Research Article Open Access
Synthesis of A Novel Ferrocene Derivative and Cytotoxicity to A549, HCT116 and MCF-7 Cell Lines
Jianping Yong1*, Xiao Wang2, Xiaoyuan Wu2, and Canzhong Lu1,3*1Xiamen Institute of Rare-earth Materials, Chinese Academy of Sciences, Xiamen, 361021, China
2Department of Pharmacy, Bao’an People’s Hospital Affiliated to Southern Medical University, Shenzhen, Guangdong, 518101, China
3Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, China
- *Corresponding Author:
- Jianping Yong
Xiamen Institute of Rare-earth Materials, Chinese Academy of Sciences
Xiamen, 361021, China
Fax: +86-59183705794
Email: czlu@fjirsm.ac.cn
Canzhong Lu
Xiamen Institute of Rare-earth Materials
Chinese Academy of Sciences, Xiamen, 361021
China
Fax: +86-59183705794
E-mail: jpyong@fjirsm.ac.cn
Received date: November 04, 2016; Accepted date: January 02, 2017; Published date: January 04, 2017
Citation: Yong J, Wang X, Wu X, Lu C (2017) Synthesis of A Novel Ferrocene Derivative and Cytotoxicity to A549, HCT116 and MCF-7 Cell Lines. J Infect Dis Ther 5:1000311. doi:10.4172/2332-0877.1000311
Copyright: © Yong J, et al This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Based on our previous results, a ferrocene derivative 1 was synthesized in this study and characterized by 1H NMR, 13C NMR, MS and XRD methods. And then, the cytotoxicity to A549, HCT116 and MCF-7 cell lines was evaluated using the MTT method. The results showed that this compound exhibited good cytotoxicity to A549, HCT116 and MCF-7 cell lines.
Keywords
Ferrocene derivative; Synthesis; Reaction mechanism; Antitumor activity
Introduction
Cancer is a major health problem worldwide. The death caused by cancer mainly is lung cancer, breast cancer, liver cancer, carcinoma of colon and rectum. Some small molecule anticancer agents have been approved by the U.S. Food and Drug Administration (FDA) in clinics and some are currently in clinical trials [1,2]. However, cancer chemotherapy is still highly inadequate. Thus, it is urgent to discover novel anticancer agents.
Ferrocene is a potential pharmacophore for drug design and drug discovery, for it is a neutral, chemically stable and nontoxic molecule [3]. Some ferrocene derivatives are potential metal-based anticancer agents [4].
In recent years, our researching group is devoted to design and synthesize the small molecule libraries for the development of anticancer agents [5,6]. We have reported some ferrocene derivatives containing isoxazole moiety and their in vitro anticancer activity against A549, HCT16 and MCF-7 cell lines [7]. In our previous work, we synthesized a novel structure of ferrocene derivative and evaluated its anticancer activity against A549, HCT16 and MCF-7 cell lines [8].
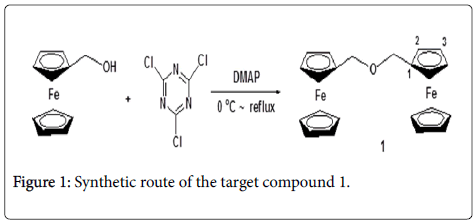
To find the most potent anticancer agents based on the ferrocene core, another new structure of ferrocene derivative 1 was synthesized in present work. The synthetic route was outlined in Figure 1. The target compound was confirmed by 1H NMR, 13C NMR, MS and XRD methods. And then, the cytotoxicity to A549, HCT116 and MCF-7 cell lines was preliminarily evaluated.
Experimental
General methods
Melting point was determined on XT-5 (Benjing Keyiecopti Instrument Factory) apparatus equipped with a microscope and value is uncorrected. 1H and 13C NMR spectra was recorded on a 400 MHz Bruker AVANCE III spectrometer in CDCl3, the chemical shifts are expressed in ppm relative to tetramethylsilane (TMS) as the internal standard; ESI-MS was performed on a DECAX-30000 LCQ Deca XP (70 eV). The reaction was monitored by thin layer chromatography(TLC) on silica gel plates at 254 nm under a UV lamp using petroleum or ethyl acetate as eluent. Column chromatography separation were obtained on silica gel (100-200 mesh). All solvents were of analytical grade and used without further purification unless specially stated.
The process for the preparation of the target compound 1
Ferrocenemethanol (0.864 g, 4 mmol) was added into a 50 mL onenecked round-bottom flask with 10 mL dry THF, the mixture was stirred in a cold bath, cyanuric chloride(TCT) (0.184 g, 1 mmol) in 10 mL dry THF was slowly added to the reaction system using a syringe, the mixture was stirred in the cold bath for 30min. Subsequently, DMAP (0.366 g, 3 mmol) in 10 mL dry THF was also slowly added to the reaction system using a syringe, the mixture was stirred in the cold bath for an additional 30 min. Then, temperature naturally rose to room temperature for 8 h. The completion of the reaction was indicated by simple TLC analysis, the solvent was evaporated under the reduced pressure, and the residue was directly purified by column chromatography (Petroleum ether/EtOAc: 5:1→2:1) to obtain the target compound 1. 0.663 g, light yellow solid, yield, 80%, Mp.: 121-123°C, 1H NMR (400 MHz, CDCl3) δ (ppm): 4.12 (s, 10H, 2 × C5H5), 4.14 (s, 4H, 2 × CH2), 4.23 (s, 4H, 2 × 2H of C5H4), 4.27 (s, 4H, 2× 2H of C5H4); 13C NMR (100MHz, CDCl3) δ (ppm): 68.5, 69.4, 76.7, 77.0, 77.4; ESI-MS(m/e, 100%) 415([M+1]+, 100); Anal.calcd. for C22H22 Fe2O: C63.77; H5.31; Found C63.75; H5.36.
The cytotoxicity to A549, HCT116 and MCF-7 cell lines
The cytotoxicity to A549, HCT116 and MCF-7 cell lines of compound 1 was evaluated using the MTT method. The cancer cell lines were cultured in Dulbecco’s Modified Essential Medium (DMEM) supplemented with 10% fetal bovine serum and 1.5% antibiotic/ antimycotic(Life Technologies, Invitrogen, USA) and maintained in CO2 incubator at 37°C, at 5% CO2 and 95% atmospheric humidity. The mixture of DMSO, PBS and DMEM was used as a negative control and gefitinib was used as the positive control. The detailed process see the reference [7].
Results and Discussion
Chemistry
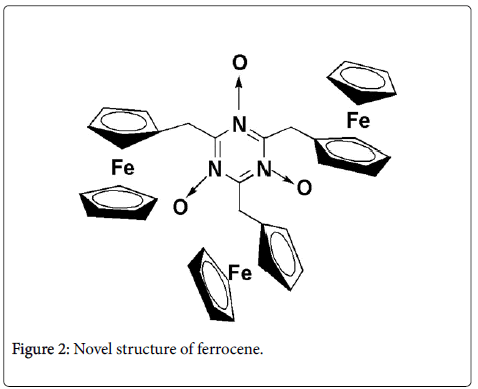
In our previous study, we added the ferrocenemethanol to the cyanuric chloride solution in THF, we obtained a novel structure of ferrocene derivative (Figure 2), and we have proposed the reaction mechanism of forming this compound [8].
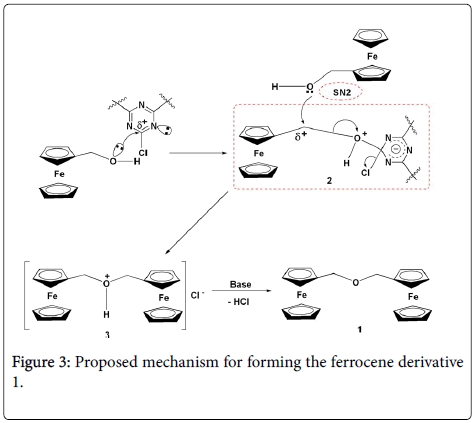
But in current study, we added the cyanuric chloride to the ferrocenemethanol solution in THF, we obtained another novel ferrocene derivative 1. From this result, we can propose the reaction mechanism for forming this new ferrocene derivative 1 (Figure 3): Firstly, the ferrocenemethanol and cyanuric chloride formed the intermediate 2. Then, another ferrocenemethanol molecule reacted with the intermediate 2 by SN2-type reaction to form intermediate 3, the intermediate 3 was neutralized with DMAP to obtain the target compound 1.
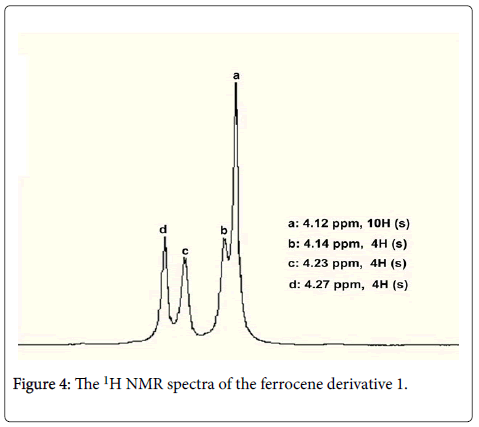
The target compound 1 was confirmed by 1H and 13C NMR and mass spectrometry. The 1H NMR of ferrocene derivative 1 showed in Figure 4, there showed three kinds of proton signals for ferrocene core of its derivative 1, the protons of C5H4 showed two singlets at 4.23 and 4.27 ppm respectively, while the protons of C5H5 showed a singlet at 4.12 ppm; The protons of CH2 showed a singlet at 4.14 ppm. In 13C NMR, there also showed five kinds of carbon signals, the carbon atoms of two CH2 showed at 76.7 ppm, the carbon atoms of two C5H5 showed at 68.5 ppm, the carbon atoms of two C-1 of C5H4 showed at 77.4 ppm, four carbon atoms of C-2 showed at 69.4 ppm, four carbon atoms of C-3 showed at 77.0 ppm.
X-Ray crystallographic study
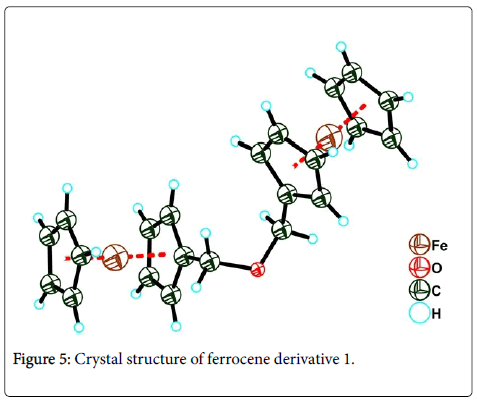
X-ray diffraction data for the ferrocene derivative 1 was collected on a Siemens Smart CCD diffractometer equipped with a graphitemonochromated CuKα radiation(λ=1.54178Å). The structure was solved by direct method using the program SHELXL-97 and refined by full-matrix least-squares techniques on F2 with SHELXL-97 program package [9]. The molecular structure showed in Figure 5 (CCDC Number: 1487772).
The cytotoxicity to A549, HCT116 and MCF-7 cell lines
The cytotoxicity to A549, HCT116 and MCF-7 cell lines of the compound 1 was evaluated using the MTT method. The anticancer efficacy was comparable with the reference drug gefitinib, and the results were summarized in Table 1. It can be seen from the Table 1 that this compound exhibited good cytotoxicity to A549, HCT116 and MCF-7 cell lines.
| Compd. | IC50 (μM) | ||
|---|---|---|---|
| A549 | HCT116 | MCF-7 | |
| Ferrocene derivative 1 | 455.8 | 66.41 | 133.8 |
| Gefitinib | 17.9 | 21.55 | 20.68 |
Table 1: The cytotoxicity to A549, HCT116 and MCF-7 cell lines of ferrocene derivative 1.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51672271).
Disclosure
Part of this article has been previously published in Med. Chem., 2016, 12, 426-431.
References
- Grosios K, Traxier P (2003) Tryosine kinase targets in drug discovery. Drug Fut 28: 679-697.
- Qu SH (2012) Second-generation irreversible epidermal growth factor receptor(EGFR) tyrosine kinase inhibitors(TKIs): A better mousetrap? A review of the clinical evidence. Crit Rev Oncol Hematol 83: 407-421.
- Staveren DRV, Metzler-Nolte N (2014) Bioorganometallic chemistry of ferrocene. Chem Rev 104: 5931-5985.
- Braga SS, Silva AMS (2013) A new age for iron: Antitumoral ferrocenes. Organometallics 32: 5626-5639.
- Lu CZ, Yong JP (2013) Quinazoline derivatives and application thereof. PCT/WO2013143057A1.
- Lu CZ, Yong JP (2014) Thieno[2,3-d]pyrimidine derivatives, preparation method and use thereof. PCT/WO2014043866A1.
- Yong JP, Lu CZ, Wu XY (2014) Synthesis of isoxazole moiety containing ferrocene derivatives and preliminarily in vitro anticancer activity. Med Chem commun 7: 968-972.
- Yong JP, Wu XY, Liao JZ, Lu CZ, Liu XL (2016) Synthesis, Structure characterization of a novel ferrocene derivativ and preliminarily anticancer activity. Med Chem 12: 426-431.
- Sheldrick GM (1997) SHELXS-97, Program for Solution of Crystal Structures, University of Gottingen, Germany.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 4319
- [From(publication date):
February-2017 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 3390
- PDF downloads : 929