Review Article Open Access
Synaptic Function and Dysfunction in Alzheimer's Disease
Origlia N1* and Domenici L1,2*1Neuroscience Institute of the National Council of Research (CNR), 56124 Pisa, Italy
2Department of Applied Clinical Sciences and Biotechnology (DISCAB), University of L’Aquila, 67100 L’Aquila, Italy
- Corresponding Authors:
- Luciano Domenici
Department of Applied Clinical Sciences and Biotechnology (DISCAB)
University of L’Aquila, 67100 L’Aquila, Italy
Tel: +390503153193
E-mail: domenici@in.cnr.it - Nicola Origlia
Neuroscience Institute of the National Council of Research (CNR)
56124 Pisa, Italy
Tel: +390503153193
E-mail: origlia@in.cnr.it
Received date: May 20, 2017; Accepted date: June 20, 2017; Published date: June 27, 2017
Citation: Origlia N, Domenici L (2017) Synaptic Function and Dysfunction in Alzheimer’s Disease. J Alzheimers Dis Parkinsonism 7:341. doi:10.4172/2161- 0460.1000341
Copyright: © 2017 Origlia N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Alzheimer’s disease (AD) is a multifactorial neurodegenerative disease, primarily affecting the elderly. Pathophysiological mechanisms have been elucidated in the past decades. First of all, AD is progressive leading to cognitive deficits till dementia. Pathologically, AD features synaptic dysfunction with changes of neuronal circuitry, progressive accumulation of protein aggregates such as the beta amyloid and tau. Herein we critically review neurobiological processes and factors involved in AD, in light of recent results on synaptic dysfunction and impairment of neuronal activity.
Keywords
Synaptic transmission; Synaptic plasticity; Neuronal activity; Beta amyloid; Tau
Introduction
Alzheimer's disease (AD) is a neurodegenerative progressive disease of the elderly leading to dementia. The world Alzheimer report (Alzheimer’s disease International, global impact of dementia) of 2015 indicated that 46.8 million people worldwide are living with dementia; this number is expected to double every 20 years [1]. There are two forms of AD.
1. Early onset Familial Alzheimer Disease (eFAD). Abnormalities of the amyloid precursor protein (APP) that render it more amyloidogenic, or defects of processing normal APP cause genetic forms of AD. The literature estimates that eFAD accounts for approximately 2% of all people with dementia (approximately 3-5% of all Alzheimer cases) [1,2]. In these patients, autosomal dominant AD usually develops before age 65 due to mutations of the APP gene on chromosome 21 or the presenilin 1 and 2 genes (PSEN1 and PSEN2) on chromosomes 14 and 1, respectively.
2. Sporadic AD (SAD, late-onset). SAD is very common in the elderly (approximately 70% of patients with dementia are attributed to SAD [1]). The cause of SAD is unknown. The vast majority of SAD is not genetically inherited although some genes such as the APOE may act as a major risk factor [3].
Vascular diseases such as hypertension and brain ischemia [4,5], diabetes [6,7] and obesity [8], traumatic brain injury [9], mood disorders [10] represent risk factors for SAD.
The neuropathological changes of AD brain include classical hallmarks such as the senile plaques and neurofibrillary tangles, and dystrophic neurites containing hyperphosphorylated tau [11-13]. Additional changes are represented by astrogliosis [14], microglial cell activation [14,15] and inflammatory reaction [16]. Senile plaques with amyloid cores in the brain of AD patients are often described in close proximity to microvessels with amyloid angiopathy [17].
Whereas considerable heterogeneity exists in the degree to which cognitive functions are affected in patients with AD, learning/memory impairment is almost invariably reported in AD [18,19]; typically, declarative memory is impaired and this quite often represents the initial cognitive deficit in AD. Indeed, the initial brain areas involved in the neurodegenerative progression of AD are the entorhinal cortex, hippocampus and temporal cortex [20,21], i.e., crucial areas for learning/memory. The hypothesis has been advanced that impairment of the entorhinal cortex initiates cortical-hippocampal dysfunction in AD [22]. The olfactory bulb, anterior olfactory nucleus, orbitofrontal cortex and parahippocampal cortices receiving olfactory input are all also affected early in AD [23]. Thus, odor recognition performance, in particular the ability to identify familiar odors, in association with episodic memory is impaired early in AD [24].
In addition to eFAD and SAD there are patients with cognitive decline unusual for their age that does not affect daily living (for example difficulty in remembering names of individuals, misplacing keys and spectacles or difficulty in remembering phone numbers, messages and appointments, therefore mostly verbal episodic memory deficit). This clinical state is called mild cognitive impairment (MCI). Some MCI patients progress to AD (roughly 15%/year; [25]), others progress to vascular dementia, but others remain stable or revert to normal, indicating that MCI has diverse causes and represents a heterogeneous group of patients. MCI patients can be further subdivided in: MCI patients with an amnestic profile [26] (impaired episodic memory retention and retrieval) and MCI patients with an anamnestic profile susceptible to be converted in AD.
AD pathogenesis: Amyloid-dependent mechanisms and synaptic dysfunction
The pathogenesis of AD is characterized by formation of senile plaques and neurofibrillary tangles considered as hallmarks of AD. Aggregates of β-amyloid protein (Aβ) are the principal component of senile plaques [27]. Electron microscopy studies revealed that dense-core plaques comprise aggregates of Aβ, extracelullar filaments (dystrophic neuritis) as well as abnormal mitochondria and dense bodies of probable mitochondrial and lysosomal origin [27-29].
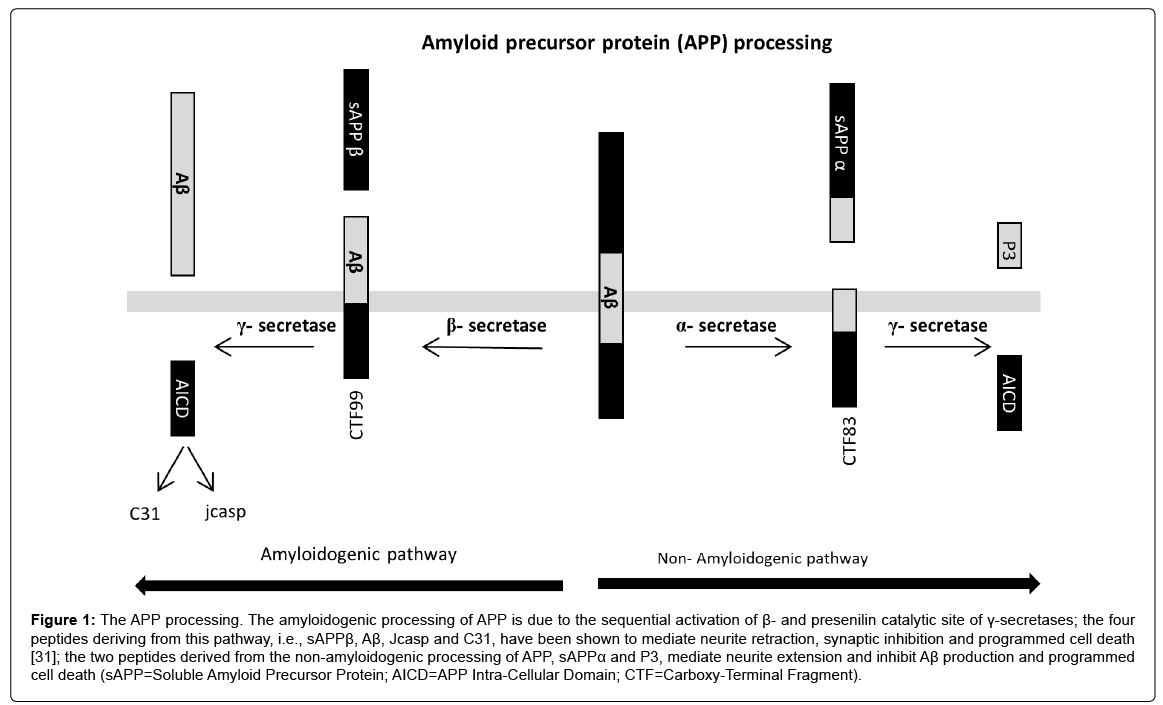
Aβ peptides start to be generated in considerable amounts by the cleavage of amyloid precursor protein (APP) due to sequential activation of β- and presenilin catalytic site of γ-secretases (Figure 1) for causes that are still unknown in SAD. The four peptides derived from the amyloidogenic processing of APP, i.e., sAPPβ, Aβ, Jcasp and C31, have been shown to mediate neurite retraction, synaptic inhibition and programmed cell death [30]. APP cleavage by α and β-secretases generates soluble secreted fragments (sAPPα and sAPPβ) and membrane-associated carboxy-terminal fragments (CTF), which preclude Aβ generation; the two peptides derived from the nonamyloidogenic processing of APP, sAPPα and CTF, mediate neurite extension, and inhibit Aβ production and programmed cell death [31]. Aβ can be found in different compositions of monomers, oligomers or fibrils [32]; in particular, increasing Aβ level tends to form monomers, which aggregate into oligomers, prefibrillar assemblies (protofibrils) and amyloid fibrils in a concentration-dependent manner. Toxic Aβ peptides are formed by 36-43 amino acids; the 42 amino acid peptide (Aβ42) is one of the most neurotoxic amyloidogenic fragments [33,34] and represents the chief component of senile plaques.
Figure 1: The APP processing. The amyloidogenic processing of APP is due to the sequential activation of β- and presenilin catalytic site of γ-secretases; the four peptides deriving from this pathway, i.e., sAPPβ, Aβ, Jcasp and C31, have been shown to mediate neurite retraction, synaptic inhibition and programmed cell death [31]; the two peptides derived from the non-amyloidogenic processing of APP, sAPPα and P3, mediate neurite extension and inhibit Aβ production and programmed cell death (sAPP=Soluble Amyloid Precursor Protein; AICD=APP Intra-Cellular Domain; CTF=Carboxy-Terminal Fragment).
Studies in AD animal models and patients have highlighted a dichotomy between behavioral deficits and neuropathologic findings. Impaired memory and synaptic loss occur before extensive deposition of amyloid in the brains of AD-type murine models and AD patients [35-37].
These observations suggest that early in AD, when levels of amyloid are low, mechanisms amplifying and focusing the effects of amyloid on cellular targets contribute to neuronal dysfunction. It is known that soluble synthetic or naturally produced oligomeric or oligomeric Aβ extracts from cerebral cortex of AD patients are capable of inhibiting hippocampal long-term potentiation (LTP) [38-42], a form of long-term synaptic plasticity thought to underlie learning and memory in the hippocampus and parahippocampal cortices [43]. Furthermore synthetic Aβ formed by dimers and trimers [44] in the low concentration of nanomolar range was capable of inhibiting LTP in the entorhinal cortex (EC) [45]. The EC represents a crucial site for memory formation as it integrates spatial information processed from the medial EC neurons with non-spatial information processed from the lateral EC neurons [46-48]. The involvement of the EC in cognitive processes is relevant for neurodegenerative disorders such as the AD, as it is one of the earliest affected brain regions [49]. This might be the consequence of a particular vulnerability of the superficial layer II neurons, that are susceptible to the deleterious consequences of aging and AD [50]. Interestingly, increasing synthetic Aβ42 concentration induces activation of microglial cells with pro-inflammatory cytokines that progressively affects synaptic transmission, AMPA current and long term depression (LTD, a second form of long term synaptic plasticity), in addition to LTP [51]. Indeed, an increasing level of Aβ42 has been shown to induce synaptic transmission impairment by regulating glutamate receptors trafficking [52,53]. Interestingly, an increase in endogenous Aβ level induced by inhibition of extracellular Aβ degradation causes pre-synaptic enhancement increasing glutamate release [54]. Thus, progression of synaptic dysfunction as well as cognitive decline by accumulation of extracellular Aβ is likely to result in alterations of pre- and post-synaptic proteins [55]. Activation of receptors such as the receptor for advanced glycation end products (RAGE) by Aβ [56] accounts for progress of synaptic dysfunction, development of inflammatory and, possibly, oxidative processes, leading cells to degenerate [51]. Concerning the receptor signaling pathways mediating synaptic dysfunction, it has been reported that Aβ impairs LTP in the hippocampus through JNK, cyclin-dependent kinase 5 (CDC5), p38 mitogen-activated protein kinase (MAPK) [41]. In particular, low level of synthetic oligomeric Aβ42 inhibits LTP in hippocampus and entorhinal cortex through phosphorylation of p38 MAPK in neurons [45,57,58]. Increasing Aβ induces specific phosphorylation of p38 MAPK and JNK in neuronal and non-neuronal cells along with the induction of pro-inflammatory cytokines, such as the IL-β; as above reported in this condition basal synaptic transmission and long-term synaptic plasticity are affected [51].
Aβ-dependent toxicity is also mediated by internalization of Aβ peptides into neuronal cells, which contributes to disrupt neuronal functions [59,60]. One important question concerns the mechanism underlying Aβ transport from the extracellular to intracellular space. It has been reported that RAGE expressed in brain endothelial cells mediates Aβ transport across the blood brain barrier [61]. More recently, Takuma et al. [62] reported that RAGE represents at least a co-factor contributing to translocation across cell membrane of Aβ driving its transport and delivery to different subcellular spaces including mitochondria. Mitochondrial dysfunction with resultant neuronal perturbation and cognitive impairment [29,63] is considered a key feature in AD neuropathology and synapto-toxicity. Recent studies have shown that Aβ-binding alcohol dehydrogenase (ABAD), an enzyme present in neuronal mitochondria, exacerbates Aβ-induced mitochondrial and neuronal dysfunction; indeed, inhibition of the ABAD-Aβ interaction protects mitochondrial function and improves learning/memory [64]. Thus, Aβ-dependent synaptic dysfunction is mediated by several factors and mechanisms and its outcome depends on: level/accumulation of Aβ peptides, their aggregation state (monomers, oligomers, protofibrils, fibrils and plaques), signaling pathways activated in different neural cells, translocation across cell membrane and transport of Aβ peptide.
Actually, Aβ can be considered a product of APP metabolism. Indeed, Aβ level is detectable even in young healthy people but at a very low level. This result is in accordance with a supposed trophic role of low Aβ level [65]; remarkably, Puzzo et al. [66,67] showed that a very low level of exogenously applied Aβ42 (picomolar range) enhances synaptic plasticity and memory by acting through α7 nicotinic receptor and confirmed that endogenous low Aβ is necessary for synaptic plasticity and memory. Thus, the accumulation (enhanced formation and/or defective clearance) of Aβ in specific areas of the brain (parahippocampal cortices, hippocampus and neocortical areas) for unknown cause(s) can be considered as an early pathologic event in AD leading to synaptic dysfunction. An alternative intriguing possibility is that mechanisms triggering the initial conversion of Aβ soluble protein into filamentous fibrillar species, with prion-like domains, are at the origin of AD pathogenesis [68]. However, several studies suggest that pre-fibrillar species, such as the Aβ oligomers in AD, may be more detrimental than fibrillar species [42], at least during an early stage in AD. During the progression of AD, misfolded Aβ and, possibly, tau filamentous species, might be involved in the propagation from one neuron to the next and from one brain region to another [68]. Interestingly, there is a relationship between the pathogenic amyloid β-peptide species and tau pathology; for example, intracerebral administration of Aβ1-42 fibrils into a mutant tau transgenic mouse induced tau hyperphosphorylation and local neurofibrillary tangles [69].
AD pathogenesis: Tau-dependent mechanisms and synaptic dysfunction
Tau is highly expressed in neurons and is abundant in axons [70]. Tau facilitates assembly and stabilization of microtubule polymers [71,72], modulating microtubule dynamics. Thus, under physiological conditions tau is mainly expressed within neurons. Human tau has been implicated in the pathogenesis of several neurodegenerative diseases, including AD [73]. Mouse models which overexpress forms of human tau have been generated and reproduce synaptic dysfunction, cognitive impairment and neurodegeneration [74-76]. Hyperphosphorylated, insoluble, and filamentous tau proteins were shown to be the main component of neurofibrillary tangles (NFTs), a pathological hallmark of AD and other tauopathies [70]. NFTs accumulate inside the cells, disrupting the intracellular transport system. Cytoskeletal changes are visible as dystrophic neurites, pre-tangles, NFTs in the cell bodies of affected neurons in AD even before plaque formation [77,78]. The neurofibrillary tangles are composed of paired helical filaments (PHF) with hyperphosphorylated forms of tau protein as the main component; the other component is represented by misfolded tau. Bundles of these PHF are also found in neurites [20,79]. Tau hyperphosphorylation can increase abnormal folding, fragmentation, aggregation and/or the development of NFTs leading to activation of intracellular pathways involved in synaptic dysfunction and neuronal toxicity; phosphorylation of tau potentiates MAPK activation similarly to Aβ and tau is one of p38 MAPK substrates [80]. Interestingly, transgenic mouse models suggest that neuronal loss and memory impairment are associated with the presence of soluble tau protein [75] (tau oligomers). Studies on cell viability have shown that misfolding of tau leads to the aggregation of tau and the appearance of toxic tau species in the extracellular space [81,82]. The endogenous intracellular tau may be released as aggregates to the extracellular space upon neuron degeneration [81]. Extracellular tau could be toxic by increasing intracellular calcium into neighboring neurons [82]. The presence of extracellular tau can be due to other causes, for example exocytosis; the N-terminal region of tau seems to be required for its secretion [83]. Neuronal toxicity may be caused by tau aggregates, even small and soluble aggregates in the form of oligomers, which have been identified in AD brain [84]. Tau can be released into the extracellular space, as oligomers [85]. Cells can take up extracellular aggregated tau [86], thus contributing to propagation of tau pathology. Formation of tau oligomers induces synaptic dysfunction and cognitive impairments [87], suggesting that this is the tau involved in early synapto-toxicity and cognitive impairment [88]. Indeed, in AD brains loss of synapses precedes NFTs formation and correlates with cognitive deficits [89,90]. Accordingly, it has been shown that prior to the formation of aggregates; tau can bind to pre-synaptic vesicles via its N-terminal domain inducing synaptic dysfunction [91]. Development of tau pathology closely associates with progressive neuronal loss and cognitive decline. In the brains of AD patients, for instance, tau pathology follows an anatomically defined pattern [20]; tau pathology spreads in the entorhinal cortex and then accumulates within limbic areas, followed by neocortical areas. Accumulation of extracellular tau species could be involved in neuronto- neuron propagation of neurofibrillary pathology and progression of tau toxicity that spreads throughout defined pattern of brain regions. The oligomers in the extracellular space could be taken-up by healthy neurons inducing further aggregation of tau [86] and propagation of neurofibrillary pathology. Recently, it has been shown that oligomeric extracellular tau is able to interact with cell receptors resulting in synaptic dysfunction and signaling propagation that could contribute to onset of neurodegeneration [92]. Moreover, these observations point to the involvement of extracellular tau species as one of the main agent in the neuron-to-neuron propagation of neurofibrillary pathology and progression of synaptic dysfunction and cognitive impairment in AD.
AD pathogenesis: The neurotrophic factors
Selective vulnerability of basal forebrain cholinergic neurons (BFCNs) contributes to cognitive decline in AD patients [93,94]. BFCNs depend on the neurotrophin nerve growth factor (NGF) [95] for their trophism and survival [96]. Moreover, other neurotrophins, such as the brain-derived neurotrophic factor (BDNF), provide a high level of protection to neurons in brain injury and diseases [97,98]. BDNF together with its receptor TrkB (tropomyosin receptor kinase), is highly expressed in several brain areas where it acts as one of the chief regulator of synaptic plasticity and synaptogenesis [99,100]. The BDNF appears to be reduced in AD brain [101-103]. Thus, the hypothesis can be raised that neurotrophins, particularly NGF and BDNF, and their receptors, are involved in the pathogenesis of neurodegenerative diseases such as the AD. In addition, the possibilities of neurotrophinbased therapeutic approaches should be evaluated. Interestingly, BDNF prevents Aβ-dependent impairment of LTP by reducing p38 MAPK phosphorylation [104]. Similarly, human painless NGF is capable of preventing synaptic plasticity impairment in the EC of the 5xFAD mouse model, either when acutely supplied on slices or following three weeks of intranasal treatment [105].
Neuronal Activity in AD
Disrupted neuronal network increases seizure activity in AD, contributing to cognitive decline [106]. Elevated electrical activity in the hippocampus has been observed early in AD in stages preceding the formation of senile plaques [107]. Interestingly, a recent paper suggested that APP molecules may function as surface receptors modulating the Aβ signaling [108]; the Authors showed that neurons in hippocampus became hyperactive at the pre-synaptic site through APP homodimer as pre-synaptic receptor, which binds Aβ40 following a rise in its concentration. If very low Aβ level is essential for the normal dayto- day life in agreement to Puzzo et al. [66,67], thus, we can hypothesize that when the level of Aβ peptides is even slightly increased, it causes neuronal hyperactivity and neuronal functional impairment in several brain areas as also frequently reported in MCI patients [109].
Since neuronal activity increases Aβ production, it is likely that regional differences in neuronal activity may underlie early Aβ aggregation and deposition in specific brain areas such as the hippocampus and additional areas that belong to the default mode network (DMN) [110]. As reported above, the typical hallmarks of AD, such as the presence of amyloid protein and neurofibrillary tangles, are seen primarily in the EC in mild AD and “spread” to the hippocampus and other cortical areas as the disease progresses [20]. Thus, the hypothesis has been raised that neurodegeneration primarily observed in EC neurons may cause trans-synaptic deficits initiating the cortical-hippocampal network dysfunction in mouse models and human patients with AD. Indeed, in an AD mouse model, selective overexpression of mutant amyloid precursor protein (APP) predominantly in layer II/III neurons of the EC caused an aberrant excitatory cortico-hippocampal network activity leading to behavioral abnormalities [22]. Moreover, the time-course of synaptic impairment of the EC layer II in human amyloid precursor protein J20 transgenic mice (mhAPP), has been characterized. Although this murine model of AD displays diffuse amyloid accumulation in the brain, synaptic dysfunction is first observed in the intrinsic circuitry of the EC and then propagates to its main target area (i.e., the hippocampus). This suggests a precise temporal profile and an exact order of involvement of different circuitries during the progression of synaptic dysfunction in mhAPP mice, possibly corresponding to different stages of Aβ accumulation [111].
Concerning tau, its release can be stimulated by enhanced neuronal activity; Wu et al. [112] showed that increasing neuronal activity enhances release and transfer of tau in vitro and exacerbate tau pathology in vivo. As neurons within the AD brain can be hyperactive [106], thus enhanced neuronal activity may increase tau pathology. More recently, Fu et al. [113] showed that in a transgenic mouse model expressing mutant human tau predominantly in the EC, the formation of tangles in old mice was associated with excitatory cell loss and cognitive deficits in grid cell function. In addition, the tau pathology in the aged mice was accompanied by spatial memory deficits. These results suggest that in addition to Aβ, the tau protein could contribute to the synaptic alterations in the EC underlying the deterioration of spatial cognition described in AD patients.
Remarkably, later stages in AD progression are generally associated with a greater Aβ load, tau aggregation and hyperphosphorylation that exaggerate impairments in synaptic and cognitive function; AMPA and NMDA current are impaired, glutamate receptors trafficking altered and metabolism reduced. In addition, reduced synaptic activity causes detrimental effects on synapses and memory, favoring the accumulation of intraneuronal Aβ [57]. Independently of amyloid, the progressive impairment of synaptic and cognitive functions in AD is generally thought to result from a reduction in neuronal and synaptic activities in brain areas such as the entorhinal cortex and hippocampus. However, recent studies revealed a more complex picture of the neuronal defects in late stages of AD, showing both hypo- and hyper-activity in several brain regions; interestingly, hyperactive neurons were found in the vicinity of beta amyloid plaques [114].
Acknowledgement
Work described in the present manuscript is supported by the Department of Biotechnological and Applied Clinical Sciences (DISCAB), University of L’Aquila, and the scientific consortium IN-BDNF. We thank Ms. S. Wilson for revising the English style.
References
- Reitz C, Mayeux R (2014) Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88: 640-651.
- Tanzi, RE (2012) The genetics of Alzheimer disease. Cold Spring Harb Perspect Med 2: a006296.
- Liu CC, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat Rev Neurol 9: 106-118.
- Pluta R, JabÅ?on´ski M, UÅ?amek-KozioÅ? M, Kocki J, Brzozowska J, et al. (2013) Sporadic Alzheimer’s disease begins as episodes of brain ischemia and ischemically dysregulated Alzheimer’s disease genes. Mol Neurobiol 48: 500-515.
- Origlia N, Criscuolo C, Arancio O, Yan SS, Domenici L (2014) RAGE inhibition in microglia prevents ischemia-dependent synaptic dysfunction in an amyloid-enriched environment. J Neurosci 34: 8749-8760.
- Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, et al. (2008) Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J 22: 246-260.
- Adzovic L, Domenici L (2014) Insulin induces phosphorylation of the AMPA receptor subunit GluR1, reversed by ZIP and overexpression of Protein Kinase M zeta, reversed by amyloid beta. J Neurochem 131: 582-587.
- Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 10: 819-28.
- Van Den Heuvel C, Thornton E, Vink R (2007) Traumatic brain injury and Alzheimer's disease: A review. Prog Brain Res 161:303-316.
- Tsuno N, Homma A (2009) What is the association between depression and Alzheimer's disease? Expert Rev Neurother 9:1667-1176.
- Terry R, Peck A, DeTeresa R, Schechter R, Horoupian D (1981) Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol 10: 184-192.
- Mandelkow EM, Mandelkow E (1998) Tau in Alzheimer’s disease. Trends Cell Biol 8: 425-427.
- Serrano-Pozo A, Frosch MP, Masliah E, Bradley TH (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1: a006189.
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24: 173-182.
- Rogers J, Luber-Narod J, Styren S, CivinW (1988) Expression of immune system-associated antigens by cells of the human central nervous system: Relationship to the pathology of Alzheimer’s disease. Neurobiol Aging 9: 339-349.
- Pimplikar SW (2014) Neuroinflammation in Alzheimer's disease: From pathogenesis to a therapeutic target. J Clin Immunol 34: 64-69.
- Vinters HV (1987) Cerebral amyloid angiopathy. A critical review. Stroke 18: 311-324.
- Auld DS, Kornecook TJ, Bastianetto S, Quirion R (2002) Alzheimer's disease and the basal forebrain cholinergic system: Relations to beta-amyloid peptides, cognition and treatment strategies. Prog Neurobiol 68: 209-245.
- Gold CA, Budson AE (2008) Memory loss in Alzheimer's disease: Implications for development of therapeutics. Expert Rev Neurother 8: 1879-1891.
- Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta neuropathol 82: 239-259.
- Braak H, Braak E (1993) Entorhinal-hippocampal interaction in amnestic disorders. Hippocampus 3: 239-246.
- Harris JA, Devidze N, Verret L, et al (2010) Transsynaptic progression of amyloid-beta-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron 68: 428-441.
- Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA (2007) The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 78: 30-35.
- Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, et al. (2009) Olfactory impairment in presymptomatic Alzheimer's disease. Ann NY Acad Sci1170: 730-735.
- Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, et al. (2002) Natural history of mild cognitive impairment in older persons. Neurology 59: 198-205.
- Dubois B, Albert ML (2004) Amnestic MCI or prodromal Alzheimer’s disease? Lancet Neurol 3: 246-8.
- Dickson TC, King CE, McCormack GH, Vickers JC (1999) Neurochemical diversity of dystrophic neurites in the early and late stages of Alzheimer’s disease. Exp Neurol 1: 100-110.
- Kidd M (1963) Paired helical filaments in electron microscopy of Alzheimer’s disease. Nature 197: 192-193.
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, et al (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21: 3017-3023.
- Lu DC, Shaked GM, Masliah E, Bredesen DE, Koo EH (2003) Amyloid beta protein toxicity mediated by the formation of amyloid-beta protein precursor complexes. Ann Neurol 54: 781-789.
- Tian Y, Crump CJ, Li YM (2010) Dual role of alpha secretase cleavage in the regulation of gammasecretase activity for amyloid production. J Biol Chem 285: 32549-32556.
- Stromer T, Serpell LC (2005) Structure and morphology of the Alzheimer's amyloid fibril. Microsc Res Tech 67: 210-217.
- Eisenhauer PB, Johnson RJ, Wells JM, Davies TA, Fine RE (2000) Toxicity of various amyloid beta peptide species in cultured human blood-brain barrier endothelial cells: increased toxicity of dutch-type mutant. J Neurosci Res 60: 804-810.
- Johnson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, et al. (2002) The Alzheimer's Abeta-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci USA 99: 11830-11835.
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, et al. (1999) Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci USA 96: 3228-3233.
- Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298: 789-791.
- De Leon MJ, Desanti S, Zinkowski R, Mehta PD, Pratico D, et al. (2004) MRI and CSF studies in the early diagnosis of Alzheimer’s disease. J Int Med 256: 205-223.
- Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, et al. (2002) Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: Reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA 99: 13217-13221.
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, et al. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535-539.
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, et al. (2002) Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res 924: 133-140.
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R (2004) Block of long term potentiation by naturally secreted and synthetic amyloid betapeptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5 and p38 mitogen activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci 24: 3370-3378.
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE et al (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14: 837-842.
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE et al (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14: 837-842.
- Origlia N, Capsoni S, Cattaneo A, Fang F, Arancio O, et al. (2009) Aβ-dependent inhibition of LTP in different intra-cortical circuits of the visual cortex: The role of RAGE. J Alzheimers Dis 17: 59-68.
- Nabavi S., Fox R, Proulx CD, Lin JY, Tsien RY, et al. (2014) Engineering a memory with LTD and LTP. Nature 511: 348-352.
- Origlia N, Capsoni S, Cattaneo A, Fang F, Arancio O, et al. (2009) Aβ-dependent inhibition of LTP in different intra-cortical circuits of the visual cortex: The role of RAGE. J Alzheimers Dis 17: 59-68.
- Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, et al.(2008) Receptor for advanced glycation end-products (RAGE)-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid β-mediated cortical synaptic dysfunction. J Neurosci 28: 3521-3530.
- Knierim JJ, Lee I, Hargreaves EL (2006) Hippocampal place cells: Parallel input streams, sub regional processing and implications for episodic memory. Hippocampus 16, 755-764.
- Lisman JE (2007) Role of the dual entorhinal inputs to hippocampus: A hypothesis based on cue/action (non-self/self) couplets. Prog Brain Res 163, 615-625.
- Moser EI, Kropff E, Moser MB (2008) Place cells, grid cells and the brain's spatial representation system. Ann Rev Neurosci 31: 69-89.
- Stranahan AM, Mattson MP (2010) Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer's disease. Neural Plast 2010: 108190.
- Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7: 278-294.
- Origlia N, Bonadonna C, Rosellini A, Leznik E, Arancio O, et al. (2010) Microglial RAGE-dependent signal pathway drives Aβ-induced synaptic depression and long-term depression impairment in entorhinal cortex. J Neurosci 30: 11414-11425.
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, et al. (2005) Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8: 1051-1058.
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, et al. (2006) AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron 52: 831-843.
- Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, et al. (2009) Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci 12: 1567-1576.
- Lista S, Hampel H (2017) Synaptic degeneration and neurogranin in the pathophysiology of Alzheimer's disease. Expert Rev Neurother 17: 47-57.
- Yan SD, Chen X, Fu J, Chen M, Zhu H, et al. (1996) RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 382: 685-691.
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, et al (2004) RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J 23: 4096-4105.
- Origlia N, Arancio O, Domenici L, Yan SS (2009) MAPK, beta-amyloid and synaptic dysfunction: The role of RAGE. Expert Rev Neurother 9: 1635-1645.
- Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E (2010) Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer's disease. Acta Neuropathol 119: 523-541.
- Ripoli C, Cocco S, Li Puma DD, Piacentini R, Mastrodonato A, et al. (2014) Intracellular accumulation of amyloid-β (Aβ) protein plays a major role in Aβ-induced alterations of glutamatergic synaptic transmission and plasticity. J Neurosci 34: 12893-12903.
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S et al. (2003) RAGE mediates amyloid-beta peptide transport across the blood brain barrier and accumulation in brain. Nat Med 9: 907-913.
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, et al. (2009) RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci USA 106: 20021-20026.
- Du H, Guo L, Fang F, Chen D, Sosunov AA, et al. (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 14: 1097-1105.
- Yao J, Du H, Yan S, Fang F, Wang C, et al. (2011) Inhibition of amyloid-beta (Abeta) peptide-binding alcohol dehydrogenase-Abeta interaction reduces Abeta accumulation and improves mitochondrial function in a mouse model of Alzheimer's disease. J Neurosci 31: 2313-2320.
- López-Toledano MA, Shelanski ML (2004) Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci 24: 5439-5444.
- Puzzo D, Privitera L, Leznik E, Fà M, Staniszewski A, et al. (2008) Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci 28: 14537-14545.
- Puzzo D, Privitera L, Fa' M, Staniszewski A, Hashimoto G, et al. (2011) Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol 69: 819-830.
- Brettschneider J, Del Tredici K, Lee VM-Y, Trojanowski JQ (2015) Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nature Rev Neurosci 16: 109-120.
- Gotz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293: 1491-1495.
- VM-Y Lee, M Goedert, JQ Trojanowski, (2001). Neurodegenerative tauopathies. Annu Rev Neurosci 24: 1121-1159.
- Cleveland DW, Hwo SY, Kirschner MW (1977) Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol 116: 207-225.
- Caceres A, Kosik KS (1990) Inhibition of neurite polarity by tau antisense oligonucleotides in primary cerebellar neurons. Nature 343: 461-463.
- Iqbal K, Liu F, Gong CX, Alonso AD, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118: 53-69.
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, et al. (2007) Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci 27: 3650-3662.
- Sydow A, Van der Jeugd A, Zheng F, Ahmed T, Balschun D, et al. (2011) Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J Neurosci 31: 2511-2525.
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ (2014) Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet 5: 88.
- Kosik KS, Joachim CL, Selkoe DJ (1986) Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl Acad Sci USA 83: 4044-4048.
- Iqbal K, Wisniewski HM, Grundke-Iqbal I, Korthals JK, Terry RD (1975) Chemical pathology of neurofibrils. Neurofibrillary tangles of Alzheimer's presenile-senile dementia. J Histochem Cytochem 23: 563-569.
- Iqbal K, Zaidi T, Thompson CH, Merz PA, Wisniewski HM (1984) Alzheimer paired helical filaments: bulk isolation, solubility and protein composition. Acta Neuropathol 62:167-177.
- Correa SA, Eales, KL (2012) The role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative disease. J Signal Transduct 64907.
- Gomez-Ramos A, Dıaz-Hernandez M, Cuadros R, Hernandez F, Avila J (2006) Extracellular tau is toxic to neuronal cells. FEBS Lett 580: 4842-4850.
- Gomez-Ramos A, Diaz-Hernandez M, Rubio A, Miras-Portugal MT, Avila J (2008) Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci 37: 673-681.
- Kim W, Lee S, Jung C, Ahmed A, Lee G, et al. (2010) Interneuronal transfer of human tau between Lamprey central neurons in situ. J Alzheimer’s Dis 19: 647-664.
- Saman S, Kim W, Raya M, Visnick Y, Miro S, et al. (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287: 3842-3849.
- Sahara N, Maeda S, Takashima A (2008) Tau oligomerization: A role for tau aggregation intermediates linked to neurodegeneration. Curr Alzheimer Res 5: 591-598.
- Frost B, Jacks RL, Diamond MI (2009) Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem 284: 12845-52.
- Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Clos AL, Jackson GR, et al. (2011) Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol Neurodegener 6: 39.
- Di J, Cohen LS, Corbo CP, Phillips GR, El Idrissi A, Alonso AD (2016) Abnormal tau induces cognitive impairment through two different mechanisms: Synaptic dysfunction and neuronal loss. Sci Rep 6:20833.
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, et al. (1991) Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 30: 572-580.
- Coleman PD, Yao PJ (2003) Synaptic slaughter in Alzheimer's disease. Neurobiol Aging 24: 1023-1027.
- Zhou L, McInnes J, Wierda K, Holt M, Herrmann AG, et al. (2017) Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun 8: 15295.
- Fá M, Puzzo D, Piacentini R, Staniszewski A, Zhang H, et al. (2016) Extracellular tau oligomers produce an immediate impairment of LTP and memory. Sci Rep 6: 19393.
- Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408-414.
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, et al. (1982). Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 215: 1237-1239.
- Levi-Montalcini R (1952) Effects of mouse tumor transplantation on the nervous system. Ann N Y Acad Sci 55: 330-344.
- Mufson EJ, Kroin JS, Sendera TJ, Sobreviela T (1999) Distribution and retrograde transport of trophic factors in the central nervous system: Functional implications for the treatment of neurodegenerative diseases. Prog Neurobiol 57: 451-484.
- Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer's disease. Aging Dis 6: 331-341.
- Criscuolo C, Fabiani C, Cerri E, Domenici L (2017) Synaptic dysfunction in Alzheimer's disease and glaucoma: From common degenerative mechanisms toward neuroprotection. Front Cell Neurosci 11: 53.
- Ernfors P, Bramham CR (2003) The coupling of a trkB tyrosine residue to LTP. Trends Neurosci 26: 171-173.
- Bramham CR, Panja Dm (2014) BDNF regulation of synaptic structure, function and plasticity. Neuropharmacology 76: 601-602.
- Connor B, Young D, Yan Q, Faull RL, Synek B, et al. (1997) Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res 49: 71-81.
- Michalski B, Fahnestock M (2003) Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer's disease. Brain Res Mol Brain Res 111: 148-154.
- Peng S, Wuu J, Mufson EJ, Fahnestock M (2005) Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem 93: 1412-1421.
- Criscuolo C, Fabiani C Bonadonna C, Origlia N, Domenici L (2014) BDNF prevents amyloid-dependent impairment of LTP in the entorhinal cortex by attenuating p38 MAPK phosphorylation. Neurobiol Aging 36: 1303-1309.
- Capsoni S, Malerba F, Carucci NM, Rizzi C, Criscuolo C, et al. (2017) The chemokine CXCL12 mediates the anti-amyloidogenic action of painless human nerve growth factor. Brain 140: 201-217.
- Larner AJ (2010) Epileptic seizures in AD patients. Neuromolecular Med 12: 71-77.
- Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, et al. (2012) Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 109: 8740-8745.
- Fogel H, Frere S, Segev O (2014) APP homodimers transduce an amyloid-beta-mediated increase in release probability at excitatory synapses. Cell Rep 7: 1560-1576.
- Bakker A, Krauss G, Albert MS, Speck C, Jones LR, et al. (2012) Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74: 467-474.
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101: 4637-4642.
- Criscuolo C, Fontebasso V, Middei S, Stazi M, Ammassari-Teule M, et al. (2017) Entorhinal cortex dysfunction can be rescued by inhibition of microglial RAGE in an Alzheimer’s disease mouse model. Sci Rep 7: 42370.
- Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, et al. (2016) Neuronal activity enhances tau propagation and tau pathology in vivo. Nat Neurosci 19: 1085-1092.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 2978
- [From(publication date):
August-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 2178
- PDF downloads : 800