Case Report Open Access
Sweet Syndrome and Pulmonary Tuberculosis in a Crohn’s Disease Patient Treated with Anti-TNFα
Daniel Trabulo*, Cristina Teixeira, Suzane Ribeiro, Cláudio Martins, Joao Mangualde, Fatima Augusto, Isabelle Cremers and Ana Paula OliveiraGastroenterology Department, Hospital de Sao Bernardo, Centro Hospitalar de Setúbal, E.P.E. Setúbal, Rua Camilo Castelo Branco, 2910-446, Setúbal, Portugal
- *Corresponding Author:
- Dr. Daniel Trabulo
Gastroenterology department
Hospital de São Bernardo – Centro Hospitalar de Setúbal
Rua Camilo Castelo Branco 2910-446 Setúbal, Portugal
Tel: 00351968148206
E-mail: danieltrabulo@yahoo.com
Received date: February 23, 2015; Accepted date: March 1, 2015; Published date: March 10, 2015
Citation: Trabulo D, Teixeira C, Ribeiro S, Martins C, Mangualde J, et al. (2015) Sweet Syndrome and Pulmonary Tuberculosis in a Crohn’s Disease Patient Treated with Anti-TNFα. J Gastrointest Dig Syst 5:262. doi:10.4172/2161-069X.1000262
Copyright: ©2015 Trabulo D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
A 36-year-old man with Crohn’s Disease (CD), under infliximab therapy, was admitted with fever and skin lesions on the face, trunk and upper limbs. Skin biopsy was consistent with Sweet Syndrome (SS). He was treated with corticosteroids, with transient clinical improvement, but without healing of skin lesions. After 2 weeks, the fever relapsed and the patient complained of night sweats. Chest X-ray and CT-scan revealed pulmonary diffuse micronodular pattern with a condensation suggestive of pulmonary tuberculosis. Tuberculin test and IGRA were positive. Bronchoalveolar lavage culture was positive for M. tuberculosis. The patient started anti-tuberculosis standard regimen and discontinued anti-TNFα therapy. During treatment, there was clinical and radiological worsening and development of CD flare. We admitted an immune reconstitution inflammatory syndrome and anti- TNFα was reintroduced after 2 months, with improvement in CD symptoms, complete healing of skin lesions and resolution of TB. To our knowledge, this is the first case reported in the literature that presents the association between SS and pulmonary tuberculosis in a patient on anti-TNFα treatment for CD, complicated with IRIS. Early recognition of this association is essential for a effective treatment. Diagnosis and therapy of SS and pulmonary tuberculosis in a patient with CD are herein discussed.
Abstract
A 36-year-old man with Crohn’s Disease (CD), under infliximab therapy, was admitted with fever and skin lesions on the face, trunk and upper limbs. Skin biopsy was consistent with Sweet Syndrome (SS). He was treated with corticosteroids, with transient clinical improvement, but without healing of skin lesions. After 2 weeks, the fever relapsed and the patient complained of night sweats. Chest X-ray and CT-scan revealed pulmonary diffuse micronodular pattern with a condensation suggestive of pulmonary tuberculosis. Tuberculin test and IGRA were positive. Bronchoalveolar lavage culture was positive for M. tuberculosis. The patient started anti-tuberculosis standard regimen and discontinued anti-TNFα therapy. During treatment, there was clinical and radiological worsening and development of CD flare. We admitted an immune reconstitution inflammatory syndrome and anti-TNFα was reintroduced after 2 months, with improvement in CD symptoms, complete healing of skin lesions and resolution of TB.
To our knowledge, this is the first case reported in the literature that presents the association between SS and pulmonary tuberculosis in a patient on anti-TNFα treatment for CD, complicated with IRIS. Early recognition of this association is essential for a effective treatment. Diagnosis and therapy of SS and pulmonary tuberculosis in a patient with CD are herein discussed.
Keywords
Sweet syndrome; Tuberculosis; Anti-TNFα therapy; Crohn’s disease; Immune reconstitution inflammatory syndrome
Introduction
Sweet Syndrome (SS) is considered to be a skin manifestation of a systemic disease. Mycobacterium infections associated with SS have been documented in only a few reports [1-3], but M. tuberculosis infection showing SS is an extremely uncommon association [4]. SS can also represent a reactive response to Inflammatory Bowel Disease (IBD).
Biologic therapies, including monoclonal antibodies against TNFα, have proved to be an effective treatment for IBD. However, there is an increased risk of opportunistic infections, such as re-activation of latent tuberculosis (TB), usually within 3 months of treatment, and de novo active TB infection [5]. Several guidelines recommend screening for active and latent TB prior to all anti-TNFα therapy. There is increasing evidence that all patients on anti-TNFα should undergo Interferon gamma release assay (IGRA) for detection of latent TB as it may offer improved screening efficacy [6].
There is a lack of data and conflicting guidance regarding stopping anti-TNFα therapy if active TB develops under this treatment. In fact, patients may experience a flare in the underlying disease. Controversy also exists over the timing of reintroduction of anti-TNF-α therapy [7]. Several case reports have shown that early reintroduction can be done safely, and may even improve resolution of the inflammatory response [8,9].
We present a rare case of SS as a manifestation of pulmonary TB in a patient with Crohn’s Disease (CD) under infliximab treatment, complicated with immune reconstitution inflammatory syndrome following the introduction of anti-tuberculosis therapy.
Case Report
A 36-year-old man with a 18-year history of CD (Montreal classification A2L3B2) presented to a hospital with fever, red eye and multiple skin lesions on the head, neck and upper body, with progressive increase in size and number, tender and non-pruriginous. The patient had been treated with infliximab 5 mg 8/8 weeks for the last 8 years with clinical remission. The symptoms appeared 4 weeks following the last infusion and lasted for one week. He also reported loss of appetite and noticed weight loss (5 kg). He denied gastrointestinal symptoms, dyspnea, cough, spuctum, drug consumption, recent travels or other relevant history.
He was diagnosed with bacterial conjunctivitis and treated with topic dexamethasone and antibiotics. He also performed a chest X-ray (described as normal) and was referred to our Institution.
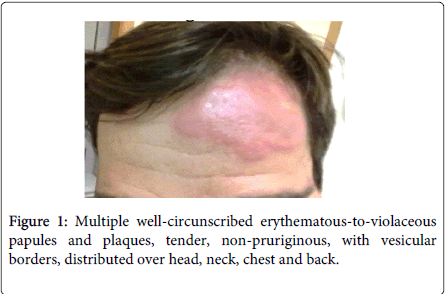
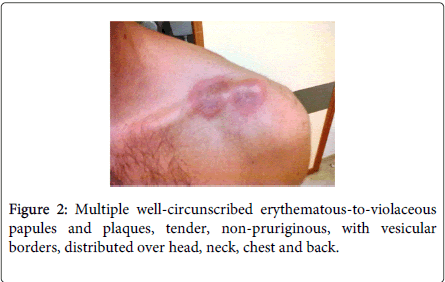
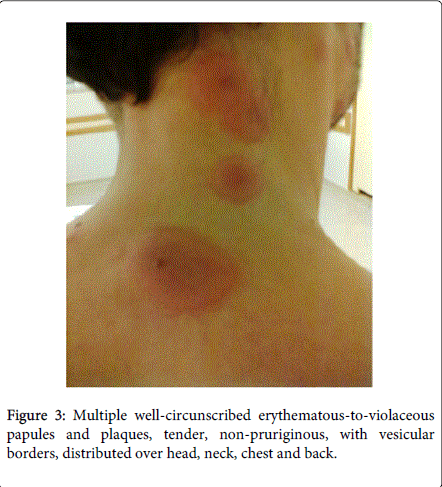
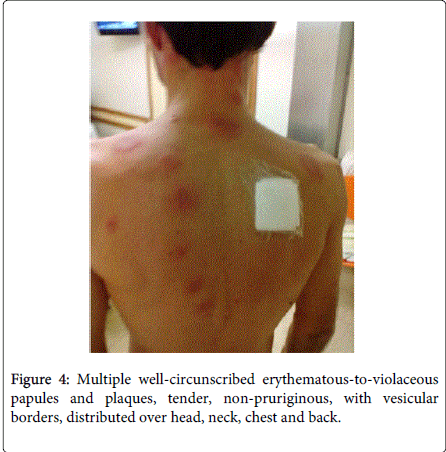
Physical examination demonstrated fever (38.5ºC), conjunctivitis and multiple well-circumscribed erythematous-to-violaceous papules and plaques with vesicular borders, distributed in an asymmetrical fashion over his head, neck, chest, back, and arms (Figures 1-4).
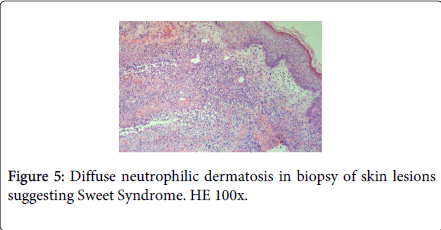
Laboratory studies revealed white blood cells 12000/μL, C-reactive protein (CRP) 357 mg/dL, erythrocyte sedimentation rate (ESR) 30 mm/h, lactic dehydrogenase level (LDH) 307 U/L and albumine 3.2 g/dL. Blood cultures were sterile. The following tests were negative: autoimmunity, Human Immunodeficiency virus, Cytomegalovirus, Epstein-Barr virus, Venereal Disease Research Laboratory, Toxoplasm, Ricketsia and Borrelia serologies. Biopsy of the skin lesions demonstrated a diffuse neutrophilic dermatosis (Figure 5). The diagnosis of Sweet Syndrome was established as an extra-intestinal manifestation of CD. The patient was treated with prednisone 60 mg/day. After 48 hours of treatment, the fever subsided, and inflammatory parameters decreased.
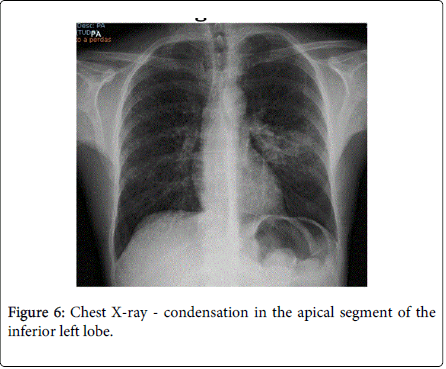
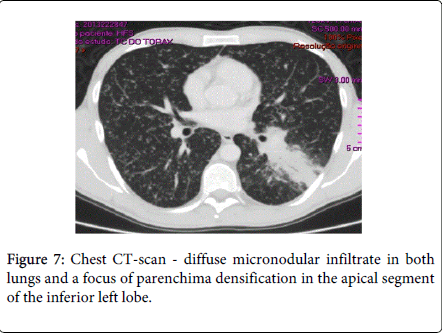
After 2 weeks of follow-up, the patient reported for the first time night sweats; fever relapsed (39ºC) and there was no significant improvement of skin lesions. Blood tests revealed persistence of inflammatory parameters (CRP=13.1 mg/dL; ESR=33 mm/h), worsening of LDH (357 U/L) and albumin levels (5 g/dL) and. Chest X-ray and CT-scan disclosed a diffuse micronodular infiltrate in both lungs and a parenchyma densification in the apical segment of the inferior left lobe (Figures 6 and 7). IGRA test (Quantiferon) was positive. The patient underwent bronchoscopy with brochoalveolar lavage (BAL) and Ziehl-Neelssen staining was positive for acid–alcohol resistant bacilli. Of note, standard screening for active and latent TB (chest X-ray and tuberculin skin test) was negative, prior to initiation of infliximab, 8 years ago. In addition, the patient had been vaccinated with BCG vaccine at birth.
The diagnosis of pulmonary TB was considered and standard anti-TB drug regimen was started (isoniazid 300 mg/d, rifampicin 600 mg/d, pyrazinamide 2 g/d, ethambutol 5 g/d). Infliximab and corticosteroids were discontinued. BAL culture was positive for M. tuberculosis sensible to all anti-TB drugs.
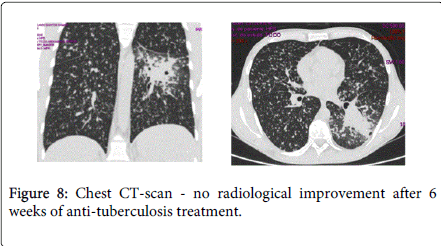
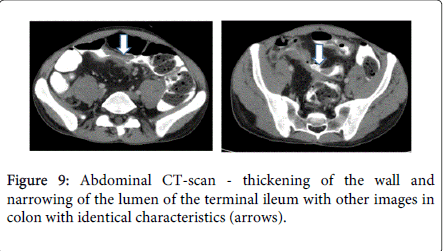
After 6 weeks of anti-TB treatment, skin lesions improved but the fever subsisted and there was no radiological improvement on chest CT-scan (Figure 8). In addition, the patient complained with pain in the right lower quadrant, abdominal distension and diarrhea (10 liquid stools daily). Fecal calprotectin level was 2590 mg/kg (normal<50 mg/kg). Abdominal CT-scan revealed thickening of the wall and narrowing of the lumen of the terminal ileum, with other images in colon with identical characteristics (Figure 9). Colonoscopy revealed a partial stenosis with 3 cm length at 15 cm from the anal verge and ulcerated stenosis of the ileo-cecal valve. Histology was compatible with active CD, without dysplasia. The patient was also evaluated by an Ophtalmologist due to decrease of visual acuity and was diagnosed with choroidal granuloma.
We considered IRIS to M. tuberculosis after discontinuing anti-TNFα, based on clinical and radiological worsening of TB on standard treatment. Furthermore, there was a flare of CD.
Infliximab was reintroduced after two months of anti-TB treatment. Skin lesions completely healed. The patient completed 9 months of anti-TB treatment with full clinical and radiological resolution. He remains asymptomatic after 1 year follow-up, without clinical or endoscopic activity of CD.
Discussion
Sweet’s Syndrome (SS), also known as Acute Febrile Neutrophilic Dermatosis, should be regarded as a cutaneous reactive response to an underlying systemic disease. It is characterized by fever, peripheral leucocytosis with neutrophilia, raised ESR and tender erythematous skin lesions with a diffuse neutrophilic dermal infiltrate [10,11]. Conjunctivitis or episcleritis occur with variable frequency [12].
SS has been associated with malignancies in about 20 to 25% of patients [13]. Most malignancies are hematopoietic, especially myelodysplastic syndromes and acute myeloid leukemia. Fifteen percent are due to solid tumors including breast, genitourinary and gastrointestinal malignancies [13]. Other causes include viral infections (chronic active hepatitis, cytomegalovirus, human immunodeficiency virus) bacterial infections (Streptococcus, Yersinia, Salmonella) or mycobacterial infections; autoimmune and collagen vascular diseases (rheumatoid arthritis, systemic lupus erythematous); medications (furosemide, hydralazine, lithium, oral contraceptive pills, trimethoprim-sulfamethoxazole); pregnancy; and inflammatory bowel disease (Crohn’s Disease and Ulcerative Colitis) [10-13].
The pathogenesis of SS is poorly understood. The condition is neutrophil mediated, as evidenced by its histopathologic appearance, associated neutrophilia, and response to medications that affect neutrophil activity [14] The association of exogenous granulocyte colony-stimulating factors (G-CSF) with the development of SS also supports the importance of neutrophils in the underlying process. G-CSF suppresses apoptosis and prolongs the survival of neutrophils. Its levels are increased in peripheral blood in patients with SS. Related endogenous cytokines such as interleukine-6 or 8 may also be responsible for immunopathologic and clinical manifestations [13]. In addition, some studies have suggested a role for tumor necrosis factor (TNF), and others have suggested an imbalance of type 1 helper T cells [15,16].
Currently, there are less than 40 cases reported in the literature of the association between SS and IBD [10]. It is more prevalent in Crohn’s Disease (CD) than in Ulcerative Colitis (UC) and is more common in young females (87-90%). Usually it does not reflect IBD activity [10-14]. As previously mentioned, it appears that SS is not initiated by the underlying disease such as IBD but rather shares with it a concurrent pathogenic mechanism [13]. Colonic or perianal disease are seen in almost all patients [10]. Standard treatment consists in prednisone 40-60 mg/day with gradual tapering off over 4-6 weeks. One-third of patients suffer relapse of symptoms [10,11].
An association between SS and infections has also been described, mainly of the respiratory tract. SS and Mycobacterium non-tuberculosis infections have been documented in literature [1], but association with M. tuberculosis and pulmonary TB is even more rare, with only a few reports found [3,4].
We considered that our patient had SS as a primary manifestation of pulmonary TB as a result of a state of immunosuppression caused by anti-TNFα treatment. However, we cannot exclude the possibility of an association between SS and CD. Indeed, the fact that appearance of skin lesions concurred with constitutional symptoms (fever, weight loss and night sweats) and analytical and radiological findings suggestive of pulmonary tuberculosis, lead us to conclude that SS was a manifestation of mycobacterial infection and not a simply result of CD. Furthermore, the patient was in clinical remission of his CD before the appearance of skin lesions and there was no clinical improvement with initial corticosteroid therapy.
Our case underlines the importance of TB surveillance and recognition of active infection in CD patients receiving long-term anti-TNFα therapy. Nevertheless, CD patients are at serious risk of severe infections, even in immunocompetent ones [15] probably as a result of impaired autoimmune mechanisms with a genetic component and response to environmental and intestinal microbial factors [16,17]
In fact, the proportion of IBD patients treated with anti-TNFα antibodies has been increasing and the use of these drugs is associated with reactivation of TB [18,19]. Although the mechanism is not fully understood, it has been linked to the failure of cell recruitment, granuloma formation and clearance of mycobacterial infection [6]. Infliximab is the anti-TNFα more frequently associated with TB [6,20]. Most cases occur in the first 6 months of treatment, with a median of 12 weeks [21]. As previously seen in cases of marked immunossuppression, the pattern of TB is atypical, with extrapulmonary being the most frequent form (56%), followed by disseminated form (24%) [21].
The introduction of latent TB screening protocols in candidate patients to anti-TNFα led to a decrease in TB incidence. Keane et al. reported a prevalence of 70:147.000 patients treated with infliximab[6]. Other studies described a prevalence of tuberculosis in 65% in IBD patients treated with anti-TNFα [21]. In Portugal, which has an intermediate incidence of TB (22 cases/100.000 habitants), a recent study showed a prevalence of 25 in 765 patients treated with anti-TNFα [22].
Countries with intermediate and high incidence of TB must be aware of latent TB diagnosis and reinfection. There is no gold standard test for latent TB infection and its treatment before anti-TNFα drugs may not be sufficient to protect from disease [2,23]. Current guidelines recommend performing a tuberculin skin test (TST) [5]. TST is considered positive if induration is ≥10mm in patients not previously exposed to immunosuppressors and ≥5 mm in previously immunosuppressed patients [22]. In patients already undergoing immunosuppressive therapy it is advised to repeat this test 1-2 weeks after initial negative screening [5]. In this group of patients, IGRA is in general more sensitive than TST but false negative results may also occur [2,23]. Since IGRA sensitivity and specificity is controversial and its positive and negative values are yet to be defined, it cannot be used as a single test. Therefore, it is recommended to use both tests to increase sensibility [5,24]. In fact, according to recent Portuguese recommendations, patients candidates for anti-TNFα, should undergo a chest x-ray, TST and IGRA [24]. Annual testing is recommended for patients with epidemiological risk of TB during treatment with biologic agents [24,25]. Our patient had a negative chest x-ray and TST prior to immunossuppression. However, he did not perform an IGRA test, nor repeated TST.
Treatment for latent TB should be started in patients with epidemiological risk factors, chest-ray sequelae of untreated previous TB or positive TST and/or IGRA, after exclusion of active TB [5,24]. Isoniazid for 9 months is the most consensual therapeutic option [24]. Anti-TNFα must be stopped after the diagnosis but can be resumed safely after one month in latent TB [25,26] or two months in active TB [5,27]. In our case, after anti-TNFα withdrawal, there was a flare of CD and paradoxical clinical and radiological worsening of TB on standard treatment. This phenomenon is a rare complication known as IRIS, which is thought to arise from an increased T cell response to TB antigens [7,28]. In our report, anti-TNF-α therapy was successfully reintroduced at 2 months with improvement in CD symptoms and resolution of TB. Early reinstitution of anti-TNFα therapy during treatment for active TB is likely to be safe and may reduce the likelihood of IRIS [7].
Conclusion
To our knowledge, this is the first case reported in the literature that presents the association between SS and pulmonary tuberculosis in a patient under anti-TNFα treatment for CD. The risk of TB in these patients remains a major concern in our country. The choice of latent TB screening protocol may have to be adapted to the geographic area. More studies are necessary to determine how TB surveillance should be performed in these patients.
References
- Chen HH, Hsiao CH, Chiu HC (2004) Successive development of cutaneous polyarteritis nodosa, leucocytoclastic vasculitis and Sweet's syndrome in a patient with cervical lymphadenitis caused by Mycobacterium fortuitum. Br J Dermatol 151: 1096-1100.
- Singh RK (2002) Acute febrile neutrophilic dermatosis following tuberculous infection. J Assoc Physicians India 50: 1322-1323.
- Serirat O Thaipisuttikul Y (2011) Sweet's syndrome associated with Mycobacterium tuberculosis and cervical cancer: a case report. J Med Assoc Thai 94 Suppl 2: S119-122.
- Karmakar PS Sherpa PL, Ray AN, Saha BK, Santra T, et al. (2013) Sweet's syndrome: a very rare association with pulmonary tuberculosis. J Infect Dev Ctries 7: 417-420.
- Rahier JF, Magro F, Abreu C (2014) Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 8: 443-468.
- Keane J Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, et al. (2001) Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345: 1098-1104.
- O'Dowd C, Kewin P, Morris J, Cotton M (2011) Tuberculosis complicated by immune reconstitution inflammatory syndrome in a patient on anti-TNFalpha therapy for Crohn's disease. BMJ Case Rep.
- Matsumoto T, Tanaka T, Kawase I (2006) Infliximab for rheumatoid arthritis in a patient with tuberculosis. N Engl J Med 355: 740-741.
- Aslanidis S, Pyrpasopoulou A, Douma S, Petidis K (2008) Is it safe to readminister tumor necrosis factor alpha antagonists following tuberculosis flare? Arthritis Rheum 58: 327-328.
- Marzano AV, Ishak RS, Saibeni S, Crosti C, Meroni PL, et al. (2013) Autoinflammatory skin disorders in inflammatory bowel diseases, pyoderma gangrenosum and Sweet's syndrome: a comprehensive review and disease classification criteria. Clin Rev Allergy Immunol 45: 202-210.
- Rappaport A Shaked M, Landau M, Dolev E (2001) Sweet's syndrome in association with Crohn's disease: report of a case and review of the literature. Dis Colon Rectum 44: 1526-1529.
- Vaz A Kramer K, Kalish RA (2000) Sweet's syndrome in association with Crohn's disease. Postgrad Med J 76: 713-714.
- Mustafa NM Lavizzo M (2008) Sweet's syndrome in a patient with Crohn's disease: a case report. J Med Case Rep 2: 221.
- Cohen PR Kurzrock R (2003) Sweet's syndrome revisited: a review of disease concepts. Int J Dermatol 42: 761-778.
- Kumar G, Bernstein JM, Waibel JS, Baumann MA (2004) Sweet's syndrome associated with sargramostim (granulocyte-macrophage colony stimulating factor) treatment. Am J Hematol. Jul 76: 283-285.
- Kemmett D Gawkrodger DJ, Wilson G, Hunter JA (1988) Sweet's syndrome in Crohn's disease. BMJ 297: 1513-1514.
- Sciaudone G Pellino G, Guadagni I, Somma A, D'Armiento FP, et al. (2011) Disseminated Cryptococcus neoformans infection and Crohn's disease in an immunocompetent patient. J Crohns Colitis 5: 60-63.
- Lawrance IC, Rogler G, Bamias G, Breynaert C, Florholmen J, et al. (2014) Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis S1873-9946.
- Latella G Rogler G Bamias G3, Breynaert C4, Florholmen J5, et al. (2014) Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis 8: 1147-1165.
- Dimakou K Papaioannides D, Latsi P, Katsimboula S, Korantzopoulos P, et al. (2004) Disseminated tuberculosis complicating anti-TNF-alpha treatment. Int J Clin Pract 58: 1052-1055.
- Jauregui-Amezaga A Turon F, Ordás I, Gallego M, Feu F, et al. (2013) Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis 7: 208-212.
- Abreu C, Magro F, Santos-Antunes J (2013) Tuberculosis in anti-TNF-alpha treated patients remains a problem in countries with an intermediate incidence: analysis of 25 patients matched with a control population. J Crohns Colitis 7: 486-492.
- Lalvani A Millington KA (2008) Screening for tuberculosis infection prior to initiation of anti-TNF therapy. Autoimmun Rev 8: 147-152.
- Duarte R Campainha S, Cotter J, Rosa B, Varela P, et al. (2012) Position paper on tuberculosis screening in patients with immune mediated inflammatory diseases candidates for biological therapy. Acta Reumatol Port 37: 253-259.
- Fonseca JE, Lucas H, Canha£o H, Duarte R, Santos MJ et al. (2008) Recommendations for the diagnosis and treatment of latent and active tuberculosis in inflammatory joint diseases candidates for therapy with tumor necrosis factor alpha inhibitors: March 2008 update. Acta Reumatol Port 33: 77-85.
- Solovic I Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, et al. (2010) The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement. Eur Respir J 36: 1185-1206.
- (2005) British Thoracic Society Standards of Care. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 60: 800-805.
- Shim TS1 (2014) Diagnosis and Treatment of Latent Tuberculosis Infection due to Initiation of Anti-TNF Therapy. Tuberc Respir Dis (Seoul) 76: 261-268.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 16285
- [From(publication date):
April-2015 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11702
- PDF downloads : 4583