Synergistic Effects of Alzheimer’s Disease and Parkinsonism on Olfactory Impairment
Received: 19-Mar-2019 / Accepted Date: 02-Apr-2019 / Published Date: 09-Apr-2019 DOI: 10.4172/2161-0460.1000464
Abstract
Background: Olfactory dysfunction is frequent in both Alzheimer’s Disease (AD) and Parkinson’s disease.
Objective: To examine whether the coexistence of parkinsonism in patients with Mild Cognitive Impairment (MCI) or mild AD synergistically affects olfactory impairment.
Methods: Olfaction was evaluated in three patient groups and age-matched controls. The patient groups consisted of amnestic MCI (Mini–Mental State Examination (MMSE) ≥ 24) or mild AD (MMSE 20-23) without parkinsonism (n: 64), parkinsonism free of cognitive impairment (n: 62), and MCI or mild AD with parkinsonism (n: 70). The odor-stick identification test for Japanese (OSIT-J) was used to count the numbers of correct answers, responses of indistinguishable, and responses of odorless (anosmia). Cognitive function was evaluated by MMSE, Clinical Dementia Rating Scale, NIA–AA criteria, MRI, and 123I-IMP SPECT. Parkinsonism was diagnosed using the Unified Parkinson Disease Rating Scale (UPDRS)-III and 123I-FP-CIT dopamine transporter SPECT.
Results: MCI or mild AD with parkinsonism group had a significantly lower percentage of correct answers than did MCI or mild AD without parkinsonism group (p: 0.0045) or parkinsonism group (p: 0.0015). The frequency of anosmia was significantly higher in MCI or mild AD with parkinsonism group than that in parkinsonism group (p: 0.0039). There were no significant differences in the number of responses of indistinguishable among the three patient groups (p>0.05). All the olfactory categories on the OSIT-J in the three patient groups showed significantly worse scores than did the age-matched controls (p<0.01).
Conclusion: The coexistence of parkinsonism in MCI or mild AD may synergistically exacerbate olfactory impairment.
Keywords: Alzheimer’s disease; Coexistence; Mild cognitive impairment; Olfaction; Olfactory impairment; Parkinson’s disease; Parkinsonism; Synergistic effect
Abbreviations
AD: Alzheimer’s Disease; CDR: Clinical Dementia Rating; 3D-SSP: three-dimensional Stereotactic Surface Projection; 123I-FPCIT: 123Iodine-labelled N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4- iodophenyl) nortropane; 123I-IMP: 123I-N-isopropyl-p-iodoamphetamine; MCI: Mild Cognitive Impairment; MMSE: Mini-Mental State Examination; MRI: Magnetic Resonance Imaging, NIA-AA criteria: criteria of the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease; PD: Parkinson’s Disease; SPECT: Single Photon Emission Tomography; UPDRS: Unified Parkinson Disease Rating Scale; VOI: Volume of Interest; VSRAD: Voxel-based Specific Regional Analysis system for Alzheimer’s Disease
Introduction
Olfactory dysfunction is one of the earliest clinical features or preclinical symptoms commonly observed in neurodegenerative diseases, such as Mild Cognitive Impairment (MCI) [1], Alzheimer’s Disease (AD) [2,3], and Parkinson’s Disease (PD) [4]. It is widely accepted that the smell identification test is a sensitive method of detecting odor identification ability at the preclinical stage of AD [5] and PD [6]. However, the possible synergistic effects of the coexistence of AD and PD on the progression of olfactory impairment remain to be elucidated. In the present study, the author examined whether the coexistence of parkinsonism in patients with MCI or mild AD is synergistically involved in the exacerbation of olfactory impairment.
Subjects and Methods
This study was approved by the ethics committee of Agano City Hospital, and informed consent to participate in the clinical investigation was obtained from all the patients or their caregivers.
Patients and control individuals
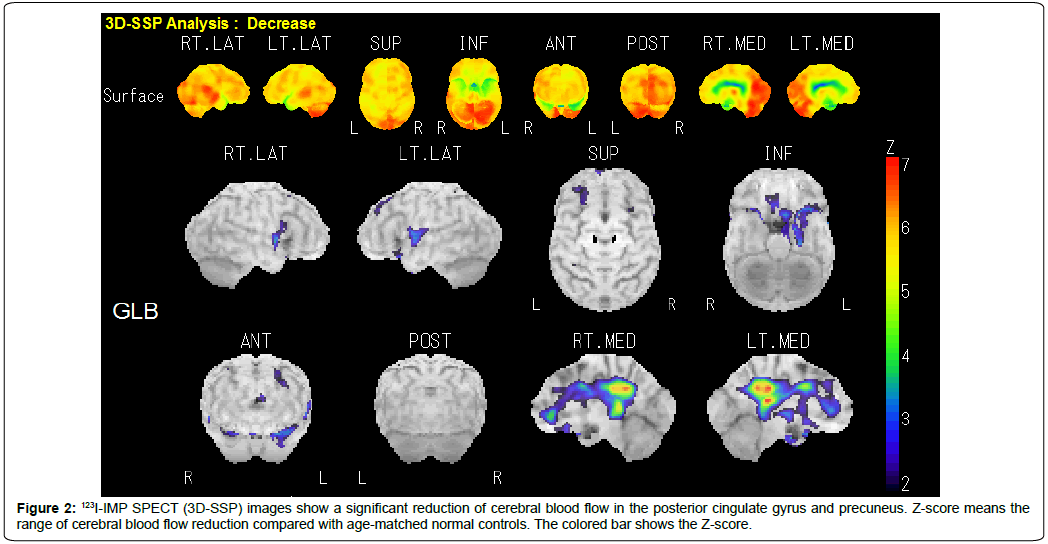
Ambulatory outpatients whose cognitive decline had been recognized by themselves or their caregivers were examined using the Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR) Scale, and the criteria of the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease (NIA–AA criteria) [7,8]. One hundred and thirty-four patients with mild cognitive reduction (MMSE ≥ 20) were consecutively investigated by MRI (Figure 1A and Figure 1B) and 123I-N-isopropyl-p-iodoamphetamine (123I-IMP) single photon emission tomography (SPECT) (three-dimensional stereotactic surface projection: 3D-SSP) (Figure 2). MRI (1.5 Tesla) was performed to rule out cognitive impairment and parkinsonian signs due to cerebral organic changes such as cerebral infarction, small vascular diseases (leukoaraiosis, amyloid angiopathy, lacunar infarcts and microbleeds), brain tumor, corticobasal syndrome, or progressive supranuclear palsy (Figure 1A). Brain MRI images were also analyzed to evaluate the degree of atrophy of medial temporal structures involving the entire region of the entorhinal cortex, hippocampus, and amygdala, determined as a target Volume of Interest (VOI), using Voxel-based Specific Regional Analysis system for Alzheimer’s Disease (VSRAD) software (Figure 1B), which is useful for diagnosing very mild AD [9]. The degree of medial temporal atrophy was obtained from the averaged VSRAD Z-score on the target VOI, with higher scores indicating more severe medial temporal atrophy (0~1: no atrophy; 1~2: mild; 2~3: moderate; 3~: severe). 123I-IMP SPECT (3D-SSP) was carried out to detect a possible reduction in cerebral blood flow, particularly in the posterior cingulate gyrus and precuneus. Exclusion criteria for the present study included: evidence of neurodegenerative diseases other than AD, such as frontotemporal dementia, argyrophilic grain disease and dementia with Lewy bodies; metabolic disturbances such as hepatic, renal, pulmonary and endocrine dysfunctions; vitamin deficiency; epilepsy; and psychiatric disease. Patients were eligible only if they had a history of cognitive decline that was gradual at the onset of the disease and then became progressive over a period of at least 12 months. Thus, a sample of Japanese outpatients meeting MMSE, CDR, NIA–AA criteria, MRI and 123I-IMP SPECT (3D-SSP) for amnestic MCI or mild AD were selected as pertinent subjects in a routine clinical setting.
A neurological examination to detect parkinsonism was performed with special emphasis on resting tremor and rigidity at the first visit, followed by monthly check-ups for at least one year to confirm the presence of parkinsonism. The diagnosis of PD was based on three specific symptoms: rigidity, bradykinesia, and resting tremor. Each patient was assessed according to the Criteria of the United Kingdom Brain Bank [10] and of a subscale of the motor examination (part III) of the Unified Parkinson Disease Rating Scale (UPDRS) [11]. In this study, parkinsonism was clinically defined as present if rigidity and resting tremor were concurrently present and the total score of UPDRS part III was ≥3.
Thus, patients with MCI or mild AD (n: 64), patients with parkinsonism free of cognitive impairment (n: 62), and patients with MCI or mild AD with parkinsonism (n: 70) were enrolled. Due to their generally mild parkinsonism, none of them were treated with antiparkinsonian medication. Out of 70 patients with MCI or mild AD with parkinsonism, 61 (87.1%) underwent 123Iodine-labelled N-(3-fluoropropyl)-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (123I-FP-CIT) dopamine transporter (DaTSCAN) SPECT (Figure 3), as did 51 (82.3%) out of 62 patients with parkinsonism. As agematched controls, 112 healthy individuals, 68 to 92 years of age, served. Co-morbid medical conditions, such as chronic rhinitis, use of nasal vasoconstrictors, severe head injury, intracranial surgery, surgery in the nasal cavity, seasonal allergies, or other current respiratory infection, as well as heavy smoking served as exclusion criteria.
Assessment of olfactory function
Deficits in olfactory function are detected by various smell tests. The most widely used are psychophysical identification tests such as the University of Pennsylvania Smell Identification Test (UPSIT) [12]. However, odor identification is strongly influenced by social and cultural factors, and many odors are not universally recognized. Therefore, the odorants used in a smell identification test should be familiar to the test population. The Odor-Stick Identification Test for Japanese (OSIT-J) (Daiichi Yakuhin Sangyo, Tokyo), whose odor items are selected because they are familiar to Japanese people, can be an effective tool to detect olfactory dysfunction in Japanese subjects [13]. The average estimated odorant familiarity score was significantly higher for the OSIT-J than the CC-SIT [13], while a significant correlation between the OSIT-J identification rate and the Cross-Cultural Smell Identification Test (CC-SIT) has been reported [14]. Thus, in this study, the OSIT-J was used for the evaluation of olfaction.
The OSIT-J includes 12 daily odorants familiar to Japanese individuals: Japanese cypress (hinoki), India ink, rose, perfume, cooking gas, menthol, sweaty socks, curry, Japanese orange, condensed milk, roasted garlic, and timber (wood). Each subject sniffed all 12 odorants applied to paraffin paper one by one, and then selected 1 of 6 choices for each odorant. Three of the choices were different smells that were not the actual smell of the odorant, 1 choice was the smell of the odorant, and the other 2 choices were “indistinguishable” (i.e., detectable but not recognized) and “odorless” (i.e., anosmia). The OSIT-J score was calculated in three categories as the number of correct answers, the number of responses of indistinguishable, and the number of responses of odorless.
Statistical analysis
The data were expressed as means (Standard Deviation: SD). Because the test for sample variance was equal in MMSE score and years of schooling, the data obtained were subjected to the unpaired Student’s t-test. The data related to age and sex was assessed by ANOVA and χ2 test, respectively. The data of non-parametric samples in UPDRS part III scores in two patient groups (parkinsonism, MCI or mild AD with parkinsonism) were analyzed by the Wilcoxon rank sum test. The data of non-parametric samples in VSRAD Z-scores and smell test scores in three patient groups (MCI or mild AD without parkinsonism, parkinsonism, and MCI or mild AD with parkinsonism) were assessed by the Kruskal-Wallis test, and when differences were significant, statistical evaluation was performed by the Wilcoxon rank sum test with Bonferroni correction. The data were analyzed using SAS 9.4 (SAS Institute, Cary, NC). Differences were considered significant if P<0.05.
Results
Age-matched control individuals
The mean (SD) age of cognitively normal age-matched controls was 78.4 (7.0) (range 68-92, n: 112), and 72 (64.3%) were female (Table 1). The mean (SD) numbers of correct answers, responses of indistinguishable and responses of odorless on the OSIT-J were 6.9 (1.7), 0.7 (1.2), and 0.2 (0.5), respectively (Table 2).
| Baseline features | Age-matched controls (n=112) | MCI or mid AD without parkinsonism (N=64) | Parkinsonism (n=62) | MCI or mild AD with parkinsonism (n=70) | P -value |
|---|---|---|---|---|---|
| Age (years), range | 78.4 ± 7.0 (68-92) | 79.5 ± 7.7 (64-97) | 78.2 ± 7.6 (60-92) | 80.2 ± 5.7 (67-91) | NS |
| Sex, female (%) | 72 (64.3%) | 38 (59.4%) | 36 (58.1%) | 47 (67.1%) | NS |
| Mean MMSE score | 24.3 ± 3.6 | 24.4 ± 3.3 | NS | ||
| Mean years of schooling | 9.8 ± 2.2 | 9.1 ± 2.1 | NS | ||
| Mean UPDRS part III score | 5.5 ± 2.0 | 6.0 ± 2.6 | NS | ||
| VSRAD z-score | normal range: 0~1 | 1.7 ± 0.85 | 1.08 ± 0.61 (n=57) | 1.82 ± 1.20 | p<0.01* |
Data shown are mean ± standard deviation.
p values were assessed by ANOVA, χ2 test, Student's t-test, or Wilcoxon rank sum test.
*Significant difference detected by Wilcoxon rank sum test with Bonferroni correction
MCI or mild AD without parkinsonism> parkinsonism (p<0.001),
MCI or mild AD with parkinsonism> parkinsonism (p=0.0003)
MMSE: Mini-Mental State Examination; UPDRS: Unified Parkinson Disease Rating Scale; VSRAD: Voxel-based Specific Regional Analysis System for Alzheimer’s Disease; MCI: mild cognitive impairment; AD: Alzheimer's disease; NS: not significant.
Table 1: The clinical and examination data on controls, MCI or mild AD without parkinsonism, parkinsonism, and MCI or mild AD with parkinsonism.
| OSIT-J score | Age-matchded controls (n=112) | Patient Groups | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| MCI or mild AD without parkinsonism (N=64) | Parkinsonism (n=62) | MCI or mild AD with parkinsonism (n=70) | ||||||
| mean ± SD | median [25th, 75th percentile] | mean ± SD | median [25th, 75th percentile] | mean ± SD | median [25th, 75th percentile] | |||
| correct answers | 6.9 ± 1.7 | 4.3 ± 2.9 | 4 [2-7] | 4.2 ± 2.0 | 4 [3-5] | 2.8 ± 2.7 | 3 [0-4] | p<0.01a |
| responses of indistinguishable | 0.7 ± 1.2 | 1.7 ± 2.2 | 1 [0-3] | 1.5 ± 1.6 | 1 [0-3] | 1.9 ± 2.2 | 1 [0-3] | NS |
| responses of odorless | 0.2 ± 0.5 | 1.9 ± 3.1 | 1 [0-2] | 0.9 ± 1.7 | 0 [0-1] | 3.6 ± 4.7 | 1 [0-7] | p<0.01b |
Data shown are mean ± standard deviation and median [25th, 75th percentile (interquantile range)]
*Significant difference detected by Wilcoxon rank sum test with Bonferroni correction
aMCI or mild AD with parkinsonism>MCI or mild AD without parkinsonism(p=0.0045), MCI or mild AD with parkinsonism>parkinsonism (p=0.0015)
bMCI or mild AD with parkinsonism> parkinsonism (p=0.0039)
All three olfactory categories were significantly impaired in the patient groupsas compared with the age-matched controls (p<0.01).
OSIT-J: The odor-stick identification test for Japanese; MCI: Mild Cognitive Impairment; SD: Standard Deviation
Table 2: Comparison of the smell test data between controls and the patient groups
Patients with MCI or mild AD without parkinsonism
The clinical details of the 64 patients consisting of 35 patients with MCI (MMSE ≥ 24, CDR 0.5) and 29 patients with mild AD (MMSE 20-23) are shown in Table 1. The mean (SD) age of the enrolled patients was 79.5 (7.7) (range 63-97, n: 64), and 59.4% (n: 38) were female. The mean (SD) MMSE score was 24.3 (3.6). The mean (SD) educational attainment was 9.8 (2.2) years (range 6-13). The averaged (SD) VSRAD Z-score on the target VOI was 1.70 (0.85) (n: 64), consisting of 1.52 (0.82) (n: 35) for the patients with MCI and 1.92 (0.84) (n: 29) for those with mild AD (p>0.05).
The mean (SD) numbers of correct answers, responses of indistinguishable, and responses of odorless were 4.3 (2.9), 1.7 (2.2), and 1.9 (3.1), respectively (Table 2).
Patients with parkinsonism
A total of 62 patients with parkinsonism free of cognitive impairment were enrolled. The mean UPDRS part III score was 5.5 (2.0) (range 3-11) (Table 1). All the patients showed the early stage of PD at a Hoehn and Yahr stage of 1 or 2. The clinical profiles and examination results of these patients are shown in Table 1. The mean (SD) age was 78.2 (7.6) (range 60-92), and 58.1% (n: 36) were female. The averaged (SD) VSRAD Z-score was 1.08 (0.61) (n: 57), significantly lower than that in the patients with MCI or mild AD without parkinsonism (1.70 [0.85], n: 64) (p<0.001). Fifty-one out of 62 patients (82.3%) underwent DaTSCAN imaging and showed pre-synaptic dopamine transporter reduction in the bilateral posterior putamen compatible with early stage PD. The mean (SD) numbers of correct answers, responses of indistinguishable, and responses of odorless were 4.2 (2.0), 1.5 (1.6), 0.9 (1.7), respectively, and none of these were significantly different from the corresponding values in the patients with MCI or mild AD without parkinsonism (p>0.05) (Table 2).
Patients with MCI or mild AD with parkinsonism
Seventy patients were investigated. The mean (SD) UPDRS part III score was 6.0 (2.6) (range 3-12, n: 70) (Hoehn and Yahr stage: 1 or 2), which was not significantly different from that in the patients with parkinsonism (p>0.05) (Table 1). The clinical profiles and examination results of these 70 patients are shown in Table 1. The mean [SD] age was 80.2 [5.7] (range 67-91), which was not significantly different from that of their counterparts without parkinsonism or that of the patients with parkinsonism, and 67.1% (n: 47) were female. The mean (SD) MMSE score was 24.4 (3.3) (n: 70), which was not significantly different from that of the patients with MCI or mild AD without parkinsonism (p>0.05). The mean educational attainment was 9.1 (2.1) years (range 6-12), showing no significant difference from that of the patients with MCI or mild AD without parkinsonism (p>0.05). The averaged (SD) VSRAD Z-score was 1.82 (1.20) (n: 70), which was not significantly different from that in the patients with MCI or mild AD without parkinsonism (1.70 [0.85]) (p>0.05) but significantly higher than that in the patients with parkinsonism (1.08 [0.61], n: 57) (p: 0.0003). Fiftyfour patients with parkinsonism who underwent DaTSCAN imaging showed pre-synaptic dopamine transporter reduction in the posterior putamen, with egg-shaped degeneration patterns [15] (Figure 3), while 7 patients exhibited dopamine transporter reduction predominantly in the caudate nucleus.
The mean (SD) number of correct answers on the OSIT-J was 2.8 (2.7), significantly lower than that in the patients with MCI or mild AD without parkinsonism (4.3 [2.9]) (p: 0.0045) or that in the patients with parkinsonism (4.2 [2.0]) (p: 0.0015) (Table 2). The mean (SD) number of responses of indistinguishable was 1.9 (2.2), and this value was not significantly different from that in the patients with MCI or mild AD without parkinsonism or that in the patients with parkinsonism (p>0.05) (Table 2). The mean (SD) number of responses of odorless was 3.6 (4.7), which was significantly higher than that in the patients with parkinsonism (0.9 [1.7]) (p: 0.0039), while no significant difference in this value was recognized between the MCI or mild AD with parkinsonism group and the MCI or mild AD without parkinsonism group, or between the MCI or mild AD without parkinsonism group and the parkinsonism group (p>0.05) (Table 2).
The olfactory functions for all three olfactory categories (correct answers, responses of indistinguishable, and responses of odorless) in the three patient groups were significantly impaired as compared with those in the age-matched controls (p<0.01) (Table 2).
Differences in smell-test results between men and women
In the age-matched controls, no significant sexual differences were recognized in the mean (SD) numbers of correct answers, responses of indistinguishable or responses of odorless (p>0.05). The mean (SD) number of responses of indistinguishable was significantly higher in men (2.9 [2.8], n: 23) than in women (1.5 [1.6], n: 47) in patients with MCI or mild AD with parkinsonism (p<0.05), but there was no significant difference by sex in the mean (SD) numbers of correct answers or responses of odorless among the three patient groups (p>0.05).
Discussion
In this study, the patients with MCI or mild AD accompanied by parkinsonism showed more severe olfactory impairment than was observed in those with MCI or mild AD without parkinsonism, or those with parkinsonism free of cognitive impairment. These findings suggest that synergistic effects of MCI or mild AD and parkinsonism can exacerbate olfactory impairment through a synergistic interaction between α-synuclein and AD pathology, providing new clinical insight into the interaction between AD and PD. A higher-than-expected degree of olfactory deficits in patients with MCI or mild AD in an outpatient setting may suggest the coexistence of parkinsonism.
Olfactory impairment is associated with the pathogenesis of the neurodegenerative diseases of MCI [1], AD [1-3], and PD [4]. Moreover, olfactory dysfunction predicts incident amnestic MCI and progression from MCI to AD [1,3,16]. In AD, olfactory identification impairment has been related to severity of illness; both reduced hippocampal volume and decreased AD signature cortical thickness by MRI [17,18]; and reduced Blood Oxygen Level-Dependent (BOLD) signals in the primary olfactory cortex, hippocampus, and insula regions by functional MRI [18]. Regarding odor identification deficits in AD, neurofibrillary tangles are found in the olfactory bulb, anterior olfactory nucleus, olfactory tract, entorhinal cortex, amygdala and CA1/subiculum area of the hippocampus [19,20], whereas the β-amyloid burden is not directly associated with the AD-related olfactory identification deficits, as measured by the Pittsburgh Compound B (PiB) PET study [21]. The observation of impaired olfaction in MCI or mild AD in this study agrees with the previous reports that impaired olfaction is one of the earliest symptoms of AD [1-3]. Moreover, the observation of a reduced volume of medial temporal structures by VSRAD in the present study is consistent with the previous reports showing reduced hippocampal volume by MRI [17,18].
PD is associated with a similarly high prevalence rate of olfactory dysfunction, ranging from 45% to 90% of patients, and involves all olfactory domains [4,22]; these results are also congruent with the present study. Regarding the pathogenesis of olfactory impairment in PD, PD-related pathology begins in the olfactory bulb, anterior olfactory nucleus, and the dorsal motor nucleus of the glossopharyngeal and vagal nerves, where Lewy bodies first develop [23]. Olfactory impairment seems to be primarily due to Lewy body pathology, including increased phosphorylated-α-synuclein immunoreactivity in the olfactory bulb, particularly in the anterior olfactory nucleus [23,24]. Correlations are also observed between Brief Smell Identification Test scores and Lewy body pathology within the limbic and neocortical brain regions [25], which is partly compatible with the present study in that the volume of the medial temporal structures was slightly reduced in the patients with parkinsonism by MRI (VSRAD).
In previous reports, anosmia has been detected in approximately 40% of patients with PD, 20% to 40% of those with AD, and 65% of those with the Lewy body variant of AD, and the incidence of anosmia in PD patients and AD patients increased with an increase in cognitive dysfunction [26,27]. While the early changes associated with olfactory system processing have been reported to include decrements in the volume of the hippocampus, piriform cortex, anterior olfactory nucleus, and frontal poles of the brain [28,29], in voxel-based morphometry in MRI, anosmia is associated with a significant decrease in gray matter volume in areas of the limbic system such as the hippocampus, parahippocampus, nucleus accumbens, and piriform cortex, which are correlated with olfaction-related structures [30]. In this study, the prevalence rate of anosmia was significantly higher in the patients with MCI or mild AD accompanied by parkinsonism than in the patients with parkinsonism. Also in the present study, the volume of medial temporal structures involving the entire region of the entorhinal cortex, hippocampus, and amygdala was decreased in all the 3 patient groups, as measured by MRI (VSRAD), which is in line with the previous reports [9,17,26].
Many clinicopathological reports have noted that AD and PD share a common pathogenesis. Moreover, nigral degeneration with Lewy body formation suggesting the coexistence of PD has been reported in 18-85% of autopsy-confirmed cases of AD patients with parkinsonism [31,32]. In addition, up to half of carefully research-diagnosed patients with AD may have unsuspected Lewy-related pathology at autopsy [33]. The present author has recently reported a high prevalence of parkinsonism in patients with MCI or mild AD [34]. Conversely, in PD patients with dementia, in addition to cortical Lewy body pathology, AD pathology has been associated with cognitive impairment [35]. Up to 50% of PD patients with dementia will meet the pathological criteria for a secondary diagnosis of AD [36], which may act synergistically with Lewy body pathology. In early PD, worse baseline olfaction was associated with a lower Aβ42 and a higher tau/Aβ42 ratio in cerebrospinal fluid, as well as long-term cognitive decline [37]. Data from animal studies suggest that synergistic interactions between tau, α-synuclein, and Aβ promote their mutual aggregation and accumulation, thereby accelerating cognitive dysfunction [38], and that the severity of α-synuclein pathology is influenced by AD pathology [39].
Further studies are warranted to elucidate whether the coexistence of MCI or mild AD and parkinsonism may synergistically exacerbate olfactory impairment.
Acknowledgement
The author wishes to thank Mr. Kentaro Yamato (Department of Public Health, Juntendo University Graduate School of Medicine, Tokyo; an employee of Takeda Pharmaceutical Company Limited, Tokyo) for statistical analysis.
Conflict of Interest/Disclosure Statement
The author has no conflict of interest to report.
References
- Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, et al. (2016) Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 73: 93-101.
- Doty RL, Reyes PF, Gregor T (1987) Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull 18: 597-600.
- Tabert MH, Liu X, Doty RL, Serby M, Zamora D, et al. (2005) A 10-item smell identification scale related to risk for Alzheimer's disease. Ann Neurol 58: 155-160.
- Doty RL (2012) Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8: 329-339.
- Vassilaki M, Christianson TJ, Mielke MM, Geda YE, Kremers WK, et al. (2017) Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann Neurol 81: 871-882.
- Doty RL, Bromley SM, Stern MB (1995) Olfactory testing as an aid in the diagnosis of Parkinson's disease: development of optimal discrimination criteria. Neurodegeneration 4: 93-97.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, et al. (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7: 263-269.
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, et al. (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement7: 270-279.
- Matsuda H, Mizumura S, Nemoto K, Yamashita F, Imabayashi E, et al (2012) Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer Disease. Am J Neuroradiol 33: 1109-1114.
- Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease.J Neurol Neurosurg Psychiatry 51: 745-752.
- https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=1365499
- Doty RL, Shaman P, Dann M (1984) Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 32: 489-502.
- Saito S, Ayabe-Kanamura S, Takashima Y, Gotow N, Naito N, et al. (2006) Development of a smell identification test using a novel stick-type odor presentation kit. Chemical Senses 31: 379-391.
- Doty RL, Marcus A, Lee WW (1996) Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 106: 353-356.
- Kahraman D, Eggers C, Schicha H, Timmermann L, Schmidt M (2012) Visual assessment of dopaminergic degeneration pattern in 123I-FP-CIT SPECT differentiates patients with atypical parkinsonian syndromes and idiopathic Parkinson's disease.J Neurol 259: 251-260.
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, et al. (2000) Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry 157: 1399-1405.
- Thomann PA, Dos Santos V, Seidl U, Toro P, Essig M, et al (2009) MRI-derived atrophy of the olfactory bulb and tract in mild cognitive impairment and Alzheimer's disease.J Alzheimers Dis 17: 213-221.
- Velayudhan L (2015) Smell identification function and Alzheimer's disease: a selective review. Curr Opin Psychiatry 28: 173-179.
- Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA (2007) The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry78: 30-35.
- Kovács T, Cairns NJ, Lantos PL (2001) Olfactory centres in Alzheimer's disease: Olfactory bulb is involved in early Braak's stages.Neuroreport12: 285-288.
- Bahar-Fuchs A, Chételat G, Villemagne VL, Moss S, Pike K, et al. (2010) Olfactory deficits and amyloid-β burden in Alzheimer's disease, mild cognitive impairment, and healthy aging: A PiB PET study. J Alzheimers Dis 22: 1081-1087.
- Haehner A, Boesveldt S, Berendse HW, Mackay-Sim A, Fleischmann J, et al. (2009) Prevalence of smell loss in Parkinson's disease--a multicenter study. Parkinsonism Relat Disord 15: 490-494.
- Braak H, Rub U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible route by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 119: 517-536.
- Zapiec B, Dieriks BV, Tan S, Faull RLM, Mombaerts P, et al. (2017) A ventral glomerular deficit in Parkinson's disease revealed by whole olfactory bulb reconstruction. Brain 140: 2722-2736.
- Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, et al. (2011) Lewy bodies and olfactory dysfunction in old age. Chem Senses 36: 367–373.
- Tarakad A, Jankovic J (2017) Anosmia and ageusia in Parkinson's disease. Int Rev Neurobiol 133: 541-556.
- Olichney JM, Murphy C, Hofstetter CR, Foster K, Hansen LA, et al. (2005) Anosmia is very common in the Lewy body variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 76: 1342-1347.
- Kemper T (1984) Neuroanatomical and neuropathological changes during age and dementia. Clinical Neurology of Aging. Oxford University Press, New York.
- Wang J, You H, Liu JF, Ni DF, Zhang ZX, et al. (2011) Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am J Neuroradiol 32: 677-681.
- Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas-Lichius O, et al. (2010) Anosmia leads to a loss of gray matter in cortical brain areas.Chem Senses 35: 407-415.
- Leverenz J, Sumi SM (1986) Parkinson's disease in patients with Alzheimer's disease. Arch Neurol 43: 662-664.
- Joachim CL, Morris JH, Selkoe DJ (1988) Clinically diagnosed Alzheimer's disease: Autopsy results in 150 cases. Ann Neurol 24: 50-56.
- Toledo JB, Cairns NJ, Da X, Chen K, Carter D, et al. (2013) Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta Neuropathol Commun1: 65.
- Sasaki. S (2018) High prevalence of parkinsonism in patients with MCI or mild Alzhei2013mer's disease. Alzheimers Dement 14: 1615-1622.
- Libow LS, Frisina PG, Haroutunian V, Perl DP, Purohit DP (2009) Parkinson's disease dementia: A diminished role for the Lewy body. Parkinsonism Relat Disord15: 572-575.
- Irwin DJ, Lee VM, Trojanowski JQ (2013) Parkinson's disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci 14: 626-636.
- Fullard ME, Tran B, Xie SX, Toledo JB, Scordia C, et al. (2016) Olfactory impairment predicts cognitive decline in early Parkinson's disease. Parkinsonism Relat Disord 25: 45-51.
- Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM (2010) Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci 30: 7281-7289.
- Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, et al. (2017) Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: A retrospective analysis. Lancet Neurol 16: 55-65.
Citation: Sasaki S (2019) Synergistic Effects of Alzheimer’s Disease and Parkinsonism on Olfactory Impairment. J Alzheimers Dis Parkinsonism 9: 464. DOI: 10.4172/2161-0460.1000464
Copyright: © 2019 Sasaki S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3204
- [From(publication date): 0-2019 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 2445
- PDF downloads: 759