Research Article Open Access
Switching to Telbivudine for Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients with Poor Response to Interferon-Alpha Therapy: A Two-Center Retrospective Study
Feng Qian1, Zhi-lin Niu2, Ming Li1, Hai-yan Wang1, Li Zhu1, Xue-hua Zhang1, Xiu-juan Shen1, Xiang Zhu1, Sheng-li Gao2 and Chuan-wu Zhu1*
1Department of Hepatology, The Affiliated Infectious Disease Hospital of Soochow University, Suzhou, China
2Department of Infectious Diseases, The Affiliated Wujiang Hospital of Nantong University, Suzhou, China
- Corresponding Author:
- Chuan-wu Zhu
Department of Hepatology
The Affiliated Infectious Disease Hospital of Soochow University
Suzhou, 215007, China
Tel: +86-512-65180193
Fax: +86-512-65291020
E-mail: zhuchw@126.com
Received Date: November 06, 2014; Accepted Date: January 19, 2015; Published Date: January 28, 2015
Citation: Qian F, Niu ZL, Li M, Wang HY, Zhu L, et al. (2015) Switching to Telbivudine for Hepatitis B e Antigen-Positive Chronic Hepatitis B Patients with Poor Response to Interferon-Alpha Therapy: A Two-Center Retrospective Study. J Gastrointest Dig Syst 5:254. doi:10.4172/2161-069X.1000254
Copyright: © 2015 Qian F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background/Purpose: The long-term efficacy of Telbivudine (LdT) between patients with poor response to interferon-alpha (IFN-α) therapy and those without antiviral history is unknown. Herein, we investigated and compared the outcome of LdT therapy in these patients.
Methods: Two groups of Chinese patients who received LdT treatment were enrolled, one with poor response to IFN-α and then switching to LdT (IFN-LdT group), and the other one without antiviral history (LdT group). The data of biochemical, serological and virological responses within 36 months of treatment were collected.
Results: The cumulative probability of ALT normalization, HBV DNA undetectability, HBeAg or HBsAg loss, and LdT-resistant mutation at 36 months was not found to be of significant difference between the two groups, respectively. However, the cumulative probability of HBeAg seroconversion at 36 months was 61.55% in IFN-LdT group, which was significantly higher than 40.68% in LdT group (p=0.047). There was significant difference in the cumulative probability of serum HBeAg<100 S/CO (p=0.010) between the two groups. IFN-α treatment (p=0.042) and serum HBeAg<300 S/CO (p=0.031) were identified as independent factors influencing serum HBeAg<100 S/CO.
Conclusions: This study revealed that LdT therapy produced a preferable efficacy in IFN-refractory patients than in antiviral-naive patients.
Keywords
Hepatitis B, Chronic; Hepatitis B e Antigens; Telbivudine; Interferon-alpha; Treatment outcome
Introduction
Chronic Hepatitis B virus (HBV) infection causes a substantial burden of chronic liver disease, and is still a serious public health problem. The clearance of Hepatitis B surface antigen (HBsAg) predicts a resolved HBV infection, but which occurs very rarely in patients with chronic Hepatitis B (CHB), so the duration of HBV infection is almost lifelong. The loss of Hepatitis B e antigen (HBeAg) in HBeAg-positive CHB patients reflects a significant improvement of clinical outcome. Moreover, appearance of HBeAg seroconversion (defined as HBeAg clearance and antibody to HBeAg development) is more likely to be associated with the clearance of serum HBsAg [1,2], and thus HBeAg seroconversion confers a favourable long-term outcome with a very low risk of cirrhosis or hepatocellular carcinoma (HCC) in the absolute majority of patients [3].
Currently, two main types of antiviral agents have been widely used for the treatment of CHB: interferon-alpha (IFN-α, conventional or pegylated) and nucleoside/nucleotide analogs (NAs), which include lamivudine (LAM), adeforvir dipivoxil (ADV), entecavir, telbivudine (LdT) and tenofovir, but any one of these agents is rarely effective to eradicate HBV infection from the hosts completely. In order to improve antiviral efficacy, many kinds of therapeutic approaches such as sequential therapy with IFN-α following a NA or vice versa, and combination therapy with two NAs have been studied. To some extent, some satisfactory outcomes have been obtained but the contradictive results have also been reported [4-10].
IFN-α is an immunomodulator that can stimulate the host immunity, and can also suppress viral replication. A meta-analysis from 15 randomized controlled studies showed that IFN-α therapy had a significant effect on the development of HBeAg or HBsAg seroconversion and on the normalization of ALT [11], but the unsatisfactory effect could frequently be seen in real life practice. De novo combination therapy with IFN-α plus a NA also did not produce better results than monotherapy with IFN-α alone [12,13]. Therefore, there still remains a challenge to treat the patients with IFN-refractory CHB. Although some therapeutic strategies have been tried to improve the outcome for these patients, a highly recommended therapy has not been obtained yet.
Material and Methods
Enrollment of patients
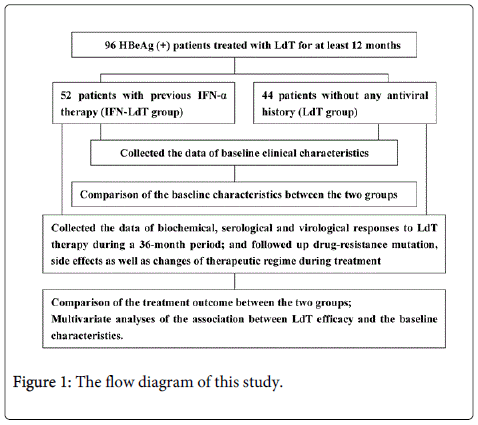
This was a two-center retrospective study. The flow diagram of this study was shown in Figure 1. The patients were selected from those with HBeAg-positive CHB who were treated with LdT therapy for at least 12 months at the Affiliated Infectious Disease Hospital of Soochow University and the Affiliated Wujiang Hospital of Nantong University, Suzhou, China, between January 2008 and Febuary 2014. All of them were NA treatment-naïve patients. They were divided into two groups: one was composed of patients with poor response to prior IFN-α therapy and then switching to LdT treatment (IFN-LdT group), and the other one consisted of those receiving LdT without antiviral history (LdT group). The patients with poor response to IFN-α therapy was defined as those with HBV DNA load>4 log10 copies/ml, abnormal ALT level (>40 IU/L) and without HBeAg loss during 6 to 12 months of IFN-α treatment. The data of biochemical, serological and virological markers within 36 months of LdT treatment were collected, but once a therapeutic regime was altered for a patient, for example, whose LdT monotherapy was changed to ADV, entecavir, or LdT plus ADV due to some certain reasons, the further data collection would be stopped. The exclusion criteria were as follows: (1) Patients with decompensated liver cirrhosis or HCC. (2) Patients coinfected with Hepatitis A, C, delta, or E viruses, as well as human immunodeficiency virus. (3) Patients with other liver diseases induced by autoimmunity, drug abuse (including excessive alcohol consumption) and so forth.
This study was reviewed and approved by the Ethics Committee of the Affiliated Infectious Disease Hospital of Soochow University and the Affiliated Wujiang Hospital of Nantong University, Suzhou, Jiangsu province, China. The code and date of ethical approval from the two hospital was SZWY-2013-5 (November 2013) and WJH-2014-2 (January 2014), respectively.
LdT therapy
All patients received oral LdT 600 mg once daily. The biochemical markers were monitored monthly after the start of treatment and then once every three months; HBV DNA loads and serological markers were monitored once during the first three months of treatment and then once every 3-6 months during treatment, according to Chinese guideline of prevention and treatment for chronic Hepatitis B [14,15]. The patients were all routinely monitored for these tests during treatment, and blood cell counts, alpha-fetoprotein, as well as abdominal ultrasound examination were also checked once every 6-12 months. In addition, if a virological breakthrough developed (defined as serum HBV DNA increasing by 1 log10 above nadir after achieving virological response and with a good compliance during continued treatment) in a patient, whose blood sample would be routinely collected and YMDD (tyrosine-methionine-aspartate-aspartate) motif mutation would be detected.
Routine laboratory tests
The routine biochemical markers, for example, liver function tests, renal function tests, creatine phosphokinase (CK) and so on (Hitachi Model 7600 Series Automatic Analyzer, Japan), HBV serological markers (ARCHITECT i2000SR, Abbott Laboratories Ltd, USA), and quantitative HBV DNA levels by real-time polymerase chain reaction (LightCycler 480 System, Roche, Penzberg, Germany) were detected for all patients before and after LdT treatment. In addition, the HBV genotypes by fluorescence polymerase chain reaction (Applied Biosystems 7300 Real-time PCR Systems, USA), other virological Hepatitis markers such as Hepatitis A, B, C, Delta and E viruses (ARCHITECT i2000SR, Abbott Laboratories Ltd, USA), as well as the antibodies in autoimmune liver diseases were also examined before treatment.
Statistical Analysis
All results were presented as mean ± standard deviation (SD), median (range) or number (%). The normality of data distribution was tested using the Kolmogorov-Smirnov test. Pearson chi-squared test and independent-samples t test were used to compare the baseline characteristics before LdT treatment. The cumulative probability of treatment outcome and LdT-resistant mutation were calculated using the Kaplan-Meier method and the differences between the curves were tested using the log-rank (Mantel-cox) chi square test. The multivariate analysis was tested using a stepwise Cox regression. Statistical analyses were performed using IBM SPSS statistics 20.0, and P<0.05 was considered as statistically significant.
Results
Study patients
A total of 96 consecutive adult Chinese patients were enrolled into this study, 52 of whom were in IFN-LdT group, who previously received conventional IFN-α (39 cases), or pegylated IFN-α-2a or -2b (13 cases) treatment for 6-12 months; and 44 of whom were in LdT group. In IFN-LdT group, the therapy was directly switched to LdT treatment following poor response to IFN-α in 32 patients, while the other 20 patients were initiated on LdT therapy after discontinuation of IFN-α for 1-3 months of drug-free period.
The baseline data in IFN-LdT group were gathered before the initiation of LdT therapy. During LdT treatment, the median observation time was 36.0 (12-36) months in IFN-LdT group, and 34.5 (12-36) months in LdT group, respectively. There was no significant difference between the two groups (p=0.089). Table 1 showed the baseline clinical data of the two groups, and the differences were not significantly different between the two groups, except for a higher level of HBV DNA at baseline in LdT group.
| Characteristic | IFN-LdT (n=52) | LdT (n=44) | p-value |
|---|---|---|---|
| Male, No. (%) | 37(71.15) | 30(68.18) | 0.752* |
| Age, yr | 29.8 ± 7.5 | 30.5 ± 7.6 | 0.639† |
| ALT, IU/L | 152.5 ± 86.4 | 190.8 ± 157.6 | 0.135† |
| HBeAg, S/CO | 455.4 ± 216.9 | 516.6 ± 236.7 | 0.190† |
| HBV DNA, log copies/ml | 6.77 ± 0.64 | 7.08 ± 0.79 | 0.033†,‡ |
| HBV genotypes, No. (%) | 0.964* | ||
| Type B | 22 (42.3) | 19 (52.3) | |
| Type C | 27 (51.9) | 23 (43.2) | |
| Other | 3 (5.8) | 2 (4.5) |
Table 1: Baseline Clinical Characteristics of the Two Groups. Data are presented as number (%) or mean ± SD. ALT, alanine aminotransferase; HBeAg, Hepatitis B e antigen; HBV, Hepatitis B virus; IFN, interferon; LdT, telbivudine. *Chi square test; †Independent-samples t-test; ‡p<0.05.
Clinical course
During the course of data collection, we followed up each patient and found that 33 patients still maintained LdT treatment, 10 had stopped the therapy, 3 had switched to Entecavir therapy, and 6 had been given LdT or LAM plus ADV treatment in IFN-LdT group; and there were 22, 7, 9 and 6 patients who maintained LdT, stopped the therapy, switched to Entecavir or to ADV, and received LdT or LAM plus ADV treatment in LdT group, respectively. There were 5 patients in IFN-LdT group and 8 patients in LdT group who emerged LdT-resistant mutation. In addition, 20 patients (38.46%) in IFN-LdT group and 15 patients (34.09%) in LdT group experienced an elevated CK level during the observation period, and the level decreased spontaneously or with oral vitamin B complex treatment; this adverse effect did not cause any clinical manifestations (such as myopathy, peripheral neuritis and so on). There was no patient who developed liver function decompensation or HCC during treatment.
Efficacy of LdT treatment
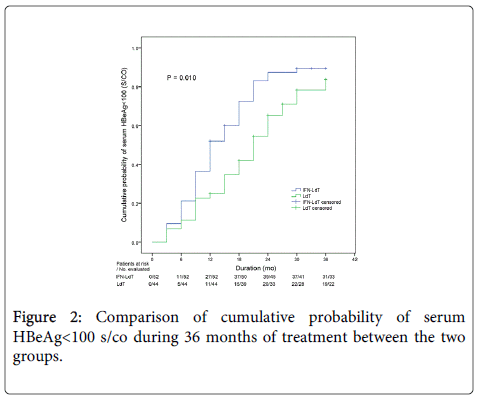
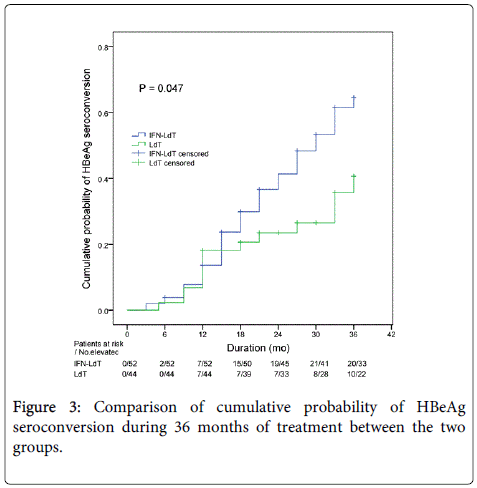
During an observation period of 36 months, the outcomes of LdT treatment in the two groups were shown in Table 2. The cumulative rate of ALT normalization and HBV DNA undetectability in IFN-LdT group was respectively higher than that in LdT group at 12, 24 and 36 months, but the difference of cumulative probability was respectively not statistically significant between the two groups (all p>0.05). The cumulative probability of serum HBeAg<100 S/CO was remarkably higher in IFN-LdT group compared with that in LdT group (p=0.010, Figure 2). However, although the cumulative rates of HBeAg loss in IFN-LdT group were also higher than those in LdT group at 12, 24 and 36 months, respectively, the cumulative probability did not reach to a statistically significant difference between the two groups (p>0.05). There were 29 patients in IFN-LdT group and 14 patients in LdT group who obtained HBeAg seroconversion during the observation, the cumulative rate of HBeAg seroconversion at 12, 24 and 36 months in IFN-LdT group was significantly higher than that in LdT group, respectively, and the difference between the two groups was markedly different (p=0.047, Figure 3). The result also demonstrated that the time to achieving HBeAg seroconversion was significantly shorter in IFN-LdT group than in LdT group.
| 12 months | 24 months | 36 months | p-value | |
|---|---|---|---|---|
| ALT normalization (<40 IU/L) | 0.248* | |||
| IFN-LdT | 59.62 | 88.28 | 92.97 | |
| LdT | 59.1 | 81.44 | 84.09 | |
| HBV DNA undetectability (<2.7 log copies/ml) | 0.112* | |||
| IFN-LdT | 65.38 | 87.69 | 95.08 | |
| LdT | 52.27 | 76.25 | 90.95 | |
| HBeAg<100 (S/CO) | 0.010*,† | |||
| IFN-LdT | 51.92 | 87.35 | 89.46 | |
| LdT | 22.73 | 65.25 | 83.71 | |
| HBeAg loss (<1 S/CO) | 0.361* | |||
| IFN-LdT | 21.15 | 41.81 | 69.34 | |
| LdT | 13.64 | 33.08 | 54.89 | |
| HBeAg seroconversion | 0.047*,† | |||
| IFN-LdT | 13.66 | 36.67 | 61.55 | |
| LdT | 18.18 | 23.49 | 40.68 | |
| HBsAg loss (<0.05 IU/ml) | 0.708* | |||
| IFN-LdT | 0 | 2.22 | 10.22 | |
| LdT | 0 | 2.5 | 6.93 | |
Table 2: Cumulative Probabilities of Antiviral Response During LdT Treatment. Data are presented in percent. ALT, alanine aminotransferase; HBV, Hepatitis B virus; HBeAg, Hepatitis B e antigen; HBsAg, Hepatitis B surface antigen; IFN, interferon; LdT, telbivudine. *Log-rank (Mantel-cox) chi square test; †p<0.05.
Although the cumulative rate of HBsAg loss at 36 months was 10.22% in IFN-LdT group and 6.93% in LdT group, respectively, the difference was not significantly different (p>0.05, Table 2). Additionally, the differences of outcome of LdT treatment did not exhibit statistically different between subgroups previously treated with conventional (39 cases) and pegylated (13 cases) IFN-α therapy (p>0.05), and between subgroups received LdT directly following IFN-α (32 cases) and initiating a delayed therapy (20 cases) after IFN-α discontinuation (p>0.05).
HBV DNA breakthrough and LdT-resistant mutation
There were 5 patients in IFN-LdT group and 8 patients in LdT group who developed virological breakthrough during LdT continued treatment, and all of 13 patients could be detected with genotypic resistance to LdT. The cumulative probability of HBV YMDD mutation at 12, 24 and 36 months in LdT group was nearly two times greater than that in IFN-LdT group, respectively, but the difference was not found to be statistically significant between the two groups (p>0.05, Table 3).
| 12 months | 24 months | 36 months | p-value | |
|---|---|---|---|---|
| YMDD motif mutation | 0.292* | |||
| IFN-LdT | 1.92 | 5.97 | 13.1 | |
| LdT | 4.55 | 10.01 | 22.73 | |
Table 3: Cumulative Probability of LdT-resistant Mutation During LdT Treatment. Data are presented in percent. YMDD, tyrosinemethionine- aspartate-aspartate; IFN, interferon. *Log-rank (Mantelcox) chi square test.
Baseline parameters associated with LdT efficacy
During LdT treatment, the COX regression analyses showed that the ALT normalization, HBV DNA undetectability and HBeAg seroconversion for all patients were not associated with age, gender, baseline serum HBeAg level, HBV DNA load, ALT level, HBV genotypes and prior IFN-α treatment (all p>0.05). Through further analysis among the above variables, we found that two variables were identified as independent factors influencing serum HBeAg<100 S/CO: IFN-α treatment (p=0.042) and baseline HBeAg level<300 S/CO (p=0.031, Table 4).
| Factors | Category | Exp (B) | 95% CI | p-value |
|---|---|---|---|---|
| IFN therapy | 1:IFN-experienced | 0.624 | 0.390-1.000 | 0.042*,‡ |
| 2: IFN-free | ||||
| Serum HBeAg (S/CO) | 1: ≥300 | 1.887 | 1.059-3.364 | 0.031*,‡ |
| 2: <300 |
Table 4: Multivariate Analysis of Baseline Factors Associated With Serum HBeAg<100 (S/CO). †Baseline factors including: age, gender, ALT level, HBV DNA level, serum HBeAg level, HBV genotype, and IFN therapy. HBeAg, Hepatitis B e antigen; IFN, interferon. *Cox regression analysis; ‡p<0.05.
Discussion
The main advantages of IFN-α therapy include the finite duration of treatment, absence of resistance, and higher rates of HBeAg and HBsAg seroconversion with 12 months of therapy [2]. For patients not responding to IFN-α, a sequential NA therapy is the best alternative in the subsequent antiviral treatment [16]. Limited studies have been conducted to evaluate the efficacy of LdT treatment for patients with poor response to IFN-α therapy, and reported that LdT was efficient and safe for these patients [17,18]. In the present study, we investigated the outcome of LdT during 36 months of treatment in 52 Chinese patients who failed to respond to IFN-α, and compared with the efficacy in 44 patients without a history of antiviral treatment. In addition, the clinical factors possibly influencing the efficacy of LdT treatment were also analyzed.
Our results showed that the cumulative rates of biochemical, serological and virological responses increased with treatment time. The cumulative probability was 92.97% for ALT normalization, 95.08% for HBV DNA undetectability, 69.34% for HBeAg loss, 61.55% for HBeAg seroconversion, and 10.22% for HBsAg loss at 36 months in IFN-LdT group, respectively. The results suggested a similar clinical significance like the reports performed in published studies [6,18]. Compared with the researches mentioned above, the duration of their studies was not more than 1 year, but majority of our patients received at least 36 months of LdT treatment, and thus a longer-term outcome could be observed and analyzed. In contrast to the biochemical, serological and virological responses achieved in LdT group, we found that the cumulative rates of treatment outcome in IFN-LdT group were higher than those in LdT group. And more importantly, there were significantly more patients who obtained lower level of serum HBeAg (<100 S/CO) and HBeAg seroconversion in IFN-LdT group than in LdT group during the observation period. The outcome demonstrated that LdT was efficacious for the treatment of patients who failed to IFN-α therapy, and the efficacy of LdT in IFN-experienced patients was obviously superior to that in antiviral naïve patients.
Concerning to LdT-associated resistance mutation, our data revealed that the cumulative rate of LdT resistance at 12, 24 and 36 months in LdT group was nearly two times greater than that in IFN-LdT group at the corresponding time point. In other studies, the drug-resistance rate ranged between 8.45% and 25.1% during 1 to 2 years of LdT treatment for naïve HBeAg positive CHB patients [19,20]. Compared with those mentioned above, the virological resistances whether in LdT group or in IFN-LdT group were obviously lower during a 3-year observation period, which was probably, at least in part, due to the mutation detected in patients with virological breakthrough in this study. More importantly, the result seemed to show that lower LdT resistance occurred in IFN-experienced patients than in naïve cases, although the difference did not reach to statistical significance. The similar result could also be seen in LMV study, the emergence of LMV mutants could be prevented by pretreatment with pegylated IFN-α-2a in LMV-naïve patients [21].
Based on the comparison of outcome between the two groups, it is possible that the difference might result from the baseline clinical characteristics, particularly the higher baseline HBV DNA level existed in LdT group than in IFN-LdT group. Therefore, the association of treatment outcome with baseline characteristics for all 96 patients was analyzed. We found that none of these baseline clinical indicators was significantly associated with ALT normalization, HBeAg loss, HBeAg seroconversion, HBV DNA undetectability, HBsAg loss and LdT resistance. However, two variables comprising previous IFN-α treatment and serum HBeAg<300 S/CO were identified as independent factors influencing the lower serum HBeAg level (<100 S/CO) achieved during treatment, suggesting that the inferior outcome in LdT group was not due to a higher level of baseline HBV DNA. The baseline HBV DNA level might possibly influence the outcome but a highly potent HBV suppression with LdT therapy may eliminate the difference for these NA-naïve patients. In addition, none of the patients exhibited the status with detectable HBV DNA and simultaneously with HBeAg loss or seroconversion, so the mutation of HBV precore or basic core promoter region was not detected. The results indicated that prior IFN-α treatment was more likely to exert an influence on the efficacy of LdT therapy in IFN-experienced patients.
To our knowledge, this was the first head-to-head retrospective study comparing the outcome of LdT in patients with or without previous IFN-α treatment. Our data demonstrated that switching to LdT was significantly more efficient in patients who failed to IFN-α therapy than in treatment-naive patients. The reason may be probably due to the improvement of immunity obtained from prior IFN-α therapy for IFN-experienced patients. A similar result indicated that activated immunity could be obtained after withdrawal of IFN-α therapy and sequential treatment with LAM could achieve a higher HBeAg seroconversion [22]. Therefore, we speculated that the patients treated with IFN-α therapy, although with an unsatisfactory response, might have an increased chance to achieve a high antiviral efficacy after being switched to LdT in the subsequent treatment. Besides the possible action of LdT on the immune system [23-26], the intrinsic mechanism may to some extent involve in an immunity improvement to HBV infection after receiving IFN-α treatment. In addition, a delayed effect of IFN-α may also play a part.
In conclusion, our data demonstrated that the efficacy of switching to LdT was efficient for the treatment of HBeAg-positive CHB patients with poor response to IFN-α therapy, and the outcome was superior to that in antiviral naïve patients. Therefore, switching to LdT might be a preferable option in patients who failed to previous IFN-α treatment. The safety profile of this switching, whether directly or indirectly, was similar to that in LdT-naïve patients. However, this was a retrospective study, the sample size was not large, and YMDD mutation was not detected for all patients with detectable HBV DNA, so a randomized, controlled trial with a larger sample would be needed to confirm the outcome in this study.
Acknowledgements
We sincerely thank all the staff who took part in this study in the two centers, including doctors, nurses, and lab colleagues for their great support.
References
- Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, et al. (2007) Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 357: 2576-2588.
- European Association for the Study of the Liver (2012) EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 57: 167-185.
- Chen G, Lin W, Shen F, Iloeje UH, London WT, et al. (2006) Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. Am J Gastroenterol 101: 1797-1803.
- Liu Y, Liu L, Peng D, Li WK, DU YR, et al. (2014) Long-term efficacy and safety of telbivudine as monotherapy and as combination therapy with adefovir dipivoxil in HBeAg-positive chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi 22: 181-184.
- Zhang W, Sandeep KK, Zhang QF, Guo SH, Zhang DZ (2013) Clinical observations of sequential interferon therapy following complete response to telbivudine treatment in chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi 21: 570-574.
- Piccolo P, Lenci I, di Paolo D, Demelia L, Sorbello O, et al. (2013) A randomized controlled trial of sequential pegylated interferon-a and telbivudine or vice versa for 48 weeks in hepatitis B e antigen-negative chronic hepatitis B. Antivir Ther 18: 57-64.
- Wong CR, Trinh HN, Yip B, Nguyen HA, Garcia RT, et al. (2011) High rate of complete viral suppression with combination therapy in patients with chronic hepatitis B and prior treatment failure. J Clin Gastroenterol 45: 900-905.
- Boglione L, D'Avolio A, Cariti G, Milia MG, Simiele M, et al. (2013) Sequential therapy with entecavir and PEG-INF in patients affected by chronic hepatitis B and high levels of HBV-DNA with non-D genotypes. J Viral Hepat 20:e11-19.
- Enomoto M, Nishiguchi S, Tamori A, Kobayashi S, Sakaguchi H, et al. (2013) Entecavir and interferon-a sequential therapy in Japanese patients with hepatitis B e antigen-positive chronic hepatitis B. J Gastroenterol 48: 397-404.
- Moucari R, Boyer N, Ripault MP, Castelnau C, Mackiewicz V, et al. (2011) Sequantial therapy with adefovir dipivoxil and pegylated interferon alfa-2a for HBeAg-negative patients. J Viral Hepat 18: 580-586.
- Wong DK, Cheung AM, O'Rourke K, Naylor CD, Detsky AS, et al. (1993) Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med 119: 312-323.
- Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, et al. (2005) Peginterferon Alfa -2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 352: 2682-2695.
- Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, et al. (2004) Peginterferon Alfa -2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 351: 1206-1217.
- (2007) Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline on prevention and treatment of chronic hepatitis B in China (2005) Chin Med J (Engl) 120: 2159-2173.
- (2011) Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Zhonghua Gan Zang Bing Za Zhi 19: 13-24.
- Tornai I (2011) [Interferon-based versus direct antiviral therapy in patients with chronic hepatitis B]. Orv Hetil 152: 869-874.
- Caroleo B, Staltari O, Gallelli L, Guadagnino V (2013) Pegylated interferon/telbivudine sequential therapy in Hepatitis Be antigen negative severe chronic hepatitis B patient. J Res Med Sci 18: 368-369.
- Huang Z, Zhao Z, Zheng Y, Peng L, Lin C, et al. (2013) Efficacy of sequential use of telbivudine in hepatitis B e antigen-positive chronic hepatitis B patients with partial responses to pegylated interferon: a pilot study. J Viral Hepat 20 Suppl 1: 52-57.
- Liaw YF, Gane E, Leung N, Zeuzem S, Wang Y, et al. (2009) 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 136: 486-495.
- Zhu XF, Lu LX, Wang Y, Xu KW, Li DJ, et al. (2011) Effect and Predictive Elements for 52 Weeks' Telbivudine Treatment on Naïve HBeAg positive Chronic Hepatitis B. Hepat Mon 11: 980-985.
- Villa E, Lei B, Taliani G, Graziosi A, Critelli R, et al. (2009) Pretreatment with pegylated interferon prevents emergence of lamivudine mutants in lamivudine-naive patients: a pilot study. Antivir Ther 14: 1081-1087.
- 1Shindo M, Hamada K, Muramatsu A, Morikawa T, Okuno T (2006) Early reduction of infected hepatocytes by activated immunity at the time of interferon withdrawal hepatitis followed by lamivudine administration resulted in higher seroconversion in hepatitis Be antigen-positive patients with chronic hepatitis B. J Gastroenterol 41: 151-157.
- Shi TD, Zhang JM, Wang XF, Chen M, Sun H, et al. (2012) Effects of antiviral therapy with Telbivudine on peripheral iNKT cells in HBeAg(+) chronic hepatitis B patients. Clin Exp Med 12: 105-113.
- Ma SW, Huang X, Li YY, Tang LB, Sun XF, et al. (2012) High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol 56: 775-781.
- Pan X, Yao W, Fu J, Liu M, Li L, et al. (2012) Telbivudine improves the function of myeloid dendritic cells in patients with chronic hepatitis B. Acta Virol 56: 31-38.
- Bertino G, Ardiri AM, Calvagno GS, Bertino N, Ruggeri MI, et al. (2012) Telbivudine on-treatment HBsAg loss in naive HBeAg negative chronic hepatitis B: a case report and brief review of the literature. Clin Ter 163: e429-434.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15024
- [From(publication date):
February-2015 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10463
- PDF downloads : 4561