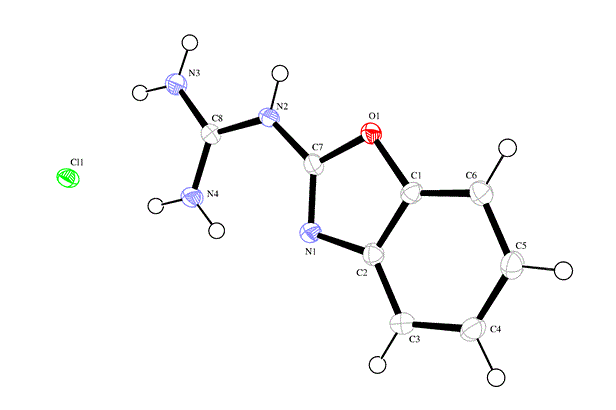 |
| Figure 1 |
 |
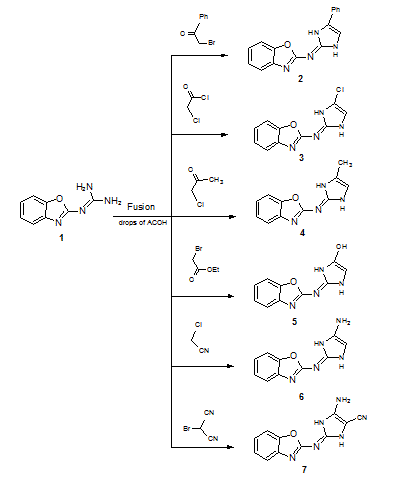 |
| Scheme 1 | Scheme 2 |
Post your comment
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15594
- [From(publication date):
August-2014 - Jul 05, 2025] - Breakdown by view type
- HTML page views : 10928
- PDF downloads : 4666
