Research Article Open Access
Surgical Site Infections in Treatment of Musculoskeletal Tumors: Experience from a Single Oncologic Orthopedic Institution
Barbara Rossi1*, Carmine Zoccali1, Luigi Toma2, Virginia Ferraresi3 and Roberto Biagini11Oncologic Orthopedics Unit, “Regina Elena”, National Cancer Institute, 00144 Rome, Italy
2Infectious Disease Unit, “Regina Elena” National Cancer Institute, 00144 Rome, Italy
3Medical Oncology Unit, “Regina Elena” National Cancer Institute, 00144 Rome, Italy
- *Corresponding Author:
- Barbara Rossi
MD, Oncologic Orthopedics Unit, “Regina Elena” National Cancer Institute
Rome, via Elio Chianesi 53, 00144, Italy
Tel: +39 -338 7777872
Fax: +39- 06-52662929
E-mail: barbararossi82@yahoo.it
Received Date: Mar 02, 2016; Accepted Date: Mar 16, 2016; Published Date: Mar 23, 2016
Citation: Rossi B, Zoccali C, Toma L, Ferraresi V, Biagini R (2016) Surgical Site Infections in Treatment of Musculoskeletal Tumors: Experience from a Single Oncologic Orthopedic Institution. J Orthop Oncol 2:108. doi:10.4172/2472-016X.1000108
Copyright: © 2016 Rossi B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Orthopedic Oncology
Abstract
Objective: Limb-sparing surgery is the mainstay treatment for musculoskeletal tumors thanks to advances in surgical techniques, imaging modalities and multimodal therapies. As patients survive longer, plastic reconstructive procedures and revision surgery are increasingly required after tumor excision. Infection rate is reported to be up to 20% after prosthetic replacement and 30-44% after pelvic resection. The purpose of this study was to investigate the incidence of surgical site infections (SSIs), identifying the causative microrganisms related to specific surgical procedures and significant risk factors for SSIs. Methods: We retrospectively reviewed 723 interventions performed between 2009 and 2015 for oncological conditions. Non neoplastic lesions, aseptic wound complications, non-skeletally mature patients were excluded. Standardised antibiotic prophylaxis was used for different surgical procedures and maintained until removal of surgical drains. Results: Without considering tumor types and surgical sites, the overall infection rate was 8.7% (63/724). Infection occurred in prosthetic reconstruction with an incidence rate of 7.8%, whereas almost half of patients having undergone pelvic surgery got infected and about 20% of patients with spinal surgery and amputations were infected. Pelvic location, malignancy and radiotherapy were related to a major risk of SSI. The causative pathogens were detected in all examined cases. The most frequent pathogens detected by culture included Staphylococcus aureus (27 cases, 47.4%) and S. epidermidis (10 cases, 17.5%). Among the S. aureus cases, 10/27 cases (37%) were methicillin-resistant S. aureus (MRSA). Sixty-three out of 130 microbial isolations (47.7%) were nosocomial ALERT organisms. Conclusion: Oncologic orthopedic surgery is burdened by frequent and challenging SSIs because of extensive soft tissues dissection, long operative times and poor skin conditions. Patients are immunosuppressed and often have concomitant comorbidities predisposing to SSIs. Monitoring of local bacterial aetiology of SSIs could help orthopedic oncologic specialized centres in achieving the optimisation of antibiotic prophylactic regimens.
Keywords
Surgical site infections; Bone and soft tissues tumors; Epidemiology; Risk factors; Orthopedic oncologic surgery; Microbial isolations; Tumor prostheses
Introduction
Limb-sparing surgery is the mainstay for treatment of musculoskeletal tumors, due to advances in surgical techniques, imaging examinations and multimodal therapies [1-4]. Reconstruction requires prosthetic implants, allograft, nails, plates or other metallic devices, vascular procedures and plastic surgery [4]. Moreover, as patients survive longer, revision surgery is increasingly required for implant failure, loosening or biomaterial overuse. Consequently, improvements in limb-salvage and prognosis have made surgical site infection (SSI) one of the most serious and discussed complications of musculoskeletal tumor surgery [2,3]. The hazard of infection can have devastating complications for all the main surgical procedures used in musculoskeletal oncology: bone resection and prosthetic reconstruction, spinal surgery, ostheosynthesis with plates, nailing, acrylic cement after curettage, allograft reconstructions; also other procedures without reconstruction such as soft tissue excisions and amputations or disarticulations. Surgical site infections are a main concern in oncologic orthopaedics and have become as challenging as recurrence, as reflected by a great number of literature reviews, monothematic meetings, multicentre prospective studies [5-8]. Literature over the last decade shows an urgent need to focus on epidemiology, early diagnosis, antimicrobial coverage for metal implants, identification of risk factors and, most of all, on the urgent need to define guidelines for antibiotic prophylaxis [7-9].
The aim of the study was to investigate the frequency and aetiology of SSIs in a single specialized Oncologic Orthopedic Center, determining whether infection is associated with particular risk factors and to compare the preliminar findings to the current literature.
Incidence and risk factors for SSIs: Background from literature
Infection rate in tumor megaprostheses is reported to be 8-35% for primary implants, 30%-43% after revision surgery and up to 22% for lower-extremity endoprosthetic reconstruction, with an average rate of 8.6-9.5% [8-12]. Infection rate in pelvic resection is 14% but increases up to 40% when it is followed by reconstruction [13,14]. The infection rate associated with excision for soft tissues sarcomas is 6-15% [3,15]. Patients affected by sarcomas have several potential risk factors for SSIs owing to tumour malignancy, depth and invasion, immunosuppression and iponutrition from neoadjuvant chemotherapy, radiotherapy, large incisions, wound complications, additional surgical procedures, along with other comorbidities such as anaemia, corticosteroids or diabetes (Table 1) [15-18].
| Malignancy | Prosthetic implants |
|---|---|
| Prior site irradiation | Trunk, pelvic and hip localization |
| Preoperative anemia | Prolonged hospitalization |
| Immunosuppression | Hematoma |
| Chemotherapy | Anticoagulation |
| Repeated accesses in hospital | Soft tissues necrosis |
| Inadequate antibiotic prophylaxis | Wound failure |
| Prolonged operative time | Drains |
Table 1: Independent risk factors associated with SSIs in musculoskeletal cancer patients [5,15-18].
From a retrospective analysis reported by Morii et al. [3] on 84 patients affected by soft tissues sarcomas, no association was actually detected between SSIs and age, chemotherapy, tumor grading or size or plastic surgery. Saddegh and Bauer [15] reviewed 103 patients with soft tissue sarcoma managed without adjuvant therapy from 1987 to 1990, finding a significant association between wound complications in deep tumors and age, tumor size and long operating time. Larger intraoperative blood loss and a trunk location were identified as statistically significant risk factors for deep infections from both aforementioned studies. Gradl et al. [17] recently reported a review of 1521 surgical procedures for bone and soft tissues tumors, finding eight independent risk factors: body mass index, age, preceding surgery, infection at another site, preexisting implants, tumor malignancy, hip location, duration of surgery. Although many different antibiotic regimens are used for prophylaxis to prevent SSIs, current practice among orthopedic oncologic surgeons seem to be in favour of long term duration therapy, from 2 to several postoperative days or at least until removal wound drains, and wide coverage against both Gram positive and negative bacteria [7-9].
Materials and Methods
All surgical activities performed at the Oncologic Orthopedic Unit of “Regina Elena” National Cancer Institute between January 2009 and December 2015 for primary malignant or benign, bony and soft tissue lesions and metastases were retrospectively reviewed. All non-previously surgically treated lesions, revisions for prosthetic aseptic loosening and re-excision for local recurrences were included. Non neoplastic lesions, aseptic wound complications, and non-skeletally mature patients were excluded. Data from each medical record were collected, including histology, age, location of surgery, surgical intervention, adjuvant treatments and other perioperative conditions as potential predictor of infection [14-17]. All surgeries were performed by the same team of surgeons. The same patterns of preoperative intravenous antibiotics were used for specific modalities of surgery (Table 2) and continued until wound drain removal (for 5 days in case of pelvic surgery, 2-3 days for all other procedures). Surgical site infections were identified in accordance with the Guidelines for Disease Control and Prevention Centers [19] as infection occurring at the site of surgery within 30 days from the operative date or up to 1 year if implant was inserted and the infection appears in relation to the surgery.
| First generation cephalosporin 1 g ì? 3 + amynoglicoside (tobramycin 100 mg x2) |
Hemipelvectomy Hip disarticulation Sacrum, pelvic resection Spinal surgery |
| Glycopeptide (teicoplanin 10-12 mg/kg) + amynoglicoside (amikacin BBK8 500 mg only for 24 h) |
Extremity long-bones resection Prosthetic reconstruction Allografts |
| First generation cephalosporin 1 g ì? 3 | Biopsy Soft tissues excision Fibula, scapula, clavicula resection Osteosynthesis (nails, plates, screws) |
Table 2: Different regimens of antibiotic prophylaxis adopted based on specific surgical procedures
All SSI diagnoses were detected from the register of Nosocomial Infection Control Group of our Institute and were compared with the medical records from clinical examination, radiographic studies and laboratory results including erythrocyte sedimentation rate, Câ¬?¬źreactive protein, white blood cell count in joint fluid analysis. Once discharged, patients were followed up at 1 month, 3 months, 6 months and at 1 year postoperatively, prolonged on the basis of the most appropriate timing of follow up for the neoplasm or until patient death. The causative microorganisms were isolated from positive wound swabs or joint bacterial culture. No patients were recalled specifically for this study; all data were obtained from the medical records. Descriptive statistics were calculated. Calculations of the relative risk were performed to assess independent risk factors for SSIs.
Results
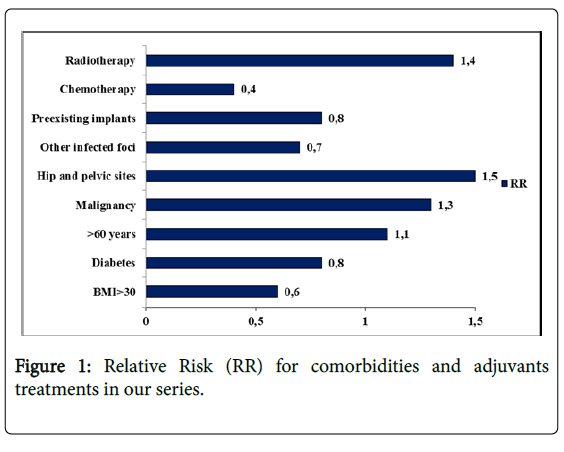
A total of 724 cases were evaluated for the inclusion of this study. Patient charactheristics are reported in Table 3. Without considering tumor types and location, 63 of these 724 cases were detected to have a SSI, the overall infection rate being 8.7%. Among recorded comorbidities and adjuvants treatments currently related to SSI, prior or adjuvant radiotherapy, malignant histotype and hip and pelvic location showed a higher relative risk (Figure 1). Infection rate for each surgical procedure is reported in Table 4. The causative pathogens were detected in all cases examined. Most infections were monomicrobial (34/63), and 29 were polymicrobial. A total of 130 microbial isolations were found in the 63 infected patients (mean 2.06 for each patient, range 1-8).
| Characteristics | Tumor origin and Location | n° patients |
|---|---|---|
| Surgical procedures | 724 | |
| Patients age (mean) | 14-85 yrs (52.5 yrs) |
|
| Tumor origin: | Primary Bone | 368 |
| Soft-tissues | 229 | |
| Metastasis | 126 | |
| Location: | Spine | 26 |
| Pelvis | 41 | |
| Extremities | 656 | |
| Risk factors for SSI [14-17]: BMI>30 Diabetes >60 years Malignancy Hip and pelvic sites Other infected foci Preexisting implants |
280/724 | |
| Chemotherapy | 365/724 | |
| Radiotherapy | 156/724 |
Table 3: Main case series’ characteristics
| Surgical procedure | n° patients | n° SSIs | SSIs rate |
|---|---|---|---|
| Extremity bone resection + prosthetic reconstruction |
128 | 10 | 7.8% |
| Pelvic resection ± prosthetic reconstruction |
36 | 17 | 47.2% |
| Soft-tissues tumors excision | 218 | 13 | 6% |
| Extremity and pelvic + bone allograft recontruction |
105 | 10 | 9.5% |
| Spinal surgery | 43 | 8 | 18.6% |
| Amputations | 54 | 10 | 18.5% |
| Nailing | 56 | 0 | 0% |
| Embolization, radiofrequency ablation | 74 | 1 | 1.4% |
Table 4: SSIs rates specifically distinguished for different surgical procedures.
The most frequent pathogens detected by culture included Staphylococcus aureus (27 cases, 47.4%) and S. epidermidis (10 cases, 17.5%). Among the S. aureus cases, 10/27 cases (37%) were methicillin-resistant S. aureus (MRSA). Sixty-three of 130 microbial isolations (47.7%) were nosocomial ALERT organisms as MRSA, Enterobacteriaceae-producing extended-spectrum β-lactamases (ESBL), vancomycin-resistant Enterococci (VRE), Acinetobacter baumanni. Table 5 shows the microbial isolations for different oncologic surgical procedures.
| Surgical procedures | MSSA MRSA |
Escherichia coli |
Klebsiella pneumoniae |
Enterococcus group D |
Enterobacter cloacae |
Proteus mirabilis |
Psuedomonas aeruginosa |
Acinetobacter baumanni |
|---|---|---|---|---|---|---|---|---|
| Extremity bone resection + prosthetic reconstruction |
4 23.5% |
4 23.5% |
3 17.6% |
0 0% |
1 5.9% |
2 10.5% |
3 17.6% |
2 11.8% |
| Soft-tissues excision | 5 31.3% | 4 25% |
0 0% |
2 12.5% |
2 12.5% |
0 0% |
1 6.3% |
2 12.5% |
| Amputations | 6 27.3% |
3 13.6% |
2 9.7% |
5 22.7% |
2 9.1% |
3 12% |
3 13.6% |
1 4.5% |
| Spinal sugery | 4 57.1% |
2 28.6% |
1 14.3% |
0 0% |
0 0% |
1 12.5% |
0 0% |
0 0% |
| Pelvic resection ± prosthetic reconstruction |
7 18.4% |
6 15.8% |
7 18.4% |
8 21.1% |
2 5.3% |
3 7.3% |
5 13.2% |
3 7.9% |
| Extremity and pelvic resection + bone allograft reconstruction |
5 37.5% |
3 21.4% |
7 18.4% |
1 7.1% |
1 7.1% |
0 0% |
4 28.6% |
0 0% |
Table 5: Absolute value and incidence rate of microbial isolations in different surgical procedures.
Discussion
The vulnerability of patients with bone and soft tissue tumors to SSIs goes beyond wound closure. Concern related to SSIs, already wellknown in conventional arthroplasty or osteosynthesis for degenerative or traumatic conditions, has largely increased in musculoskeletal tumors surgery due to a multitude of scarcely modifiable risk factors related to: 1) the complexity of surgical procedures with long operative times, wide resections with sacrifice of bone and soft tissues; 2) poor conditions of patients who are usually debilitated from the cancer itself and chemotherapy. Patients are immunosuppressed and often have comorbidities predisposed to poor local skin conditions and wound failure [3,4,15]. Infection is a major complication of oncologic surgery : it represents the most common failure of tumor endoprostheses, leading to amputation in 20% of cases. It undermines limb function and patients’ health conditions, it can delay or even prevent adjuvant treatment, thus seriously affecting the prognosis and life expectancy of cancer patients.
Similar to the data present in literature, we found that infection occurred in prosthetic reconstruction with a 7.8% incidence, in 50% of patients undergoing pelvic surgery and about 20% of patients after spinal surgery and amputations. Observational investigation on risk factors showed that pelvic site, malignant tumor and radiotherapy had a direct correlation to the development of infection. Treatment for the SSI cases observed is not discussed here because the purpose of the study was only limited to an epidemiological investigation on etiology. Microbial isolations were investigated for each modality of surgical intervention. To our knowledge, this is the first study that focuses on the correlation between a surgical technique and the causative microorganisms in surgical treatment of sarcomas.
Staphylococcus epidermidis and S. aureus account for most infections of permanently implanted material, thus prophylaxis is mainly against staphylococci [1-3,6,12]. However, having knowledge of local epidemiological data is fundamental in order to carry out the most effective antibiotic prophylaxis [7-9]. Thus, the choice on the most appropriate antibiotic should be done not only on the basis of literary reports about etiology of periprosthetic infections, but also on considering the features of local ecosystem in terms of antibiotic resistance and decisional politics from single medical institutes depending on individual plan of epidemiological surveillance.
S. epidermidis is traditionally reported to be dominant over S. aureus in periprosthetic infection [2,6,12]. In last ten years, the incidence of SSIs from S. aureus has increased from what was reported previously [1,8,18]. It may be partially explained by postoperative use of antibiotics and drains >24 hours, extension of surgical fields with soft tissue damage [18]. Large defect of quadriceps muscle and knee extraarticular resection, diabetes mellitus, lack of a gastrocnemius flap in the tibia and prolonged operation time are especially related to MRSA infection [5,18]. Different to what is reported in literature our results show that a high rate of infection from Gram negative and difficult-to-treat bacteria was found in joint prosthetic replacement after bone tumor resection of the extremities.
Already 20 years ago, Saddegh and Bauer [15] assessed the trunk and lower extremity as potential risk factors for the development of wound complications. Although anatomic location may be an independent risk factor for infection, distinction among surgical procedures is not a simple topographic distribution [17]. In our opinion, the affected site can be useful in stratifying specific surgical-related factors that could be a predictive indicator for the infection risk. In pelvic surgery, the high complication rate is attributed to the wide dead space after soft tissue excision, the proximity of rectal and urinary tract and long operative time. Wide periacetabular defects or massive reconstruction with prostheses or allograft implanted through a large wound are other contributing factors [10,16]. In surgery for soft tissue tumours, infection is related to large excision, sacrifice of even healthy tissues in order to guarantee wide margins, haematomas or sieromas and radiotherapy. Infections after osteosynthesis are less frequent but the incidence increases with open reduction, curettage and scarce tissue coverage as in tibial location. Low extremity amputations and hip disarticulations can be at risk of infections similarly to pelvic surgery especially in case of previously irradiated sites, lack of adequate bone stump coverage, complicated wounds with necrosis, eschars and consequently prolonged hospitalisation for medications and/or surgical wound revisions [3,4,16].
Gram negative nosocomial widespread is changing the scenario of orthopedic infections and patients affected from musculoskeletal cancer are at particularly high risk from colonization from multiresistant organisms, in all pre, intra- and postoperative periods [18]. Prosthetic surgery for bone tumors is usually done in highly-specialized centers where multiresistant germs are selected. The issue of SSIs troubles the oncologic field because it involves “frail” cancer patients and an equally “poor” surgery due to its complexity, extensive dissection, long and repeated operative times. Moreover, the inappropriate use of antibiotics along with migratory streams and nosocomial contaminations has selected, especially in the last decade, multiresistant germs.
Conclusion
Despite limitations and rarity of musculoskeletal tumors , further investigations are required. Our experience highlights the importance of an interdisciplinary team, involving orthopaedics, microbiologists, infectivologists, anaesthesiologists, and oncologists. Antibiotic prophylatic guidelines are still lacking in terms of molecules, dosage and duration [7,8]. Identification and stratification of patients at risk for multidrug-resistant colonization are fundamental, guidelines are expected, and antibiotic therapy should be appropriate for each single, specific case. Monitoring of local bacterial aetiology of SSIs could help orthopedic oncologic specialized centres in achieving the optimisation of antibiotic prophylactic regimens.
Acknowledgement
The authors would like to thank Dr. Grazia Prignano for collecting microbiological diagnoses and Dr. Diana Giannarelli for revising the epidemiological data.
References
- Gosheger G, Goetze C, Hardes J, Joosten U, Winkelmann W, et al. (2008) The influence of the alloy of megaprostheses on infection rate. J Arthroplasty 23: 916-920.
- Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, et al. (2010) Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol 101: 389-395.
- Morii T, Mochizuki K, Tajima T, Ichimura S, Satomi K (2012) Surgical site infection in malignant soft tissue tumors. J Orthop Sci 17: 51-57.
- Schwartz A, Rebecca A, Smith A, Casey W, Ashman J, et al. (2013) Risk factors for significant wound complications following wide resection of extremity soft tissue sarcomas. Clin Orthop Relat Res 471: 3612-3617.
- Morii T, Morioka H, Ueda T, Araki N, Hashimoto N, et al. (2013) Deep infection in tumor endoprosthesis around the knee: a multi-institutional study by the Japanese musculoskeletal oncology group. BMC Musculoskelet Disord 14: 51.
- Jeys LM, Grimer RJ, Carter SR, Tillman RM (2005) Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am 87: 842-849.
- Hasan K, Racano A, Deheshi B, Farrokhyar F, Wunder J, et al. (2012) Prophylactic antibiotic regimens in tumor surgery (PARITY) survey. BMC Musculoskelet Disord 13: 91.
- Ghert M, Deheshi B, Holt G, Randall RL, Ferguson P, et al. Parity Investigators (2012) Prophylactic antibiotic regimens in tumour surgery (PARITY): protocol for a multicentre randomised controlled study. BMJ Open 28: 2.
- Racano A, Pazionis T, Farrokhyar F, Deheshi B, Ghert M (2013) High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: a systematic review. Clin Orthop Relat Res 471: 2017-2027.
- Capanna R, Morris HG, Campanacci D, Del Ben M, Campanacci M (1994) Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg Br 76: 178–186.
- Shehadeh A, Noveau J, Malawer M, Henshaw R (2010) Late complications and survival of endoprosthetic reconstruction after resection of bone tumors. Clin Orthop Relat Res 468: 2885-2895.
- Mavrogenis AF, Pala E, Angelini A, Calabro T, Romagnoli C, et al. (2015) Infected Prostheses after Lower-Extremity Bone Tumor Resection: Clinical Outcomes of 100 Patients. Surg Infect (Larchmt) 16: 267-275.
- Abudu A, Grimer RJ, Cannon SR, Carter SR, Sneath RS (1997) Reconstruction of the hemipelvis after the excision of malignant tumours. Complications and functional outcome of prostheses. J Bone Joint Surg Br 79: 773-779.
- Angelini A, Drago G, Trovarelli G, Calabrò T, Ruggieri P (2014) Infection after surgical resection for pelvic bone tumors: an analysis of 270 patients from one institution. Clin Orthop Relat Res 472: 349-359.
- Saddegh MK, Bauer HC (1993) Wound complication in surgery of soft tissue sarcoma. Analysis of 103 consecutive patients managed without adjuvant therapy. Clin Orthop Relat Res 289: 247-253.
- Siegel HJ (2014) Management of open wounds: lessons from orthopedic oncology. Orthop Clin North Am 45: 99-107.
- Gradl G, de Witte PB, Evans BT, Hornicek F, Raskin K, et al. (2014) Surgical site infection in orthopaedic oncology. J Bone Joint Surg Am 96: 223-230.
- Manian FA (2014) The role of postoperative factors in surgical site infections: time to take notice. Clin Infect Dis 59: 1272-1276.
- Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Centers for disease control and prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control 27: 97-132.
Relevant Topics
- 3D Printing in Limb-Sparing Surgery
- Adamantinoma
- Aneurysmal Bone Cysts
- Chondrosarcoma
- Chordomas
- Cryosurgery
- Enchondroma
- Ewing√Ę‚ā¨‚ĄĘs Sarcoma
- Fibrous Dysplasia
- Giant Cell Tumor of Bone
- Immunotherapy for Osteosarcoma
- Liquid Biopsy in Orthopedic Oncology
- Malignant Osteoid
- Metastatic Bone Cancer
- Molecular Profiling of Bone Tumors
- Multilobular Tumour of Bone
- Orthopaedic Oncology
- Osteocartilaginous Exostosis
- Osteochondrodysplasia
- Osteoma
- Osteonecrosis
- Osteosarcoma
- Primary Bone Tumors
- Sarcoma
- Secondary Bone Tumours
- Targeted Therapy in Bone Sarcomas
- Tumours of Bone
Recommended Journals
Article Tools
Article Usage
- Total views: 12839
- [From(publication date):
March-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11892
- PDF downloads : 947