Research Article Open Access
Synergy Testing between Sulbactam and Meropenem/ Colistin in MDR Acinetobacter baumannii-calcoaceticus Complex Isolated from Ventilator Associated Pneumonia
Shalini Anandan*, Lydia Jennifer, Shalini Anandan, Agila Kumari Pragasam, Baby Abirami Shankar, Balaji Veeraraghavan, John Victor Peter and Shoma V RaoChristian Medical College, India
- *Corresponding Author:
- Shalini Anandan
Professor, Clinical Microbiology, Christian Medical College, India
Tel: +91-9742918016
E-mail: shalinianandan@cmcvellore.ac.in
Received date: September 05, 2016; Accepted date: October 21, 2016; Published date: October 24, 2016
Citation: Anandan S, Jennifer L, Anandan S, Pragasam AK, Shankar BA, et al. (2016) Synergy Testing between Sulbactam and Meropenem/ Colistin in MDR Acinetobacter baumannii-calcoaceticus Complex Isolated from Ventilator Associated Pneumonia. J Infect Dis Ther 4:299. doi:10.4172/2332-0877.1000299
Copyright: © 2016 Anandan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Background: A. baumannii-calcoaceticus (Abc) complex has surfaced as a major nosocomial pathogen causing blood stream infection and ventilators associated pneumonia (VAP). Carbapenems have come to be the cornerstone of treatment for Abc complex. However, there has been an increased incidence of infections with carbapenem resistant strains. To validate the clinical practice of combination antibiotic therapy, in-vitro combinations of antibiotics have been examined using checkerboard methods, E-tests, and the reference, time-kill assay.
Method: A prospective pilot study was conducted for the duration of one year. Twenty five isolates of carbapenem resistant Abc complex cultured from endotracheal aspirates of patients admitted in medical and surgical intensive care units diagnosed with ventilator associated pneumonia were collected. Isolates were tested for MIC (Minimum inhibitory concentration) by micro-broth dilution method for meropenem, sulbactam and colistin. Synergism between sulbactam plus meropenem and sulbactam plus colistin was tested by micro-broth checkerboard assay and the reference, time kill assay.
Result: Minimum inhibitory concentration ranges (μg/ml) for sulbactam, meropenem, and colistin were 16-512, 16-256, and 0.5-64, respectively. MIC50 for sulbactam, meropenem, and colistin was 128, 128 and 1, correspondingly, and MIC90 for sulbactam, meropenem, and colistin was 256, 256 and 2, respectively. In the checkerboard assay and time-kill assay, a higher percentage of synergy was noted for the combination of sulbactam plus meropenem.
Conclusion: Against multi-drug resistant (MDR) isolates of Abc complex, commendable synergy was seen with time kill assay for sulbactam plus meropenem combination. Therefore, in-vitro combinations of antimicrobial agents are most effective than the single agent against multidrug resistant organism.
Keywords
MDR Abc complex; VAP; Synergy; Checkerboard assay; Time-kill assay; Meropenem; Sulbactam; Colistin
List of Abbreviations
Abc complex: Acinetobacter baumannii calcoaceticus complex; VAP- Ventilator Associated Pneumonia; MDR: Multi Drug Resistant; MIC: Minimum Inhibitory Concentration; Etest: Epsilometer test; NABL: National Accreditation Board for Testing and Calibration Laboratories; CFU: Colony Forming Units; CLSI: Clinical and Laboratory Standards Institute; FIC: Fractional Inhibitory Concentration; NCCLS: National Committee on Clinical Laboratory Standards
Introduction
In the genus Acinetobacter, A. baumannii has proved to be the most important species accountable for infections [1]. Wide spectrum of illnesses ranging from hospital acquired pneumonia, communityacquired pneumonia, catheter related bloodstream infections, urinary tract infections related to urinary catheters, meningitis, traumatic battlefield to other wound infections have been caused by A. baumannii [2-5]. This has been made possible by its environmental adaptability [5].
Studies have described the ability of Acinetobacter spp. to exchange genetic elements, which, in turn, results in development of resistance to many classes of antimicrobial agents. Hence, the antibiotics that are used to treat life threatening infections caused by A. baumannii are carbapenems, polymyxins E and B, sulbactam, piperacillin/ tazobactam, tigecycline and aminoglycosides [5]. Among these antibiotics, carbapenems occupy a distinguished place in the empirical therapy [6]. However, over the past several years, there has been a sharp increase in the development of resistance to carbapenems. This is mediated by the production of carbapenemases, expression of efflux pumps and porin channel loss [5].
To overcome resistance, combination therapy has been used against multi-drug resistant organisms to achieve synergy, which, in turn can boost the efficacy of treatment [7]. Extensive research has been conducted on understanding combination therapy for A. baumannii [8,9]. To support this clinical practice, in-vitro combinations of antibiotics have been examined using checkerboard methods, E-tests, and the reference, time-kill assay.
In the present study we aim to determine the minimum inhibitory concentration (MIC) of meropenem, colistin and sulbactam by broth micro dilution, followed by detection of synergism between sulbactam plus meropenem and sulbactam plus colistin for Abc complex isolates using the reference method of time-kill assay. Further, we will compare this synergism with checkerboard assay, which in turn will help in understanding the effective drug combinations.
In this study, we have employed in- vitro methods like checkerboard assay and the gold standard time kill assay to understand whether or not synergy exists between sulbactam plus meropenem and sulbactam plus colistin combinations against imipenem and meropenemresistant Acinetobacter baumannii - calcoaceticus complex. Even though the organism was resistant to meropenem, we hypothesized that combining meropenem with sulbactam may produce synergism in vitro . Sulbactam plus colistin was another combination that was tested in this study. Sulbactam is an intriguing antimicrobial agent belonging to beta-lactamase inhibitor group. Sulbactam has an innate activity against A. baumannii . Studies have described the effectiveness of sulbactam plus colistin combination over monotherapy for infections with A. baumannii [10-12].
Materials and Methods
Bacterial isolates
The study was conducted in the NABL (National Accreditation Board for Testing and Calibration Laboratories) accredited Department of Clinical Microbiology (M0728), Christian Medical College, Vellore, which is a tertiary health care centre in Tamilnadu, India. Twenty five consecutive non-duplicate isolates of carbapenem resistant Acinetobacter baumannii - calcoaceticus complex were collected prospectively from July 2014 to June 2015. These isolates were cultured from endotracheal aspirates of patients admitted in medical and surgical intensive care units diagnosed clinically and radiologically as ventilator associated pneumonia. In addition, these endotracheal aspirates showed a growth of ≥ 105 CFU/ml fulfilling the microbiological criteria of ventilator associated pneumonia.
Demographic details of the patients were also collected. According to the standard protocol, conventional biochemical reactions were used for the species identification of the isolates [13]. All the study isolates were tested for resistance to carbapenems using imipenem and meropenem by disk diffusion technique and the zone sizes were interpreted based on CLSI (Clinical and Laboratory Standards Institute) 2014 guidelines [14].
Minimum inhibitory concentration determination
Pure substance of sulbactam, meropenem and colistin were procured from Sigma-Aldrich. Clinical Laboratory Standards Institute (CLSI) guidelines were followed for the determination of minimum inhibitory concentration (MIC) by micro broth dilution technique [14,15].
Synergy testing
Checker board assay: The checkerboard method of synergy testing determines the activity of antimicrobial combinations tested at clinically achievable concentrations in serial twofold dilutions, in two dimensions [16]. Interpretation of the result is done by calculating fractional inhibitory concentration (FIC) for individual antibiotics at a given concentration combination. Synergy is defined as an FIC index of ≤ 0.5, indifference/ no interaction as an FIC index of >0.5 to <4 and antagonism as an FIC index of >4. When the FIC index is in the range of 0.5 to 1.0, it is considered to be non-synergistic or additive [16].
Time kill assay: Time kill assay is considered to be reference method for in-vitro synergy testing. The assay is based on minimum bactericidal concentration testing (MBT) which determines the concentration of antimicrobial agent required to kill 99.9% of an inoculum after being exposed for a period of twenty four hours. It determines the rate and extent of killing, therefore helping in predicting response to therapy [17]. A standard protocol for performing time kill assay has been laid out by National Committee on Clinical Laboratory Standards (NCCLS currently known as CLSI) in 1999 [13,14]. The assay is carried out in glass tubes containing 10ml of cation- adjusted Mueller Hinton broth, into which the inoculum and a fixed concentration of antimicrobial agents is added. One tube of sterility control, one tube of growth control, tubes for individual antimicrobial agents and combination of agents are included for each isolate.
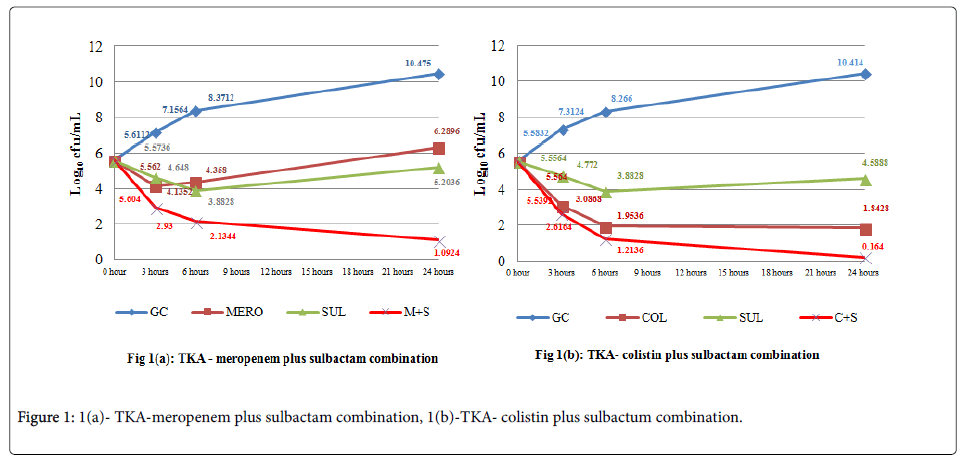
Subsequently the test isolate is inoculated and incubated for a total of 24 hours, during which 0.5 mL aliquots are serially diluted at fixed time interval of 0 hour, 3 hours, 6 hours and 24 hours and plated for colony count determination [17]. Synergy for time-kill assays is defined as a ≥ 2 log10 CFU/ml reduction in the antimicrobial combination when compared to the most active antimicrobial agent. While antagonism is described as a ≥ 2 log10 CFU/ml increment in the antimicrobial combination when compared to the most active antimicrobial agent. Bactericidal effects can also be determined in time kill assay, with a ≥ 3 log10 CFU/ml decrease in colony count than the initial inoculum at 24 hours [17]. While determining synergy, one of the antimicrobial agents is used at sub-inhibitory concentration, such that, it will not interfere with the growth of organisms when used alone.
Statistical analysis
Data entry was done using Microsoft Excel 2007 (Roselle, IL, USA).Data analysis was performed using SPSS version 16.0(SPSS Inc, Chicago, IL, USA). The two-tailed Fisher’s exact test was used to calculate the p value.
Results
Patient demographics
The age of the patients ranged between 19 years to 76 years. When observed for the age distribution, mean age was found to be 46.8 years. There were differences in the number of isolates recovered from male and female patients. The sex distribution was observed to be slightly on the higher side for males. Eighteen (72%) isolates were from males and seven (28%) were from females.
Antimicrobial susceptibility
All of the 25 (100%) study isolates were resistant to imipenem and meropenem by disk diffusion testing. Further, these isolates were also resistant to other β- lactam antibiotic like ceftazidime and betalactam/ beta- lactamase inhibitor combination piperacillintazobactam, cefoperazone-sulbactam. 4% of the isolates were susceptible to levofloxacin, 8% of the isolates were susceptible to amikacin and netilmicin. The susceptibility rates for tobramycin, cotrimoxazole and tetracycline were 12%, 16% and 24% respectively. All 25 isolates (100%) were susceptible to Polymyxin B 300.
MIC ranges (μg/mL) for meropenem, sulbactam and colistin were 16-256, 16-512, and 0.5-64 respectively. MIC50 (μg/mL) for meropenem, sulbactam and colistin was 128, 128 and 1 correspondingly. MIC90 (μg/mL) for meropenem, sulbactam and colistin was 256, 256, and 2 respectively; this data is described in Table 1. Notably, two isolates showed colistin MICs in the resistant range (64 μg/mL).
| Meropenem MIC | Colistin MIC | Sulbactam MIC | |
|---|---|---|---|
| (µg/mL) | (µg/mL) | (µg/mL) | |
| Mean MIC | 154.24 | 6.04 | 141.44 |
| MIC range | 16-256 | 0.5-64 | 16-512 |
| MIC50 | 128 | 1 | 128 |
| MIC90 | 256 | 2 | 256 |
Table 1: Mean MIC, MIC range, MIC50 and MIC90 of the isolates.
Synergism testing
Checkerboard assay: Table 2 gives the results of checkerboard assay. The synergy rates with sulbactam plus meropenem combination (52%) was higher when compared with the synergy rates of sulbactam plus colistin combination (16%), this was found to be statistically significant with a p value of 0.007, standard error of 0.123 and 95% confidence interval of 0.602. A higher percentage of indifference was observed with sulbactam plus colistin combination (84%).
| Combination | Checkerboard assay | Time-kill assay | |||||
|---|---|---|---|---|---|---|---|
| Lowest ∑FIC range* | Synergy (%) | Indifference (%) | Synergy (%) | Indifference (%) | Bactericidal effect (%) | ||
| Sulbactam + Meropenem | 0.28-1.01 | 13 (52%) | 12 (48%) | 17 (68%) | 8 (32%) | 20 (80%) | |
| Sulbactam + Colistin | 0.24-1.01 | 4 (16%) | 21 (84%) | 8 (32%) | 17 (68%) | 24 (96%) | |
| *∑FIC- Fractional inhibitory concentration Index | |||||||
Table 2: Result of checkerboard assay and time-kill assay.
Time-kill assay: Table 2 describes the results of time kill assay. Time kill assay detected higher percentage of synergy (68%) than checkerboard assay (52%) for sulbactam plus meropenem combination. Likewise, for sulbactam plus colistin combination, time kill assay (32%) detected synergy at a greater rate than checker board assay (16%). In sulbactam plus meropenem combination, sulbactam was the most active antimicrobial agent (80%), while in sulbactam plus colistin combination, colistin was the most active agent (80%). Additionally, bactericidal effect was 96% in sulbactam plus colistin combination, which was higher than sulbactam plus meropenem combination (80%).
Antagonism was not observed by either of the methods and with any of the combinations tested. Figures 1a and 1b describes the cumulative effect of sulbactam plus meropenem and sulbactam plus colistin combinations in time-kill assay on all the isolates, respectively [18].
Dose optimization
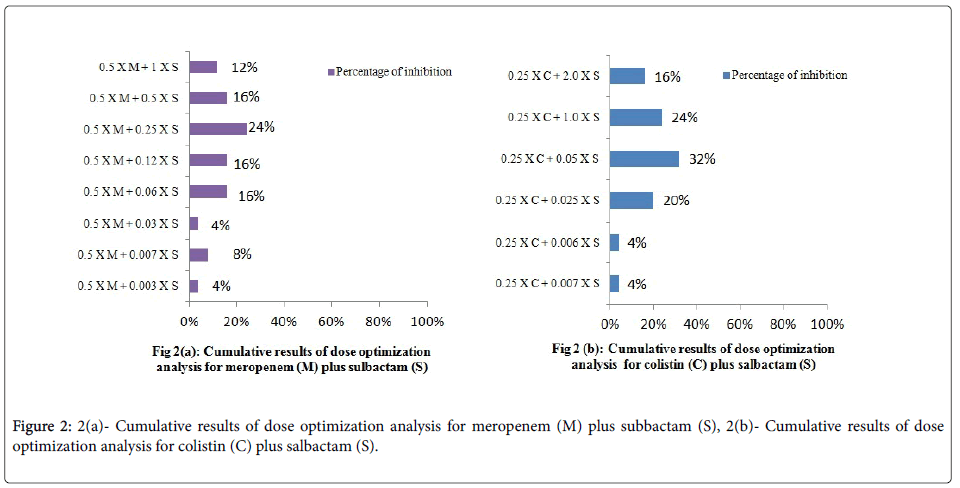
Dose optimization analysis was carried out at 0.25 × MIC of colistin and 0.5 × MIC of meropenem. For colistin, 4 %, 4 %, 20 %, 32 %, 24 % and 16 % of the inhibition was achieved at 0. 007 ×, 0.06 ×, 0.25 ×, 0.5 ×, 1.0 × and 2.0 × MIC of sulbactam, respectively. While for meropenem, 4 %, 8 %, 4 %, 16 %, 16 %, 24 %, 16 % and 12 % of the inhibition was achieved at 0.003 ×, 0.007 ×, 0.03 ×, 0.06 ×, 0.12 ×, 0.25 ×, 0.5 × and 1 × of sulbactam respectively. The results are summarized in Figures 2a and 2b.
Discussion
A. baumannii is frequently isolated from health-care associated infections especially ventilator associated pneumonia [19]. Over the last decade, carbapenem resistance in A. baumannii has been steadily increasing across the globe, and there are very few therapeutic options available for life threatening infections [20]. Studies on clinical efficacy of treatment with antimicrobial combinations on Acinetobacter species showed higher clinical response (76%) with carbapenems plus colistin combinations compared to monotherapy. Few studies also showed higher survival rate in combination therapy with carbapenem plus colistin (mortality rate of 19%) and carbapenem plus ampicillin/ sulbactam (mortality rate of 30%). Combinations of carbapenem with sulbactam did not show any statistically significant difference in microbiological eradication [21].
In the current study, the synergy rate for meropenem plus sulbactam combination was 52% with checkerboard assay. A similar study with meropenem plus ampicillin/sulbactam combination has shown a higher rate of synergy (94.1%). Further, Pongpech et al., reported 70% synergy with meropenem plus sulbactam combination with checkerboard assay [22]. In contrast, meropenem plus cefoperazone/sulbactam combination has shown a very low synergy of 8.8% [23].
Compared to meropenem plus sulbactam combination, sulbactam plus colistin combination demonstrated a higher rate of indifference (84%). Similar results have been shown by Santimaleeworagun et al. and Thamlikitkul et al. for colistin plus sulbactam combinations [24,25]. Pongpech et al. showed synergy of 53.3% with sulbactam plus colistin [22]. In this study, higher rates of synergy were observed for meropenem plus sulbactam combination when compared to sulbactam plus colistin combination, which was statistically significant (p value of 0.007).
Antagonism was not detected in both the combinations tested. However, Pongpech et al. have shown antagonism in 6.7% of the isolates against meropenem plus colistin and in 13.3% of isolates against sulbactam plus colistin combinations [22]. The difference in results from various studies for same combination with checkerboard assay could be due to discrepancies in the analysis of results and diverse characteristics of the isolates [26].
Time kill assay performed in this study using sulbactam plus meropenem combinations has shown commendable synergy of 68%. Furthermore, colistin plus meropenem combination has demonstrated synergy in 32% of isolates. Statistically significant (p value of 0.01) increased rates of synergy was observed for meropenem plus sulbactam combination when compared to sulbactam plus colistin combination. Considerable bactericidal effect of 80% and 96% was seen in sulbactam plus meropenem and sulbactam plus colistin combination, respectively.
In this study, synergy rates detected by time kill assay exceed the synergy rates detected by checkerboard assay. Meropenem plus sulbactam combination showed 68% synergy by time kill assay, whereas checkerboard assay showed 52% synergy. Similar findings were seen with the colistin plus sulbactam combination. Systematic review has also described higher rates of synergy with time- kill assay [27]. The limitations of this study are: synergism observed by time kill assay and checkerboard assay have not been compared with molecular mechanisms of resistance. The possibility of synergism being determined by the activity of the particular beta-lactamase produced by the organism and other mechanisms of resistance like efflux pump production or porin loss was not studied. Correlation of this nature might have aided in predicting the presence of synergy based on the prevailing resistance mechanism.
In dose optimization analysis, overall, 82 % and 100 % of inhibition was seen at 1 X of sulbactam for colistin plus sulbactam and meropenem plus sulbactam combinations respectively. However, colistin plus sulbactam combination required 2.0 X MIC of sulbactam for 16 % of the isolates tested. Further, for determining the ratio of two agents to be effective, extensive in-vitro studies are needed.
Conclusion
On analyzing the results of time kill assay for both the combinations (sulbactam plus meropenem and sulbactam plus colistin), we can infer that, in-vitro combinations of antimicrobial agents are more effective than a single agent against multidrug resistant organisms. Welldesigned animal model studies and clinical studies are essential to validate the findings of the present study.
Ethical Clearance and Consent for Publication
The approval for the study was obtained from the Institutional Review Board, Christian Medical College, Vellore. Institutional review Board, Minute Number: 8942 dated 07.07.2014
Conflict of Interest
The authors have declared no conflict of interest.
References
- Manchanda V, Sanchaita S, Singh N (2010) Multidrug Resistant Acinetobacter. J Glob Infect Dis 2: 291–304.
- Peng H, Tao X-B, Li Y, Hu Q, Qian L-H, et al. (2015) Health care-associated infections surveillance in an intensive care unit of a university hospital in China, 2010-2014: Findings of International Nosocomial Infection Control Consortium. Am J Infect Control 43: e83-85.
- Chittawatanarat K, Jaipakdee W, Chotirosniramit N, Chandacham K, Jirapongcharoenlap T (2014) Microbiology, resistance patterns, and risk factors of mortality in ventilator-associated bacterial pneumonia in a Northern Thai tertiary-care university based general surgical intensive care unit. Infect Drug Resist 7: 203–10.
- Zhang Z, Duan J (2015) Nosocomial pneumonia in non-invasive ventilation patients: incidence, characteristics, and outcomes. J Hosp Infect 91: 153–7.
- Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, et al. (2007) Global Challenge of Multidrug-Resistant Acinetobacter baumannii. Antimicrob Agents Chemother 51: 3471–84.
- Protic D, Pejovic A, Andjelkovic D, Djukanovic N, Savic D, et al.(2016) Nosocomial Infections Caused by Acinetobacter baumannii: Are We Losing the Battle? Surg Infect 17: 236–42.
- Tängdén T (2004) Combination antibiotic therapy for multidrug-resistant Gram-negative bacteria. Ups J Med Sci 119: 149–53.
- Maragakis LL, Perl TM (2008) Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis Off Publ Infect Dis Soc Am 46: 1254–63.
- Laishram S, Anandan S, Devi BY, Elakkiya M, Priyanka B, et al. (2016) Determination of synergy between sulbactam, meropenem and colistin in carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii isolates and correlation with the molecular mechanism of resistance. J Chemother 28: 297–303.
- Kalin G, Alp E, Akin A, Coskun R, Doganay M (2014) Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection 42: 37–42.
- Batirel A, Balkan II, Karabay O, Agalar C, Akalin S, et al. (2014) Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 33: 1311–22.
- Yilmaz GR, Guven T, Guner R, Kocak Tufan Z, Izdes S, et al. (2015) Colistin alone or combined with sulbactam or carbapenem against A. baumannii in ventilator-associated pneumonia. J Infect Dev Ctries 9: 476–85.
- Koneman EW (2006) Koneman’s Color Atlas and Textbook of Diagnostic Microbiology (6th edition). Lippincott Williams & Wilkins, Wolters Kluwer Company, Philadelphia, Baltimore, New York, London, Hong Kong, Sydney, Tokyo.
- Clinical And Laboratory Standards Institute (2014).Performance standards for antimicrobial susceptibility testing. Twenty fourth Informational Supplement. CLSI document M100-24. 1-230.
- Clinical and Laboratory Standards Institute (2013) Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty third Information Supplement.
- Jenkins SG, Schuetz AN (2012) Current Concepts in Laboratory Testing to Guide Antimicrobial Therapy. Mayo Clin Proc 87: 290–308.
- Doern CD (2014) When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J Clin Microbiol 52: 4124–8.
- Barry L, Craig WA, Nadler H, Reller LB, Sanders CC, et al. (1999) Methods for determining bactericidal activity of antimicrobial agents; approved guideline. NCCLS .
- Peng H, Tao X-B, Li Y, Hu Q, Qian L-H, et al. (2015) Health care-associated infections surveillance in an intensive care unit of a university hospital in China, 2010-2014: Findings of International Nosocomial Infection Control Consortium. Am J Infect Control 43: e83-85.
- Poirel L, Nordmann P (2006) Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 12: 826–836.
- Ko W-C, Lee H-C, Chiang S-R, Yan J-J, Wu J-J, et al. (2004) In vitro and in vivo activity of meropenem and sulbactam against a multidrug-resistant Acinetobacter baumannii strain. J Antimicrob Chemother 53: 393–395.
- Pongpech P, Amornnopparattanakul S, Panapakdee S, Fungwithaya S, Nannha P, et al. (2010) Antibacterial activity of carbapenem-based combinations againts multidrug-resistant Acinetobacter baumannii. J Med Assoc Thail Chotmaihet Thangphaet 93: 161–171.
- Ar─▒do─?an BC (2012) In vitro synergistic activity of carbapenems in combination with other antimicrobial agents against multidrug-resistant Acinetobacter baumannii. African Journal of Microbiology Research 6: 2985-2992.
- Santimaleeworagun W, Wongpoowarak P, Chayakul P, Pattharachayakul S, Tansakul P, et al. (2011) In vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemases. Southeast Asian J Trop Med Public Health 42: 890–900.
- Thamlikitkul V, Tiengrim S (2014) In vitro activity of colistin plus sulbactam against extensive-drug-resistant Acinetobacter baumannii by checkerboard method. J Med Assoc Thail Chotmaihet Thangphaet 97 Suppl 3: S1-6.
- Ozseven AG, Sesli Çetin E, Ozseven L (2012) [Do different interpretative methods used for evaluation of checkerboard synergy test affect the results?]. Mikrobiyoloji Bül 46: 410–420.
- Zusman O, Avni T, Leibovici L, Adler A, Friberg L, et al. (2013) Systematic Review and Meta-Analysis of In Vitro Synergy of Polymyxins and Carbapenems. Antimicrob Agents Chemother 57: 5104–5111.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14729
- [From(publication date):
October-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 13557
- PDF downloads : 1172