Research Article Open Access
Surfactant Assistant Enhancement of Bioremediation Rate for Hexavalent Chromium by Water Algae
| Rumki Nandi, Kakali Mukherjee and Bidyut Saha* | |
| Department of Chemistry, Bioremediation Laboratory, The University of Burdwan, Golapbag, Burdwan, Pin-713104, WB, India | |
| Corresponding Author : | Bidyut Saha Department of Chemistry, Bioremediation Laboratory The University of Burdwan, Golapbag, Burdwan, Pin 713104, WB, India Tel: +91 9476341691 Fax: +91-0342-2530452 E-mail: b_saha31@rediffmail.com |
| Received: July 07, 2015; Accepted: July 28, 2015; Published: August 04, 2015 | |
| Citation: Nandi R, Mukherjee K, Saha B (2015) Surfactant Assistant Enhancement of Bioremediation Rate for Hexavalent Chromium by Water Algae. Biochem Physiol 4:173.doi: 10.4172/2168-9652.1000173 | |
| Copyright: © 2015 Nandi R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
| Related article at Pubmed, Scholar Google | |
Visit for more related articles at Biochemistry & Physiology: Open Access
Abstract
Cr(VI) in waste water is a serious pollutant in diverse environmental conditions due to its toxicity and carcinogenicity. Bioremediation can alternatively be used to the conventional chemical treatments. Bioremediation of toxic hexavalent chromium is done by reduction of hexavalent chromium to nontoxic trivalent chromium in consequence of oxidation of organic components present in the water extract of water algae. The rate of bioremediation is increased by anionic surfactant sodium dodecylsulphate (SDS) and neutral surfactant Triton-X-100 (TX-100). In the present study micellar catalysts are used as surfactans. SDS is found to be most suitable among the catalyst. All the observations were recorded using UV-Vis, fluorescenece and FTIR spectrophotometer.
| Keywords |
| Hexavalent chromium; Non-toxic; Water algae; Water extract; Surfactant |
| Introduction |
| Accumulation of heavy metals in the environment is in consequence of rapid industrialisation. These non biodegradable heavy metals found to harm all types of living species including human. The extent of toxicity of some of these elements show dependence on their chemical nature. Chromium is one of such toxic metal. Chromium occurs naturally in water from alluvial aquifers in the western part of the Mojave Desert [1]. Chromium can exist in various oxidation states ranging from 0 to VI. Cr(III) and Cr(VI) are most important among all of them. Cr(VI) is highly mobile and excellent oxidizing agent. Cr(III) in low level helps in metabolism of glucose, lipid and protein in mammals [2], whereas its hexavalent form causes liver damage, pulmonary congestion and skin irritation resulting in ulcer formation [3]. It also found to exhibit carcinogenic and mutagenic effects on biological system in nature [4,5]. Cr(VI) is used in many industrial applications such as textile dyeing, pigmentation, chrome plating, industrial pulp, wood preservation and tanning among others. Hexavalent chromium is a common water pollutant and toxic (permissible limit of <0.05mg/L) [6]. Cr (VI) contamination in waste water is resulted from draining of water from such several chemical industries. |
| There are many conventional methods available in literature for the removal of hexavalent chromium viz electrolytic reduction, ion exchange, reverse osmosis and adsorption. However, there is also precedence of Adsorption mechanism example (electrostatic interaction) in literature [7]. These type of method reduces Cr(VI) to 300 times less toxic Cr(III) [8,9] and thereby lowering the concentration of Cr(VI). Nevertheless, these methods are very expensive and hazardous. |
| Bioremediation is an effective alternative of the conventional methods for removing of heavy metals from water. Various types of biosorbents such as fungi [10], yeast [11], bacteria [12], algae [13], and lignocellulosic material [14-17] are used for removal of hexavalent chromium. The biomass are collected from different source, dried and granulated for using as biosorbent. The removal proceeds through the reaction which undergoes a reduction coupled adsorption mechanism. In the powdered from, the biomaterials can reduce Cr(VI) completely to Cr(III) at low pH. However the aqueous part of the biomaterial also have the possible potential to remove Cr(VI) by reducing it to Cr(III). The detailed mechanism is still not well understood. |
| Blue-green algae are easily available and their high metal ion capacity inspires us to develop the hypothesis of the present work. In this work the bioremediation of hexavalent chromium is performed by water soluble part of the blue-green algae. UV, fluorescence and IR spectroscopy are used to monitor the reduction mechanism. The reaction proceeds further through ionic interaction and complex formation between the metal ion and the organic moieties present in the biomaterials. The cellulose, hemicelluloses, protein, phycocyanin and other polymers are present in the biosorbent and mainly responsible for the removal of Cr(VI). The reduction ability of the biomass originates from various functional groups like hydroxyl, carboxylate ion, aromatic nitrogen, keto- group, alkene etc. present in the structure of the biomass. The amount of Cr(III) obtained from the reduction of Cr(VI) is readily form stable and soluble organo Cr(III) complex complexed with the organic ligands present in the cellular organic compounds to [18,19]. The reduction process is accelerated by the use of surfactants. Anionic surfactant sodiumdodecyl sulphate (SDS) and neutral surfactant triton X-100 (TX-100) are used and the final product is found to be hydrated Cr(III) for both catalyzed and unanalyzed reaction. Anionic surfactant SDS shows greater catalytic effeciency than neutral surfactant, Triton X-100 (TX-100 in this reduction-biosorption process). Cr(III)-organo complex is water soluble. It is characterized by UV-VIS spectroscopy and fluorescence spectroscopy. |
| Experimental |
| Materials |
| Dried and powered blue-green algae, K2Cr2O7 (AR, BDH, India), SDS (AR, SRL, India), TX-100 (AR, SRL, India), H2SO4 (QUALIGENS, India), α-naphthol (AR, SRL, India), Methanol (AR, SRL, India), Quinoline-2-carboxaldehyde (AR, SRL, India) and all other chemicals used were highest purity available commercially. |
| Methods |
| Preparation of water extract: The blue-green alga was procured from a lake. Then it is washed thoroughly with distilled water. Then the algae were air dried and finally super granulated by a mixer grinder. One gram of the powdered algae was added to 250 ml of water and mixed thoroughly by a sonicator (Digital ultrasonic cleaner CD-4820). Then the mixture was kept for overnight. The insoluble part was filtered out by simple filtration method. A light green colored solution was obtained upon filtration as mother liquid. Stock solution (100 mg/L) of hexavalent chromium was prepared by dissolving required quantity of K2Cr2O7 in deionized water. Then a number of solutions of different concentration ranging from 40 mg/L to 1 mg/L were prepared from the stock solution. Absorbance of the prepared solutions were recorded with a UV-Vis spectrophotometer [UV- 2450 (SHIMADZU)] at 450 nm. Stock solutions (0.01 M) of chloride salts (Na+, K+, Cu2+,Zn2+, Fe2+, Al3+, Fe3+, Cr3+) and nitrate salts (Hg2+, Pb2+, Cd2+) were prepared with 10 mM Tris–HCl buffer solution (pH 7.4) for fluorescence measurement. A stock solution of RDQ (Cr3+ ion probe) was also prepared in ethanolwater (3:7). All fluorescence spectra were recorded 10 min after the addition of analyte at 25ºC with the excitation wavelength set at 520 nm (excitation/emission slit widths: 5 nm). After initial calibration, the reaction mixture was employed to the solution of RDQ for fluorescence measurement. |
| Preparation of test samples for determination of unknown concentration of Cr3+ formed in the reaction mixture (by fluorescence method): The amount of free Cr3+ formed in the solution can be directly measured by fluorescence method. The hexavalent chromium ion remained un-reacted was measured by absorbance method previously according to literature [20]. For determination of Cr3+ formed in the reaction mixture by fluorescence method; the reaction mixture in water was neutralized to adjust the pH at 7.4. Then the reaction solution at different time interval was taken and the observed change in fluorescence and UV-VIS was recorded in presence of RDQ (give fluorescence response to Cr3+ ion only, RDQ remains unaffected by Cr6+) in ethanol-water in TRIS–HCl buffer at pH = 7.4. |
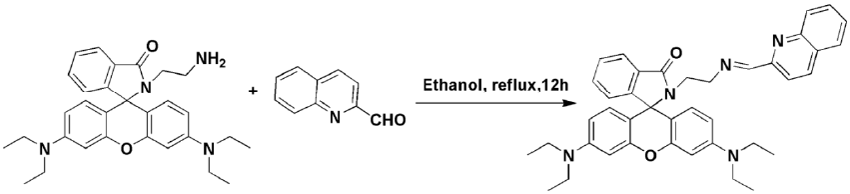
| Preparation of RDQ: RDQ is a simple Schiff base which can effectively detect Cr3+ in methanol-water system. RDQ is prepared by refluxing rhodamine ethelenediamine and quinoline-2-carboxaldehyde (details in ESI). RDQ detects Cr3+ in ethanol-water system in pH 7.4. The synthetic details for the probe RDQ is shown in Scheme1. |
| A plot of absorbance vs. concentration in a calibration curve is shown in Figure 1. |
| Initially, 5 ml of water extract of blue-green algae was mixed with 5 ml of 100 mg/L Cr(VI). The pH of this mixture was found to above 5 at that time. The reaction rate was very slow at this pH. Hexavalent chromium exists in aqueous solution as chromate (CrO42−), bichromate (HCrO4−) and dichromate (Cr2O72−). There relative distribution of these species depends on the solution pH [21]. In the present reductive removal process, the optimum pH value is found to 2. Therefore, concentrated sulphuric acid was added to adjusts the pH = 2. Final volume of the solution was adjusted to 25 ml. The mixture was centrifuged by Centrifuge-Z206A (Hermle Labortechnik GmbH) for ten minutes at 3000 rpm. After one hour of mixing the absorbance of Cr(VI) was measured at 450 nm at regular time interval (half hour) in the first day. On the second day absorbance of Cr(VI) was again measured (at 450 nm) at regular time interval (half hour). It is observed that the rate of reduction decreased with increasing time. So the next measurements were made after twenty four hours interval. The exact procedure was followed in presence of the surfactants. The all measurements were performed at 40ºC. |
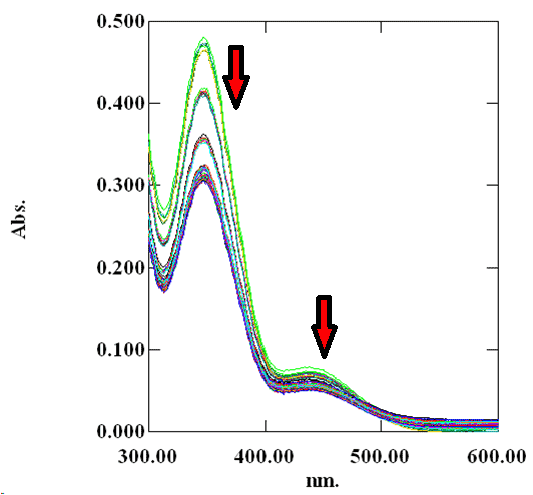
| The absorbance value of Cr(VI) at 450 nm decreased with time was clearly observed from the scanned spectra. The calibration curve (Figure 1) is useful for determination of concentrations of Cr(VI) in the solution at different time interval. In Figure 3 and Figure 4, the decrease in absorbance value of Cr(VI) by the water extract of blue-green algae in presence of surfactant TX-100 and SDS was observed. |
| Results |
| UV-VIS spectra analysis |
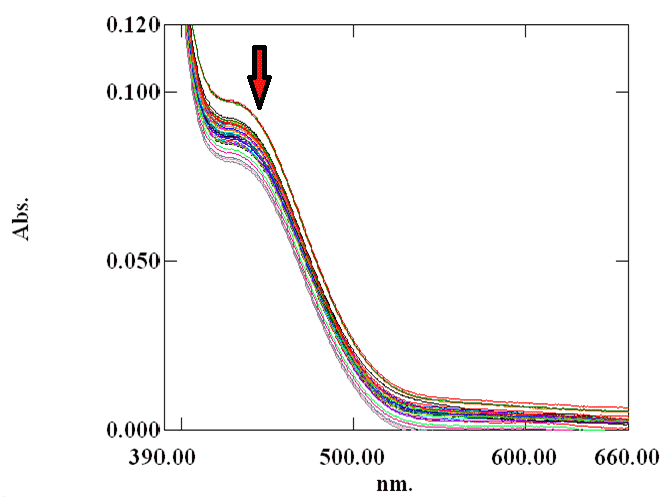
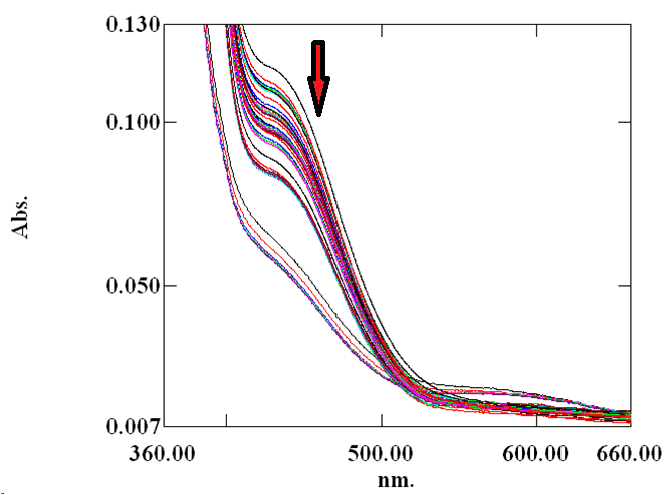
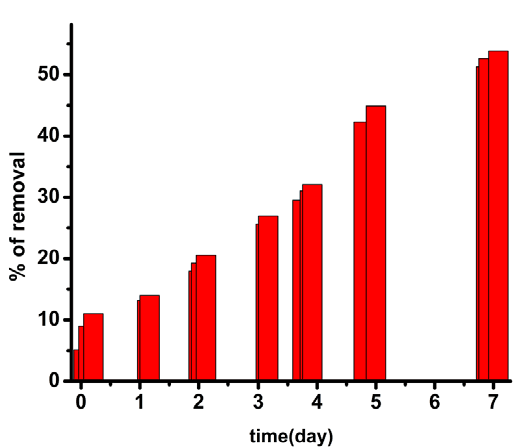
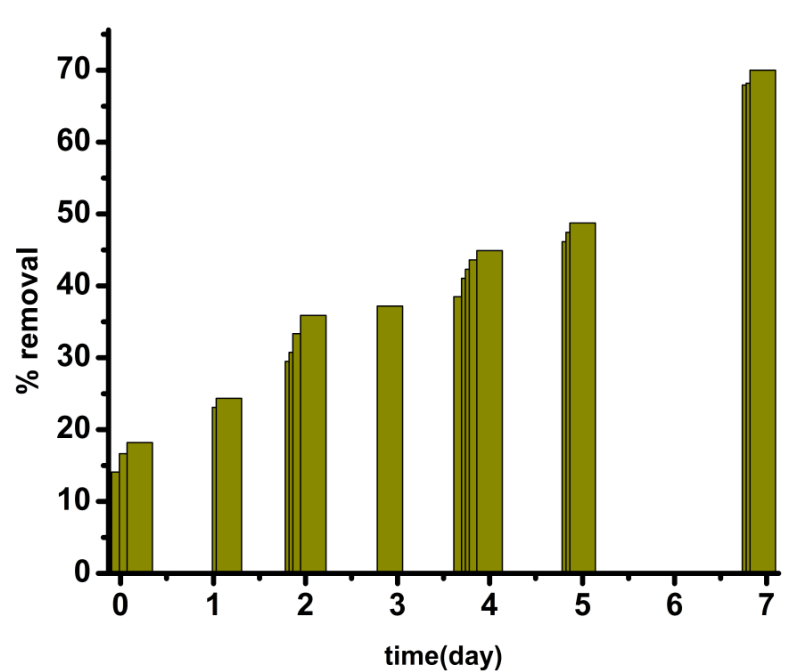
| Cr(VI) is converted to Cr(III) by the reducing components present in the water extract of blue green algae. Therefore, the absorbance value of Cr(VI) decreases with time at 450 nm initially in contact with the water extract of the biomaterial. Figure 2 showed that absorbance of Cr(VI) at 450 nm continuously decreases with time in presence of surfactant free biomaterial. From Figure 3 and Figure 4, it is clear that the absorbance of Cr(VI) at 450 nm decreases rapidly in presence of surfactants compared to surfactant free water extract of blue green algae. |
| Fluorescence spectra analysis |
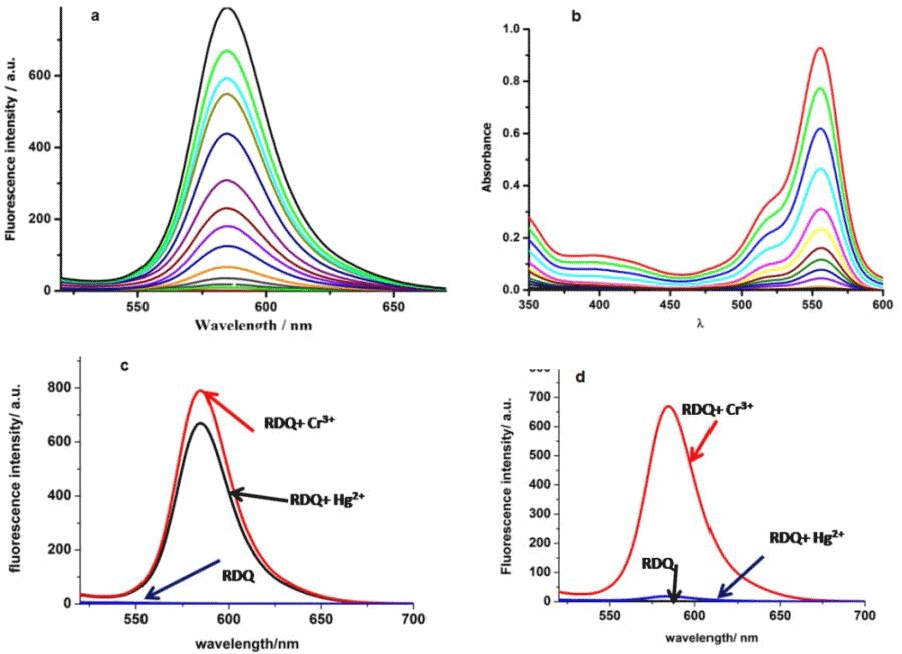
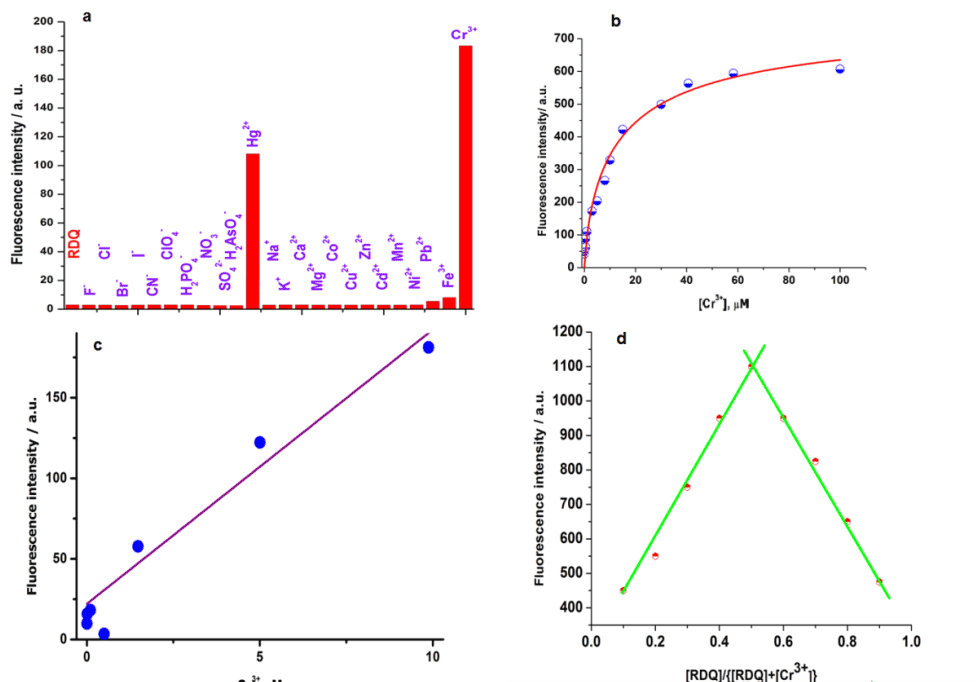
| It is well known that spirocyclic rhodamine derivatives are useful for selective sensing of analyte via strong red emission, high quantum yield, cell permeability and efficient FRET acceptor [22]. There are several reported probes based on rhodamine conjugates for selective detection of cations [23]. Figure 4c and 4d clearly indicates that upon adding KI to the reaction mixture the interferences from Hg2+ can be completely removed. For analytical detection of Cr3+ this method can be applied. Figure 5b clearly indicates that any unknown concentration of the Cr3+ can be obtained simply from the Calibration curve. For exact determination of Cr3+ ion in the reaction mixture 1ml of the reaction mixture at different time interval was taken and from the Calibration curve we found the exact concentration of Cr3+ in 1m of reaction mixture. Therefore, the amount of Cr3+ ion in mg/L can be obtained from the total volume of the reaction mixture. |
| Measurement of detection limit, binding constant and interferences |
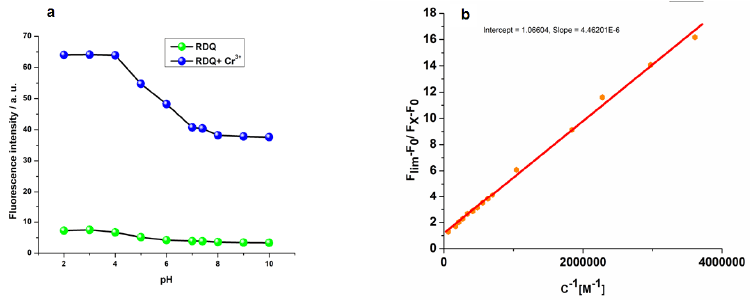
| Addition of Cr3+ to the probe enhances the emission intensity at 580 nm due to spirolactum ring opening of rhodamine ring. Changes in the emission spectra of RDQ upon gradual addition of Cr3+ are shown in Figure 8a. Emission intensities of RDQ and RDQ-Cr3+ adduct in presence of different cations are shown at Figure 9a. In competitive measurement, the solution of RDQ–Cr3+ showed almost no changes in the presence of other common metal ions including Cr6+ ion (Figure 9a). Distinctive colorimetric transition from colorless to pink and a fluorometric transition from non-fluorescent to strong pink fluorescent emission can be clearly discerned due to the selective recognition of Cr3+ ions with RDQ probe (ESI). Figure 9b shows the plot of emission intensity vs. Cr3+ concentration, the linear region (up to 10 μM Cr3+) of which (Figure 9c) is useful for determination of unknown Cr3+ concentration in any reaction mixture. Changes in absorbance of RDQ upon gradual addition of Cr3+ (at 556 nm) are presented in Figure 8b, attributed to the Cr3+ assisted spirolactam ring opening. The effect of pH on the emission intensities of free RDQ and RDQ- Cr3+ systems (Figure 10a) indicates that along pH ranging from 3.0 to 10.0 is useful for Cr3+ detection. The probe RDQ is stable in a long rang of pH and fluoresence maxima is observed in biological pH range. Thus RDQ can also be applied for detection of Cr3+ in cells. |
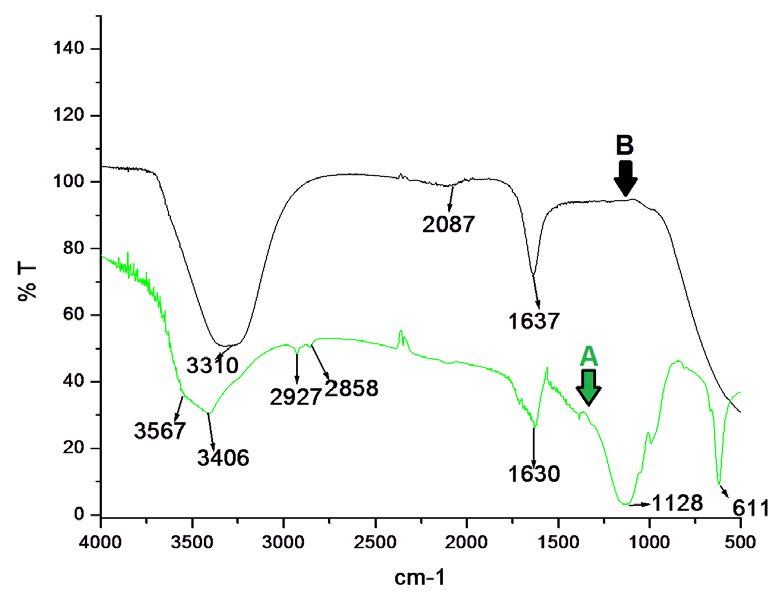
| The dissociation constant of RDQ–Cr3+was obtained from nonlinear curve fitting of the fluorescencetitration data with Benesi– Hildebrand equation [24]. The detection limit was then determined to 0 .005 μM (Figure 9c). |
| From Job’s plot (Figure 9d), 1:1 stoichiometry is con-firmed. From this information, binding constant of RDQ with Cr3+ ion is calculated and the value is found as 2.2×106 (Figure 10b). |
| IR analysis |
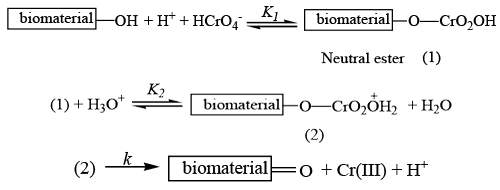
| FTIR spectra gives also proof of this reduction absorption mechanism. Peaks in the range 2850-3000 cm-1 are indication of CH2 stretching of alkyl methelene in water algae solution (chromium free) [25]. In chromium treated solution a weak band is observed at 2087 cm-1 due to C-C stretching of alkynes. This results proves that alkene carbon is changed to alkyne carbon due to oxidation by Cr(VI). In case of untreated water algae the peak due to OH group appears at 3567 cm-1. Whereas in case of Cr laden material this peak appears at 3310 cm-1 indicating the decrease in hydrogen bonding [26]. For C-O stretching of aliphatic ethers in untreated water algae solution frequency comes at 1128 cm-1 whereas the value has been shifted to 1630 cm-1 in chromium treated solution. It is likely that C-OH has been oxidized into C=O due to the increasing absorbing intensity at peak of 1628 cm (Figure 11). |
| Discussion |
| The dust form of the biomaterial upon treatment in water, the water soluble components such as proteins, peptide, phycocyanin of the biomaterial and blue green algae are mixed well into the water. At very low pH, chromium ions mainly exist in the solution as HCrO4- form and with increase in pH (pH value up to 6) various forms of chromium such as Cr2O72-, HCrO4- and Cr3O102- coexist. HCrO4- is the predominant form among all the components. At pH values greater than 7 OHpredominates as major component rather than other of the anions (e.g., CrO42-, Cr2O72- ) [27]. Cromium uptake is maximum in the pH range 2-4 according to the literature references [28]. Increase of pH causes the value of E(HCrO4-/Cr3+) to drop dramatically, thereby weakening the oxidizing power of HCrO4 - ions with respect to the compounds present in the water extract [29]. At pH 2.00-4.00 following type of reaction occurs [30] |
| Cr2O72- + 14H+ + 6e- ↔ 2Cr3+ + 7H2O E0 = 1.33V |
| HCrO4 - + 7H+ + 3e- ↔ Cr3+ + 4H2O E0 = 1.35V |
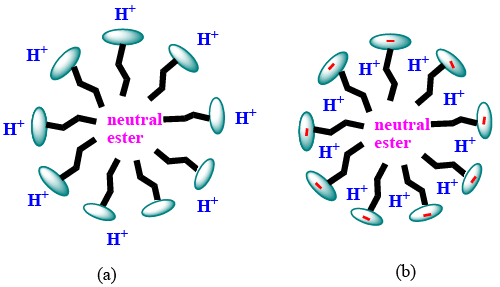
| Protonation of the functional groups present in the water extract of the blue-green algae occurs due to the low pH value of the reaction mixture. So they can not bind with Cr3+ formed by the reduction of Cr(VI) and therefore chromic ion species exist in acidic solution as bulky hydrated species [Cr(H2O)6]3+ [31]. At pH value greater than 3, Cr(III) can easily complex with the functional groups of the biomaterial by replacing protons [32]. So both proton and electron (supplied by the electron donor group of the blue green algae) was required for the reduction of hexavalent chromium. In case of surfactant catalyzed bioremediation, the concentration of surfactants are taken above their critical micellar concentration (CMC). CMC is defined as the concentration at which the surfactants begin to form globular aggregate with the hydrophilic “head” regions in contact with surrounding aqueous solvent, sequestering the hydrophobic single-tail regions in the micelle centre. So they form micelle in the solution. The reactive species responsible for the reduction of hexavalent chromium present in the solution will distribute itself between the aqueous phase and micellar phase. As the concentration of the reactive species is higher in the micellar phase than the aqueous phase, rate of Cr(VI) reduction by the water extract of blue-green algae increases in presence of surfactant. It is evident from Figure 5, Figure 6 and Figure 7. The mechanism of the reaction occurring inside the micelle can be written as |
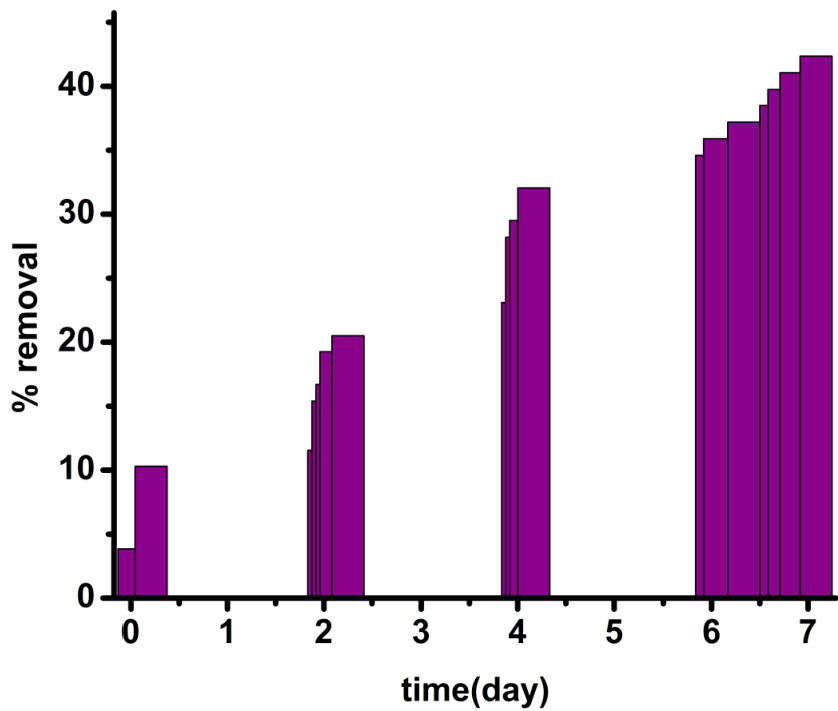
| Biomaterial first reacts with Cr(VI) and forms a neutral ester (1) [33]. Partitioning of neutral ester (Figure 12, Figure 13) occurs here in TX-100 and SDS micelles. But the maximum partitioning of proton occurs in anionic surfactant SDS due to electrostatic attraction, and intermediate in neutral surfactant TX-100. So the rate is highest in presence of SDS. In absence of surfactants, the percentage of removal is less than 50% in 170 hours whereas it goes to 70% and 53.85% in case of SDS and TX-100 respectively in 167 hours (Table S1-S3 in ESI). |
| Conclusion |
| In the present study, bioremediation of toxic hexavalent chromium is done by reduction of hexavalent chromium to nontoxic trivalent chromium by simultaneous oxidation of organic components present in the water extract of water algae. It is observed that blue-green algae has ability for bioremediation of Cr(VI). The process used is ecofriendly. Hydroxyl group, carboxylate ion, aromatic nitrogen, keto group present in the biomaterial are responsible for the removal of Cr(VI). The rate of bioremediation is increased by anionic surfactant sodium dodecylsulphate (SDS) and neutral surfactant Triton-X-100 (TX-100). In the present study micellar catalysts are used as surfactans. SDS is found to be most suitable among the catalyst. The amount of Cr3+ formed is directly measured by UV-Vis and fluorescence method. The choice of using blue-green algae is due to the fact they grows abundantly in the lake and no expensive chemicals are required to extract the active species. Further modification of the water extract and selective identification of the most active species is still challenging area of active research. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
 |
 |
 |
 |
|
| Figure 11 | Figure 12 | Figure 13 | Scheme 1 |
Relevant Topics
- Analytical Biochemistry
- Applied Biochemistry
- Carbohydrate Biochemistry
- Cellular Biochemistry
- Clinical_Biochemistry
- Comparative Biochemistry
- Environmental Biochemistry
- Forensic Biochemistry
- Lipid Biochemistry
- Medical_Biochemistry
- Metabolomics
- Nutritional Biochemistry
- Pesticide Biochemistry
- Process Biochemistry
- Protein_Biochemistry
- Single-Cell Biochemistry
- Soil_Biochemistry
Recommended Journals
- Biosensor Journals
- Cellular Biology Journal
- Journal of Biochemistry and Microbial Toxicology
- Journal of Biochemistry and Cell Biology
- Journal of Biological and Medical Sciences
- Journal of Cell Biology & Immunology
- Journal of Cellular and Molecular Pharmacology
- Journal of Chemical Biology & Therapeutics
- Journal of Phytochemicistry And Biochemistry
Article Tools
Article Usage
- Total views: 15709
- [From(publication date):
September-2015 - Nov 23, 2024] - Breakdown by view type
- HTML page views : 11249
- PDF downloads : 4460
