Symptom Prevalence of Neurodegenerative Diseases among Minorities
Received: 02-Nov-2017 / Accepted Date: 02-Nov-2017 / Published Date: 09-Nov-2017 DOI: 10.4172/2161-0460.1000397
Abstract
Background: The annual number of neurodegenerative diseases among minorities is projected to increase by 524% between 1990 and 2040 in the US and there have been no studies looking at the incidence and prevalence of signs/symptoms among different racial and ethnic minority patients with Alzheimer's dementia (AD), Parkinson's disease (PD) and Motor neuron disease (MND).
Methods: Retrospective review of all minority subgroups who presented to Mayo Clinic, Rochester (MN), with a diagnosis of AD, PD or MND between January 1st, 2000 and December 31st, 2015. We divided our study population into seven groups: Black, Asian, South Asian, Middle Eastern, Hispanic/Latino, American Indian/Alaskan Native and Native Hawaiian/Pacific Islander.
Results: From a total of 8927 patients diagnosed with a neurodegenerative disease at our institution over the 15 year time frame, 472 were minority [PD=220 (46.6%); AD=90 (19.1%) and MND=162 (34.3%)]. The most common races/ethnicity were Black or African American in 135 (28.6%), Asian in 101 (21.4%), South Asian in 69 (14.6%), Middle eastern in 60 (12.7%), Hispanic/Latino in 59 (12.5%) and American Indian/Alaskan Native/Native Hawaiian/ Pacific Islander in 48 (10.2%). For PD, there were differences in the frequency of micrographia, anosmia, levodopa induced dyskinesia, falls and dystonia, while for AD there were differences in executive dysfunction and visual spatial changes and for MND difference were present for muscle atrophy, limb fasciculation, inability to ambulate, tongue fasciculation, choking episodes and dysphagia.
Conclusion: Neurodegenerative diseases afflict all minority races and ethnicities, including some not previously reported and the frequency of presenting signs and symptoms significantly vary across different minority/ethnic groups.
Keywords: Alzheimer’s; Parkinson; ALS; Epidemiology; Minority aging; Cognitive aging
Introduction
The major neurodegenerative diseases, i.e., “the big three”, Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS), account for considerable morbidity and mortality in the United States elderly population. The elderly has been forecast to be the fastest growing segment of the population, and among them the fastest growing will be minority (non-white) groups. Between 1992 and 2040, the Census bureau projects an increase from 42.3 to 96.6 million minorities (126% increase), while the minority elderly population will increase from 3.3 to 14.1 million (327% increase) [1]. The annual number of neurodegenerative diseases deaths among minorities is projected to increase by 524% between 1990 to 2040, with the largest increase in death from PD and like diseases with a smaller projected increase from ALS [2]. Since data collection for this study, done in 1992, there have been only a few studies looking at the incidence and prevalence of major neurodegenerative diseases among minority groups both within and outside of the USA [3-9]. These studies focused on the prevalence and characteristics of the study population for these diseases. There have been, however, no studies that have assessed symptoms within minority or ethnic (minority/ethnic) groups, for specific neurodegenerative diseases or determined whether symptoms differ across various minority/ethnic groups. Furthermore, there is no data published on specific minority groups, for example, patients from the Middle East.
In this study we assessed typical and atypical (meaning less typical) presenting signs and symptoms among different minority/ethnic groups, and also compare signs/symptoms across the different racial/ ethnic groups within each of the three major neurodegenerative diseases (AD, PD and ALS). We hypothesize that the frequency of typical and atypical signs/symptoms associated with all three neurodegenerative diseases will differ across the different minority/ethnic groups.
Methods
Study design
We conducted a retrospective review of all the patients who presented to Mayo Clinic, Rochester (MN), with a diagnosis of any neurodegenerative disease between 1/1/2000 and 12/31/2015. Patients were identified using our electronic data search system and confirmed by manual review of the medical records. Demographic data for all patients was ascertained. In order to be included in this study, the patient had to be a minority, defined as any category of race or ethnicity that is differentiated from the social majority. The study was approved by the Mayo Clinic institutional review board. All included patients had signed a general informed consent form allowing their medical records to be used for research purposes.
Study definitions
For this study, we focused on the three major neurodegenerative diseases, i.e., AD, PD and ALS. An Alzheimer’s disease diagnoses for this study was based on criteria recommended by the National Institute of Aging and the Alzheimer’s Association work group [10]. A Parkinson’s disease diagnosis was based on the most recently modified Queen Square Brain Bank Criteria [11,12]. The revised El Escorial criteria were used for ALS [13].
Categorization of patients into different races
The classification system that we utilized for the different races and ethnicity for this study was motivated by the United Kingdom’s classification of races/ethnicity [14], with some modifications made based on the races/ethnicity of other racial groups in the USA, as well as other racial groups that have not been previously studied, but were identified in our cohort. As a result, we divided our study population into seven groups: Black (African American, African, Afro-Caribbean), Asian (Chinese, Japanese, all or other Asian racial/ethnic groups), South Asian (India, Pakistan, Bangladesh, Sri Lanka, any other South Asian background), Hispanic/Latino, American Indian/Alaskan Native, Native Hawaiian/Pacific Islander and Middle eastern. We included a Middle Eastern race as a separate category in our cohort because of the large number of patients that were evaluated at our institution from the Middle East, as well as to explore this specific subset of patient population given that there is no published data in the western literature on this race, to the best of our knowledge. We combined American Indian/Alaskan Native, and Native Hawaiian/ Pacific Islander, due to the small number of patients representing these races. We also combined patients of similar races born in different countries (e.g. we combined Asian patients who were born in USA with Asian patients born in a country from Asia).
Inclusion/exclusion criteria
Patients were only included in our study if they satisfied the following criteria: 1) Confirmed clinical diagnosis of AD, PD or ALS after being seen by a neurologist at Mayo Clinic, during every visit based on the definitions above. If the diagnosis of the patients changed during follow-up (based on the development of new signs/symptoms or new radiographic features), then the final diagnosis of the patients was the one during their last follow up in order to get the most accurate diagnosis; 2) were more than 18 years of age; 3) did not have dementia secondary to other causes and 4) had a definitive diagnosis up to the last follow-up. Patients were excluded if they didn’t have a definitive diagnosis at their last follow up.
Collected variables
The electronic medical records of all patients who satisfied the above criteria were reviewed and data abstracted at the time of their first presentation to our institution including patient demographics (age, sex, race/ethnicity), family history of disease, and disease duration up to the time of presentation. Specific data collected for those with a diagnosis of PD included levodopa responsiveness, as well as signs/ symptoms considered typical and atypical. For AD, we abstracted data on the Kokmen Short Test of Mental Status score at presentation, a measure of global cognition [15] as well as typical and atypical signs/ symptoms. For ALS, we included data on spinal cord and bulbar/other related signs/symptoms.
A sign/symptom was considered present if it was documented in the medical records as present, and considered absent if it was documented as absent in the medial records. If a sign/symptom was not documented it was not considered present or absent.
Statistical Analysis
Descriptive summaries were reported as median and interquartile range for continuous variables and frequencies and percentages for categorical variables. Associations between categorical variable were assessed using χ2 test or Fisher’s exact test as applicable. Wilcoxon/ Kruskal–Wallis test were used to compare continuous variables as the data was not normally distributed. All tests were two sided and p-values less than 0.05 were considered statistically significant. Statistical analysis was performed using JMP 11.0.0 (SAS Institute Inc., Cary, NC).
Results
We identified 8,927 patients who had a diagnosis of a neurodegenerative disease at least once during their visit over the specified time period. After excluding patients who were not minorities, were classified as unknown/chose not to disclose race/ethnicity, had an uncertain diagnosis, or an alternate diagnosis at last follow up, our final cohort consisted of 627 patients. Of these 627, 472 patients were diagnosed with one of the three major neurodegenerative diseases [PD in 220 (46.6%), AD in 90 (19.1%) and ALS in 162 (34.3%) patients]. The most common minority races that presented to our clinic were Black or African American in 135 (28.6%), Asian in 101 (21.4%), South Asian in 69 (14.6%), Middle eastern in 60 (12.7%), Hispanic/Latino in 59 (12.5%) and American Indian/Alaskan Native/Native Hawaiian/Pacific Islander in 48 (10.2%). The remaining 155 patients were diagnosed with a less common neurodegenerative disease.
Parkinson’s disease
The most common of the three neurodegenerative disease that present in our cohort was PD. The median age of the patients at presentation was 64.8 (IQR 58.2-73) years. The South Asian group was the youngest with a median age of 60.1 years (IQR 53.7-68.4). The median duration of the symptoms before presentation was 3 years (IQR 1.5-6) with no difference in the symptom duration before presentation across the different races and ethnicity. A positive family history of PD was most common in the South Asian group in 11 (25.6%) and least common in the Asian group (5.2%). Detailed demographics are presented in Table 1.
| Black or African American (n=135) |
Asian (n=101) |
South Asian (n=69) |
Middle east (n=60) |
Hispanic or Latino (n=59) |
American Indian/Alaskan Native/Native Hawaiian/ Pacific Islander (n=48) |
Total patients (n=472) |
|
|---|---|---|---|---|---|---|---|
| All Dementias | 55 (37.4) | 30 (20.4) | 17 (11.6) | 17 (11.6) | 20 (13.6) | 8 (5.4) | 147 |
| Alzheimer dementia | 35 (38.8) | 20 (22.2) | 9 (10.0) | 7 (7.8) | 15 (16.7) | 4 (4.4) | 90 |
| Age | 72.7 (64.7-77.3) | 73.9 (61.6-81.1) | 70.0 (59.2-77.1) | 71.1 (59.2-79.6) | 75.2 (64.5-77.7) | 62.6 (52.4-80.1) | 72 (63-78) |
| Males | 14 (40.0) | 10 (50.0) | 3 (33.3) | 5 (71.4) | 7 (46.7) | 2 (50.0) | 41 (45.6) |
| Family History* | 14 (40.0) | 4 (20.0) | 2 (22.2) | 0 (0.0) | 3 (20.0) | 3 (75.0) | 26 (28.9) |
| Duration of symptoms | 2.3 (2-4) | 2.5 (1-4.8) | 3 (1.3-6) | 2 (2-6) | 3 (1.5-4) | 2.5 (1.3-3) | 3 (1.5-4) |
| Kokeman STMS | 20 (16-25) | 19.5 (15.3-27.5) | 22 (14-26) | 10 (5.5-16) | 19 (10-26) | 11 (2-25.3) | 19 (14-25.5) |
| All Parkinsonism | 59 (21.7) | 86 (27.0) | 59 (18.6) | 39 (12.3) | 32 (10.0) | 33 (10.4) | 318 |
| Parkinson’s disease | 35 (15.9) | 58 (26.4) | 43 (19.5) | 35 (15.9) | 22 (10.0) | 27 (12.3) | 220 |
| Age* | 65.6 (57.8-72.9) | 65.0 (58.6-73.9) | 60.1 (53.7-68.4) | 67.3 (60.3-71.1) | 66.8 (62.5-73.5) | 70.2 (61.1-76.3) | 64.8 (58.2-73) |
| Males | 21 (60.0) | 29 (50.0) | 31 (72.1) | 23 (65.7) | 11 (50.0) | 13 (48.2) | 128 (58.2) |
| Family History* | 5 (14.7) | 3 (5.2) | 11 (25.6) | 7 (20.6) | 3 (13.6) | 5 (18.5) | 34 (15.6) |
| Duration of Symptoms |
3 (1-5) | 3 (1.5-5) | 3 (1.5-5) | 3 (1.5-7) | 2.75 (1.3-6.3) | 5 (2-8) | 3 (1.5-6) |
| Levodopa responsive | 25 (92.6) | 45 (97.8) | 36 (97.3) | 31 (100.0) | 19 (100.0) | 23 (100.0) | 179 (97.8) |
| All Amyotrophic Lateral Sclerosis | 65 (40.1) | 23 (14.2) | 17 (10.5) | 18 (11.1) | 22 (13.6) | 17 (10.5) | 162 |
| Age | 53.1 (46.3-62.5) | 56.2 (48.1-63.5) | 56.6 (46.9-63.6) | 55.7 (37.2-65.3) | 61.5 (50.8-67.8) | 58.1 (53.6-68.7) | 56.7 (47.9-64) |
| Males* | 34 (52.3) | 10 (43.5) | 11 (64.7) | 12 (66.7) | 17 (77.3) | 3 (17.7) | 87 (53.7) |
| Family History | 1 (1.5) | 1 (4.4) | 0 (0.0) | 1 (5.6) | 0 (0.0) | 0 (0.0) | 3 (1.9) |
| Duration of symptoms |
1.5 (1-3) | 1.5 (0.9-3) | 1.5 (1-2.2) | 1.5 (0.8-3) | 1 (0.9-2) | 1 (0.7-3.3) | 1.5 (1-2.6) |
| EMG fasciculation | 60 (96.8) | 21 (95.5) | 15 (100.0) | 16 (94.1) | 21 (100.0) | 16 (94.1) | 149 (96.8) |
Percentages in the brackets indicate the percentage of patients when compared to the total number of patients and does not indicate the proportion among the races. All symptoms with asterisk (*) have a statistically significant p-value
Table 1: Demographics of the study population with different races.
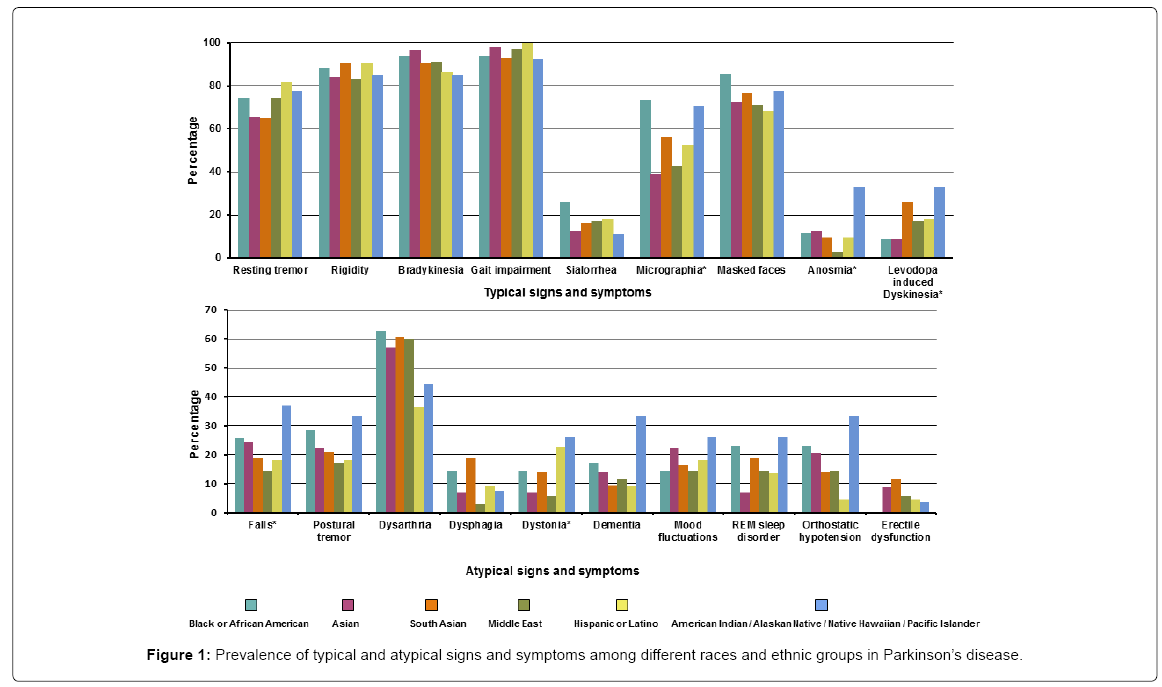
Of the PD patients, more than 90% presented with typical signs including gait impairment 211 (95.9%) and bradykinesia 202 (91.8%). Resting tremor occurred in 157 (71.4%). The symptoms that differed across the various races/ethnicity for PD were micrographia (most prevalent in the Black/African group while least in the Asian and Middle eastern groups), anosmia (most common in American Indians/ Native Hawaiian group while least prevalent in the Middle eastern group), levodopa induced dyskinesia (most prevalent in the American Indian/Native Hawaiian group and least in Black/African American and Asian groups), falls (most common in American Indian/Native Hawaiian group while least in the Asian and Hispanic groups) and dystonia (more common in the American Indian/Native Hawaiian and Hispanic group while least in Middle eastern group). Detailed signs/ symptoms are presented in Supplementary Table 1 and Figure 1.
Alzheimer’s disease
The median age of the AD patients was 72 years (IQR 63-78) with American Indians/Native Hawaiian presenting with the youngest age 62.6 years (IQR 52.4-80.1). The median duration of the symptoms before presentation was 3 years (IQR 1.5-4) with no difference in symptom duration before presentation among the different races/ ethnicities. The family history of AD was most commonly present in 3 (75%) of the American Indian/Native Hawaiian group while none of the patients from the Middle eastern group had a history of AD. Detailed demographics are presented in Table 1.
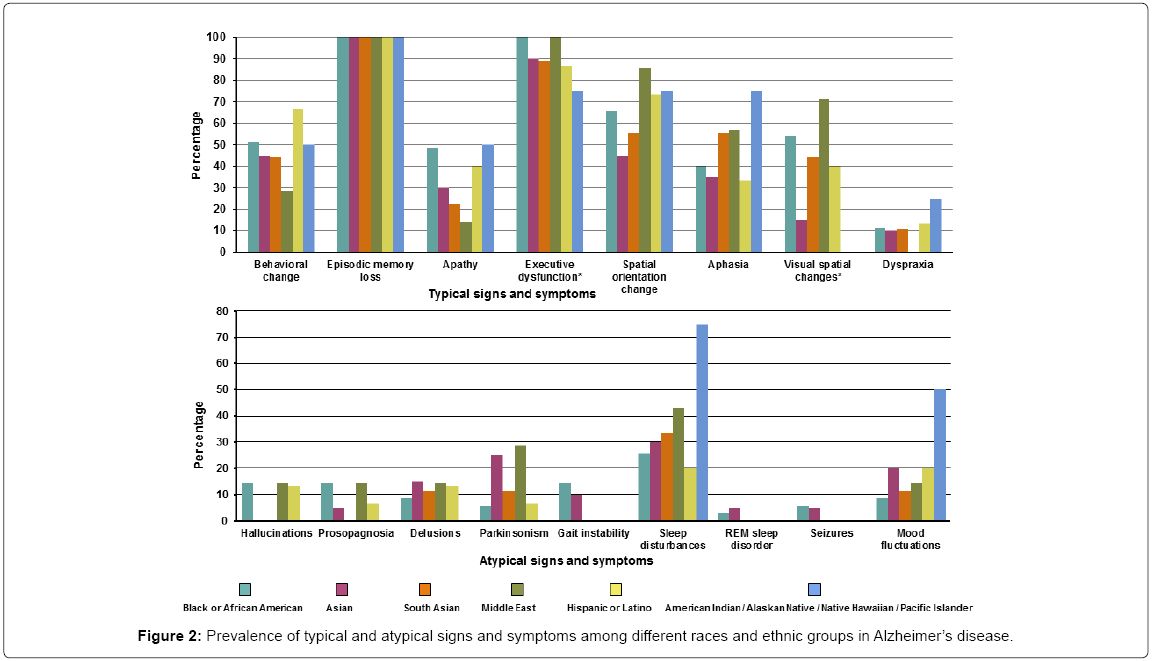
Of the patients diagnosed with AD 100% presented with episodic memory loss. Other typical and atypical symptoms were much less frequent. The typical symptoms that differed across races/ethnicity were executive dysfunction (present in all the Black/African American and Middle Eastern groups, while least in the American Indian/ Native Hawaiian groups) and visual spatial changes (most common in Middle Eastern group while least in the Asian group). None of the atypical signs and symptoms differed among races/ethnicity although the Black/African American and Middle Eastern groups accounted for the majority. Detailed signs/symptoms are presented in Supplementary Table 2 and Figure 2.
Amyotrophic lateral sclerosis
The median age of the patients was 56.7 years (IQR: 47.9-64) and the Black/African American group was the youngest at presentation, 53.1 years (IQR 46.3-62.5). The majority of patients were males, except for the Asian and American Indian/Native Hawaiian groups in which MND was present in 10 (43.5%) and 3 (17.7%) of males. The median duration of symptoms before they present to the hospital was 1.5 years (IQR 1-2.6) and again did not differ by race/ethnicity. A family history of ALS was very rare. All patients had widespread chronic active neurogenetic changes consistent with ALS on their EMGs. Detailed demographics are presented in Table 1.
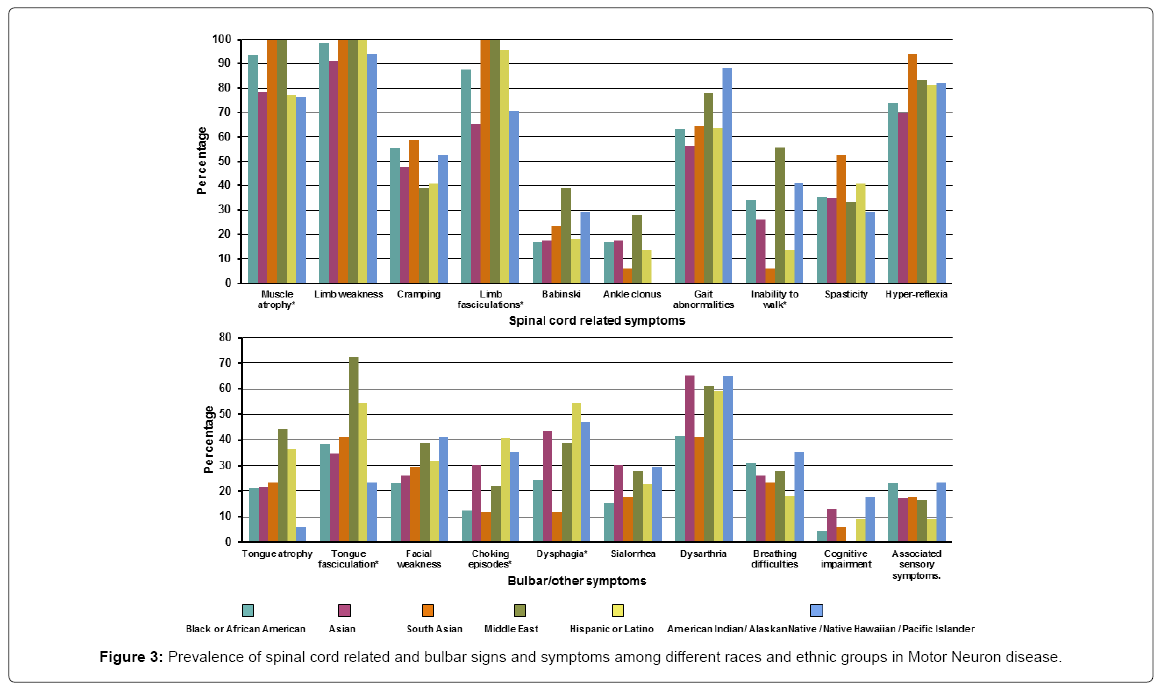
Of the ALS patients, the most common spinal cord related symptom was limb weakness occurring in almost all patients 158 (97.5%). Bulbar signs/symptoms were much less common the most frequent being dysarthria 84 (51.9%). The spinal cord related symptoms that differed across the different races/ethnicity were muscle atrophy (present in all the South Asian and Middle eastern patients while least common in the Asian, Hispanic and American Indian/Native Hawaiian groups), limb fasciculation (present in all the South Asian and Middle east patients and least common in the Asian group) and inability to walk (most common in from the Middle Eastern group while least in the South Asian group). Bulbar symptoms that varied across different races/ethnicity were tongue fasciculation (most common in the Middle Eastern group and least in the American Indian/Native Hawaiian group), choking episodes (most common in the Hispanic group while least in the South Asian group) and dysphagia (most common in Hispanics while South Asians were least affected). Detailed signs/symptoms are presented in Supplementary Table 3 and Figure 3.
Discussion
This study highlights the prevalence of, and differences between presenting signs/symptoms among minorities diagnosed with one of the three major neurodegenerative diseases (AD, PD and ALS). We show that patients from different races and ethnicities with comparable disease duration from onset have varied symptom presentations. The findings are significant as they provide relevant information to a large proportion of the USA, and worlds populations, for which such information is not known.
It has been currently estimated by the World Health Organization (WHO) that by 2025, about 75% of the estimated 1.2 billion people aged 60 years and older will reside in developing countries [16,17]. The rate of growth will be highest (around 336%) in India, China, South Asia and the Western Pacific regions, 235-393% in Latin America and Africa, and the lowest (100%) in developed regions [18]. In addition, with an increase in foreign born individuals immigrating to the USA each year (India, China, Mexico, Canada and the Philippines being the leading countries of origin for the new immigrants), it becomes extremely important to understand the prevalence of disease symptoms among the different racial/ethnic groups [19]. It is also important for practicing physicians in the USA to be familiar with the potential disparities of symptoms that minority patients may present with, as some symptoms may not be truly atypical given race or ethnicity. Furthermore, a better understanding of symptoms association with minorities improves diagnosis and prognosis.
The classification system that we utilized to segregate the different races and ethnicity in our study may not be identical to others, but we were particularly interested in exploring prevalences and differences among different races, as much as we could. Hence, we adopted the UK system of classification with some modification from the USA system of races/classification in order to have a more worldwide assessment by race as oppose to limiting race to those typically studied in the USA [14]. This was also necessary given that our institution evaluates a large international population. On-the-other hand, we had to combine some races to get some power as some of our racial categories were relatively small.
The most common neurodegenerative disorder present among the minorities in our study was PD followed by AD and ALS. This was in contrast to the overall disease incidence and prevalence of these three diseases among the US population where AD is the most common, followed by PD and then ALS [20]. The reason for this difference in ordering is not clear. With regards to disease durations, for all three neurodegenerative diseases, patients were evaluated at our institution early into the course of their illness without significant differences across the different races/ethnicity. The absence of any difference in duration makes it unlikely that our results are cofounded by disease duration, i.e., longer durations would be expected to be associated with higher prevalences.
For this study, we also focus on the Middle Eastern population as a separate subgroup, given that this group has never been previously studied. In addition, the percentage of elderly from the Middle East will double in the next ten years [21] and hence we are likely to more frequently encounter neurodegenerative diseases in this minority group, in the USA. Lastly, there is no substantial research being done in the Middle East on neurodegenerative diseases. In our cohort, the most common neurodegenerative disease encountered among patients from the Middle East was PD, followed by ALS and then AD. Middle Eastern patients with PD had a higher prevalence of atypical symptoms including dysarthria; those with ALS had a higher prevalence of limb fasciculation, tongue atrophy, tongue fasciculation, facial weakness and dysarthria, while those with AD had a higher prevalence of spatial disorientation, compared to other races/ethnic groups. The reason for these differences is not entirely clear and could be due to what is cultural acceptable to report to the physician. It is also possible that unrecognized genetic factors are playing a role. Regardless of the reason however, it behoves us to reconsider what is atypical and what is typical since a symptom that is atypical in one race may be typical for another.
Another unique group was the South Asian group. Patients within this group are typically merged with other patients from Asia in the US race classification. We opted to separate this group given that they are the second largest immigrant group in the USA after Mexicans, and account for 4.7% of the foreign born immigrants to the USA [19]. In addition to those who immigrated to the USA, a considerable number of South-Asians also consist of 2nd and 3rd generation children, who were born and raised in the USA. This further stresses the importance to examine this population as a separate group and not in combination with other populations from Asia. Similar to the Middle Easter group, patients from South-Asia were more likely to present with features considered atypical for the disease. It should be pointed out that typical and atypical are solely based on studies in non-minority populations.
The other four races and ethnicity, Blacks/African Americans, Asians, American Indian/Alaskan Native/Native Hawaiian/Pacific Islander, and Hispanics/Latinos are more commonly reported in studies from the western world, although information regarding neurodegenerative diseases is still lacking. In our study we noted that overall the most common group was Black/African American which was also true for two of the three diseases, AD and ALS. However, we noted that for PD, Black/African American was the third most common group. The differences in Black/African Americans across the three diseases argue against the notion that racial frequencies in our study simply mirrored the racial frequencies of the population evaluated at our institution. Hispanics were also well represented in our cohort, across the three diseases, but did not show any sign/symptom that significantly differed compared to races. This could be due to the fact that Hispanic is not a race but an ethnicity consisting of different races. Our Hispanic population is predominantly Caucasian.
Our study also has several limitations including its retrospective design and the fact that our institution is a major referral centre and might not be representative of the experiences at other USA centres; it might be, however, more typical of institutions in the UK. The majority of the patients that are evaluated at our institution are Caucasians (94%) and hence the prevalence of the three major diseases may not be representative of the USA population. We also ended up combining American Indian/Alaskan Native/Native Hawaiian/Pacific Islander races because of the smaller study samples, which hampered us from exploring the symptoms of those individual races. Regardless, we were still able to categorize patients, perhaps even more accurately, as we utilized information up to the time of the last visit. Despite these limitations, this is the first study that has explored the variability of different signs and symptoms in AD, PD and ALS, among major racial and ethnic groups in the USA. It is also the first study that has studied patients from the Middle East and South Asia.
Conclusion
Neurodegenerative diseases afflict all minority races and ethnicity, including races not previously studied. The frequency of presenting signs and symptoms vary across different minority/ethnic groups which challenges the current notion of what should be considered typical and atypical.
References
- Day J (1996) Population projections of the united states by age, sex, race and hispanic origin: 1995 to 2050. Curr Popul Rep, pp: 25-1130.
- Lilienfeld DE, Perl DP (1994) Projected neurodegenerative disease mortality among minorities in the United States, 1990-2040. Neuroepidemiology 13: 179-186.
- Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, et al. (2003) Incidence of Parkinson's disease: Variation by age, gender and race/ethnicity. Am J Epidemiol 157: 1015-1022.
- Llibre Rodriguez JJ, Ferri CP, Acosta D, Guerra M, Huang Y, et al. (2008) Prevalence of dementia in Latin America, India and China: A population-based cross-sectional survey. Lancet 372: 464-474.
- Willis WA, Evanoff BA, Lian M, Criswell SR, Racette BA (2010) Geographic and ethnic variation in Parkinson disease: A population-based study of US Medicare beneficiaries. Neuroepidemiology 34: 143-151.
- Das SK, Pal S, Ghosal MK (2012) Dementia: Indian scenario. Neurol India 60: 618-624.
- Barnes LL, Bennett DA (2014) Alzheimer's disease in African Americans: Risk factors and challenges for the future. Health Aff (Millwood) 33: 580-586.
- Rechtman L, Jordan H, Wagner L, Horton DK, Kaye W (2015) Racial and ethnic differences among amyotrophic lateral sclerosis cases in the United States. Amyotroph Lateral Scler Frontotemporal Degener 16: 65-71.
- Tuerk R, Sauer J (2015) Dementia in a black and minority ethnic population: Characteristics of presentation to an inner London memory service. BJ Psych Bull 39: 162-166.
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 263-269.
- Berardelli A, Wenning GK, Antonini A, Berg D, Bloem BR, et al. (2013) EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson's disease. Eur J Neurol 20: 16-34.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181-184.
- Ludolph A, Drory V, Hardiman O, Nakano I, Ravits J, et al. (2015) A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degener 16: 291-292.
- Majesty’s Stationery Office (2003) Ethnic group statistics: A guide for the collection and classification of ethnicity data.
- Kokmen E, Naessens JM, Offord KP (1987) A short test of mental status: Description and preliminary results. Mayo Clin Proc 62: 281-288.
- Kalache A, Gatti A (2003) Active ageing: A policy framework. Adv Gerontol 11: 7-18.
- World Health Organization (2002) Active ageing: A policy framework. Aging Male 5: 1-37.
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, et al. (2005) Global prevalence of dementia: A Delphi consensus study. Lancet 366: 2112-2117.
- Statistics OoI (2016) Immigration Statistics. In: Statistics OoI, ed.: U.S. Department of Homeland Security.
- Borlongan CV, Burns J, Tajiri N, Stahl CE, Weinbren NL, et al. (2013) Epidemiological survey-based formulae to approximate incidence and prevalence of neurological disorders in the United States: A meta-analysis. PLoS ONE 8: e78490.
- Abyad A (2014) Alzheimer’s in the Middle East. JSM Alzheimer’s Dis Related Dementia 2: 1012.
Citation: Singh TD, Josephs KA (2017) Symptom Prevalence of Neurodegenerative Diseases among Minorities. J Alzheimers Dis Parkinsonism 7: 397. DOI: 10.4172/2161-0460.1000397
Copyright: © 2017 Singh TD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6709
- [From(publication date): 0-2017 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 5814
- PDF downloads: 895