Research Article Open Access
Surface Plasmon Resonance Sensing of Biological Warfare Agent Botulinum Neurotoxin A
Arvind Tomar, Garima Gupta, Manglesh K. Singh, M. Boopathi, Beer Singh and Ram K. Dhaked*
Biotechnology Division, Protective Device Division, Defence Research and Development Establishment, Gwalior, India
- *Corresponding Author:
- Dhaked RK
Biotechnology Division
Defence Research & Development Establishment
Ministry of Defence, Gwalior-474002, (MP) India
Tel: +91-751-2390274
Fax: 91-751-2341148
E-mail: ramkumardhaked@hotmail.com
Received Date: March 15, 2016; Accepted Date: April 05, 2016; Published Date: April 11, 2016
Citation: Tomar A, Gupta G, Singh MK, Boopathi M, Singh B, et al., (2016) Surface Plasmon Resonance Sensing of Biological Warfare Agent Botulinum Neurotoxin A. J Bioterror Biodef 7: 142. doi: 10.4172/2157-2526.1000142
Copyright: © 2016 Tomar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
A label free real time method was developed for the detection as well as quantification of botulinum neurotoxin A (BoNT/A) using surface plasmon resonance (SPR). In the present work, antibody against rBoNT/A-HCC fragment and synaptic vesicles (SV) were immobilized on carboxymethyldextran modified gold chip. The immobilization of BoNT/A antibody and interaction of BoNT/A with immobilized antibody were in-situ characterized by SPR and electrochemical impedance spectroscopy. A sample solution containing BoNT/A antigen in the concentration ranging from 0.225 fM to 4.5 fM and 0.045 fM to 5.62 fM was interacted with immobilized antibody and immobilized SV, respectively. By using kinetic evaluation software, KD (equilibrium constant) and Bmax (maximum binding capacity of analyte) values were calculated and found to be 0.53 fM and 38.23 mo for immobilized antibody and 0.22 fM and 116.0 mo for immobilized SV, respectively. Moreover, thermodynamic parameters such as change in Gibb’s free energy (ΔG), change in enthalpy (ΔH) and change in entropy (ΔS) were determined and the values revealed that the interaction between BoNT/A antigen and BoNT/A antibody as spontaneous, endothermic and entropy driven one. In order to optimize the detection method, temperature and pH variation studies were also performed.
Keywords
Biological warfare agent; SPR; Impedance; BoNT/A
Introduction
The use of hazardous materials from chemical or biological origin as weapons for homicide was well known since ancient times. Consequently, the use of the biological agents by terrorists groups was considered as a serious threat to the safety of the world. The possibility of bioterrorism has become more credible after the 9/11 attack followed by various hoax cases of anthrax. Thus, this underscored the need for the development of rapid, easy to use, sensitive and specific detection techniques to recognize potential biological targets such as bacteria, viruses and toxins in order to counter bioterrorism.
Botulinum neurotoxins (BoNTs) are the most deadly toxins known. There are seven serologically distinct neurotoxins (BoNT/A-G) produced mainly by the Clostridium botulinum type A through G [1]. BoNTs are the causative agent of botulism, the disease inferred from flaccid muscle paralysis due to the blockage of acetylcholine release from nerve terminals at the neuromuscular junction [2]. Each of the BoNT is synthesized as a 150 kDa single polypeptide, nicked by trypsin like proteases into heavy chain (HC) [M.wt. 100 kDa] and light chain (LC) [M.wt. 50 kDa] coupled by a single disulphide bond [3]. The HC contains the cell receptor-binding (HC) and translocation (Hn) domains. Crystal structure analysis of the BoNT/A reveals that the HC clearly consists two distinct subdomains [4] that is N-terminal domain ( HCN ) and C-terminal domain ( HCC) and are speculated to bind with a protein receptor and gangliosides [5-8]. The HCC fragment in its isolated purified form can enable one to study its interactions with receptors of BoNT/A and to design the binding inhibitors to prevent the neurotoxic action.
The most associated serotypes with human food-borne infection are types A, B and E [9,10], among these, serotype A is the most poisonous toxin and can be categorized as a biological warfare agent (BWA). The oral lethal dose for a human is 1 μg/kg of body weight. Premeditated or accidental contamination of food or drink with microbial toxins like BoNT/A is not only a form of a biological attack, but also a “global public health problem” [11]. Hence, there is a need to develop rapid and sensitive identification system for BoNT/A.
Numerous analytical methods are developed to detect BoNTs including various biological assays such as mouse bioassay, enzyme linked immunosorbent assay and surface plasmon resonance based assay. Mouse bioassay is a highly sensitive assay that directly measures the amount of functional toxic BoNTs. However, it has a well-known limitation that it usually requires 24 h to 10 days to conduct experiment and requires animals. Various SPR assays were reported for the measurement of binding of BoNT/A to ganglioside GT1b [12], BoNT/A to monoclonal antibodies (MAbs) [13] including synaptic vesicles chips assay [14] and protein chip membrane capture assay [15]. A number of assays for BoNTs have been developed, aiming to equal the sensitivity of the mouse lethality assay while improving its limitation [16-25]. Though a lot of methods have been reported for BoNTs detection still better method development and improvisation are needed.
Protein chips technologies are promising one in chemical biology for detection and quantification of proteins and can be automated, multiplexed and miniaturized [26,27]. Among the different protein chip methodologies, SPR allows for real-time and label-free detection of molecular interactions between immobilized ligand on a sensor chip and analytes injected over the surface [28,15].
In continuation to our earlier studies on the development of detection methodologies for biological and chemical warfare agents [29- 31], in the present work we have developed a label-free real-time SPR optical method for the sensing of BoNT/A with carboxymethyldextran modified gold chip (CM5 chip). Interaction of BoNT/A with immobilized antibody was conducted. Moreover, interaction of BoNT/A with synaptic vesicles (rat brain lysate) was performed to check the biological activity of purified protein. The interaction between the antigen and antibody was characterized by electrochemical impedance spectroscopy (EIS) and the experimental parameters those affect SPR angle change such as temperature and pH were conducted and optimized. Finally, equilibrium constant (KD), maximum binding capacity of analyte (Bmax) and thermodynamic parameters such as change in Gibb’s free energy (ΔG), change in enthalpy (ΔH) and change in entropy (ΔS) were also deduced in this study.
Experimental
Chemicals and reagents
N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), N-Hydroxysuccinimide (NHS), phosphate buffered saline (PBS), sodium acetate; ethanolamine and hydrochloric acid (HCl) were obtained from Fluka. Glacial acetic acid, glycine and NaOH were supplied by Sigma-Aldrich. All chemicals and reagents used were of analytical grade and purification was performed wherever necessary before use.
Rat brain fractions containing Synaptic Vesicles (SVs) were obtained in one step as described earlier [32]. BoNT/A HCC gene was cloned, expressed and purified (M.Wt. 22 kDa) and BoNT/A antibody was raised by the trained biologists in our establishment. For SPR measurements, CM5 chip with a thickness of 50 nm (20 nm carboxymethyldextran coating +30 nm gold) was used which was purchased from Xantec Bioanalytics (Germany). Different buffer solutions were used in this study depending on pH [acetate buffer (pH 4.0-5.5), phosphate buffered saline (pH 6.0-7.5) and glycine-NaOH buffer (pH 8.0-9.0)] for the optimization of pH. All solutions were prepared using water from a Milli-Q system throughout the experiment.
Instruments
SPR assays were conducted using a two channel cuvette based electrochemical surface plasmon resonance system (Autolab ESPRIT, Ecochemie B.V., The Netherlands) on CM5 chip using 10 mM PBS (pH 7.4) as running buffer. The outcome of the SPR measurement was automatically monitored using a PC with data acquisition software version 4.4. All kinetic and thermodynamic parameters were evaluated using kinetic evaluation software version 5.1 (Ecochemie B.V. The Netherlands). EIS studies were carried out with Autolab PGSTAT- 302N digital potentiostat/galvanostat using FRAII module with FRA software 4.9 (Ecochemie B.V.). The EIS analyses were conducted within the frequency range of 0.1-10 kHz at open circuit potential. The pH of the buffers was measured with a EUTECH instrument pH meter (pH-1500, Singapore). All experiments were carried out at 25°C unless otherwise specified and the temperature of cuvette was controlled by a Julabo HE-4 (Germany) water bath.
Immobilization of antibody on CM5 modified gold SPR sensor chip
Prior to the immobilization of antibody on CM5 chip, 50 μL of 10 mM phosphate buffer (pH 7.4) was passed every 120s interval for 600s in order to get a stable baseline in both the channel. Sensor chip was chemically activated by the injection of 50 μl of a 1:1 mixture of 400 mM EDC and 100 mM NHS. This was followed by injection of 50 μL of BoNT/A antibody (1:500 dilution in 10 mM PBS) in channel 2 for 1,800s to get an effective immobilization over the activated dextran modified surface. Following antibody immobilization, the remaining active sites were then blocked with 50 μL of 1000 mM ethanolamine. Afterwards, 10 mM HCl was injected to achieve regeneration of immobilized sensor surface. For negative control measurements, the modified gold surface was activated with EDC/NHS and then quenched with ethanolamine in channel 1 as mentioned above and was used as blank control surface. The same procedure of immobilization used for BoNT/A was adopted for the immobilization of SV (1:500 dilution in 10 mM PBS) on CM5 as discussed above. SV protein was immobilized in both channel and bovine serum albumin (BSA) protein was used for negative control measurements.
Binding of BoNT/A to immobilized antibody and SV
A sample solution containing a selected concentration of BoNT/A in the PBS was injected in both channels from the 384 well microtiter plate and then association was performed for 500 s and dissociation was performed for 300 s. Subsequently, regeneration of the sensor surface was achieved by addition of 10 mM HCl for 120 s and then antigen was recovered in order to bring the signal to base line level so as to start a new measurement cycle [33,34].
Optimization of experimental parameters
In order to find out the effect of temperature on SPR measurements during the binding of BoNT/A with immobilized BoNT/A antibody as well as to calculate its thermodynamic parameters, temperature variation study was carried out between 10 and 37°C with a 3°C increment. pH variation study was also conducted using acetate buffer (pH 4.0-5.5), phosphate buffered saline (pH 6.0-7.5) and glycine-NaOH buffer (pH 8.0-9.0) so as to find out the optimum pH for the binding of BoNT/A with immobilized BoNT/A antibody.
Results and Discussion
Immobilization of BoNT/A antibody and SV protein on CM5 SPR sensor chip
Figures 1A and 1B represents the stepwise immobilization of BoNT/A antibody and SV protein on CM5 chip, respectively and this process comprises of nine steps. In first step, stabilization of baseline was carried out for 120s. In second step, activation of carboxyl groups on CM5 chip was performed for 900s with EDC-NHS to enable activated carboxymethylated groups on the sensor chip to bond covalently to the free amino groups of BoNT/A antibody up on its interaction. In third step, washing was conducted with PBS and the SPR angle shifted nearly to baseline [35]. In fourth step, BoNT/A antibody was injected on CM5 chip, allowed to 1800s and an increase in SPR angle is observed. In fifth step, washing was performed and in sixth step, to prevent non-specific binding and also for the blocking of unreacted NHS-ester groups on CM5 chip 1000 mM ethanolamine was used and permitted to react with sensor surface for 600s. In seventh step, washing was performed for 30s as discussed in the experimental part. In eighth step, regeneration was carried out for 120s. At last in ninth step, back to base line process was conducted for 60s. From Figures 1 and 2, a net angle change of 95.44 m° and 48 mo are observed and this ascribes the attachment of 0.79 ng/ mm2 of antibody and 0.4 ng/mm2 SV protein on CM5 chip, respectively [36].
Figure 1: Sensorgram showing different steps [(1) Baseline (2) EDCNHS activation (3) Washing (4) Antibody coupling (5) Washing (6) Deactivation (7) Washing (8) Regeneration and (9) Back to baseline] involved in the (A) immobilization of BoNT/A antibody (1: 500 dilution); (B) immobilization of SV on CM5 chip.
Interaction of BoNT/A antigen with the BoNT/A and SV immobilized sensor disc
The BoNT/A antibody immobilized sensor disc was utilized for the sensing of BoNT/A antigen by interacting with different concentration of the antigen and the net results are depicted as SPR sensorgram in Figure 2A. Figure 2A shows the sensogram corresponding to the concentration dependant angle changes, upon the interaction of various concentrations of BoNT/A with immobilized antibody and the Figure 2A depicts the corresponding calibration curve. The limit of detection (LOD) of the present method was calculated experimentally and was found to be 0.045 fM and this is the minimum concentration of BoNT/A antigen which showed the response during the interaction of BoNT/A antigen with its immobilized antibody.
Binding of different concentration of BoNT/A antigen was also carried out with immobilized SV protein in order to know the biological activity of purified protein and the sensorgrams are shown in Figure 2B. Figure 3 represents the comparative response of binding of BoNT/A and BSA with immobilized SV. BSA was used as negative control and showed 63% binding with immobilized SV in comparison of BoNT/A as shown in Figure 3.
Evaluation of kinetics involved in the antigen and antibody interaction
The affinity interactions between immobilized BoNT/A antibody and BoNT/A antigen were characterized by the equilibrium constant (KD). The data were fitted using a simple 1:1 interaction model [37], A + B = AB, where ‘A’ is the injected analyte, ‘B’ is the immobilized ligand and ‘AB’ is the analyte–ligand complex formed during the interaction process. In the SPR system, the signal R is proportional to the amount of (AB) and the Rmax is proportional to the initial (B). Hence, in this study kinetic parameters such as KD and Bmax value were calculated for binding of BoNT/A antigen with immobilized BoNT/A antibody and also for binding of BoNT/A antigen with immobilized SV protein using the software and found to be 0.53 fM and 38.23 mo and 0.22 fM and 116.03 mo, respectively. This low KD value (If KD ≤ 10 nM then it represents the high affinity interactions) represents high affinity interaction of the antigen with the immobilized antibody and also with SV protein [38]. Moreover, this KD value is lesser than earlier reported values for the BoNT/A sensing by SPR [12,13] and this confirms that the present antibody as a high affinity one due to the low KD value.
Evaluation of thermodynamic parameters
The KD obtained from kinetic evaluation software was further exploited to calculate the thermodynamic parameters such as change in Gibb’s free energy (ΔG), change in enthalpy (ΔH) and change in entropy (ΔS) associated with binding of BoNT/A antigen with immobilized antibody using Van’t Hoff [39,40] equation:
 (1)
(1)
 (2)
(2)
 (3)
(3)
where, R: universal gas constant, KA: affinity constant, T: temperature and K1, K2 are affinity constants for association at T1 and T2 temperature, respectively. ΔG, ΔH and ΔS are change in Gibb’s free energy, change in the enthalpy and change in the entropy due to the binding of BoNT/A with immobilized BoNT/A antibody, respectively.
The value of the change in Gibb’s free energy for binding of BoNT/A with immobilized BoNT/A antibody, was found to be -84.99 kJ/mol at 298 K. The negative value of ΔG indicates the spontaneous interaction of BoNT/A antigen with its antibody.
The calculated value of ΔH using Von’t Hoff Wizard in kinetic evaluation software was found to be +16.33 kcal/mol. The positive value of ΔH reveals the interaction of BoNT/A with immobilized BoNT/A antibody as endothermic [41].
The value of ΔS for binding of BoNT/A with immobilized BoNT/A antibody was calculated using software and was found to be 207.43 cal mole-1 K-1. The magnitude of TΔS value was found to be higher than ΔH indicating that the net influence of enthalpy on the BoNT/A with immobilized BoNT/A antibody is minor and the apparent gain in entropy is actually the driving force for the BoNT/A with immobilized BoNT/A antibody [42,43]. The observed positive value in entropy indicated that the interaction can be explained by Langmuir replacement reaction that exhibits Langmuir type isotherm [38]. Langmuir replacement reaction suggests that the observed positive entropy is because of desorption of water molecules from either antibody or antigen or both. The significance of desorption of water molecules from protein surfaces during ligand-protein binding was reported earlier [43].
Effect of temperature
Temperature variation study was performed in order to know the effect of temperature on SPR response during the interaction of antibody with antigen. Upon increasing temperature from 10 to 25°C an increase in SPR angle is observed as shown in Figure 4A and beyond 25°C, SPR angle decreased. Hence, 25°C is used as optimum temperature for the interaction of BoNT/A antigen with its immobilized antibody.
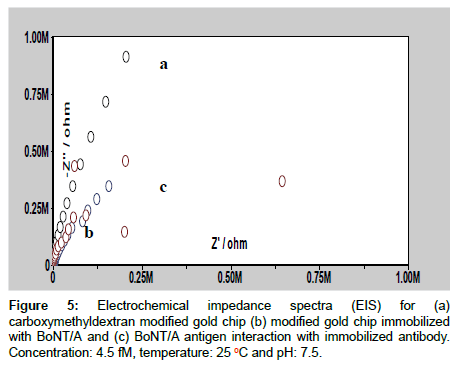
Figure 4: (A) Effect of temperature on the interaction of BoNT/A antigen with the immobilized BoNT/A antibody on carboxymethyldextran modified gold chip. Temperature range: 10 oC-37 oC, pH: 7.5 and BoNT/A antigen concentration: 4.5 fM and (B) Effect of pH on the interaction of BoNT/A antigen with the immobilized BoNT/A antibody. pH range: 4.0-9.0, temperature: 25 oC and concentration: 4.5 fM.
Effect of pH
Figure 4b shows the effect of pH on SPR angle due to the interaction of BoNT/A antigen with its immobilized antibody. It is observed from Figure 4B that SPR response exhibits a maximum at pH 7.5. This result can also be explained by considering the effect of pH on pI of BoNT/A. At pH 7.5 BoNT/A possess net positive charge due to its pI (8.6) and immobilized antibody have the negative charge due to the free carboxylic group of amino-acid, as –NH2 group of amino-acid is involved in the formation of covalent bond with –COOH group of CM5 chip. Therefore, chances of electrostatic interaction will increase and which in turn give more angle change at pH 7.5. All these observations implies that at pH 7.5, BoNT/A and its immobilized antibody interaction is more effective, hence, pH 7.5 was used for further studies.
Electrochemical impedance spectroscopic characterization
Electrochemical impedance spectroscopic study gives information on the impedance change of the electrode/solution or modified SPR disc/solution interface. The impedance can be presented as the sum of the real (Z’ ω) and imaginary (Z” ω) components that originate mainly from the resistance and capacitance of the cell and this is known as Nyquist plot [43]. In Figure 5, the semicircle diameter at higher frequency corresponds to the electron transfer limited process (Ret). Line 5a shows the AC impedance spectrum for CM5 gold disc, line 5b is AC impedance spectrum for modified gold disc immobilized with BoNT/A antibody and line 5c is AC impedance spectrum for interaction of BoNT/A with immobilized antibody. After the addition of BoNT/A the diameter of semi-circle is decreased and this is owing to the acceleration of electron transfer due to interaction of antigen with antibody. In addition to the above, Ret is most influential and direct parameter to reflect the in-situ changes those are occurring on the modified disc/electrolyte interface, hence, fit and simulation method was adopted to find out the Ret value for CM5 gold disc and antibody immobilized CM5 gold chip before and after antigen interaction, the Ret values are found to be 2.79 MΩ, 466 kΩ and 440 kΩ, respectively. The decrease in Ret value after the interaction of antigen with antibody directly confirms an increase in electron transfer due to the effective binding of antigen with antibody because of the presence of good interaction at this pH (7.5) as reported earlier for an antigen antibody interaction [44].
Conclusion
A label free real time SPR detection methodology for BoNT/A was developed using a carboxymethyldextran modified sensor chip. The effective interaction between the antigen and antibody was confirmed based on EIS data as a decrease in charge transfer resistance was observed due to the effective interaction between BoNT/A antigen and antibody. The LOD of the developed method is 0.045 fM. The SPR sensor gram of the binding of BoNT/A with immobilized SV confirmed the biological activity of purified protein. The kinetic parameters such as KD and Bmax values were calculated and found to be 0.53 fM and 38.23 mo for immobilized antibody and 0.22 fM and 116.0 mo for immobilized SV, respectively. The KD value of 0.53 fM implies and classifies this antibody as a high affinity one. The negative value of change in Gibbs free energy indicates the spontaneous nature of interaction of BoNT/A antigen with immobilized antibody and SV and the positive value of ΔH revealed the interaction of BoNT/A with immobilized antibody as endothermic. The positive value of TΔS was found to be greater than ΔH indicating the interaction of BoNT/A with its immobilized antibody as entropy driven. This study gives inputs for the development of SPR based sensors using antigen and antibody interaction for BoNT/A and other BWAs.
References
- Sakaguchi G (1982) Clostridium botulinum toxins. Pharmacol 19: 165-194
- Hambleton P(1992) Clostridium botulinum toxins: a general review of involvement in disease, structure, mode of action and preparation for clinical use. J Neurol 239:16-20
- Turton K, Chaddock JA, Acharya KR(2002) Botulinum and tetanus neurotoxtins: structure, function and therapeutic utility. Trends BiochemSci 27: 552-558.
- Lacy DB, Tepp W, Cohen AC, Gupta BRD, Stevens RC (1998) Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat StructBiol5: 898-902.
- Kitamura M,Takamiya K, AizawaS,Furukawa K(1999)Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. BiochimBiophysActa 1441: 1-3.
- Kozaki S, Kamata Y,Watarai S, Nishiki T, Mochida S (1998) Ganglioside GT1b as a complementary receptor component for Clostridium botulinumneurotoxins. MicrobPathog 25: 91-99.
- Li L, SinghBR (1998) Isolation of synaptotagmin as a receptor for types A and E botulinum neurotoxin and analysis of their comparative binding using a new microtiter plate assay. J Nat Toxins 7: 215-226.
- GinalskiK, Venclovas C, Lesyng B, FidelisK (2000)Structure-based sequence alignment for the beta trefoil subdomain of the clostridial neurotoxin family provides residue level information about the putative ganglioside binding site. FEBS Lett 482: 119-124.
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, et al. (2001) Botulinum toxin as a biological weapon: medical and public health management. JAMA 285: 1059-1070.
- Bigalke H, Rummel A(2005) Medical aspects of toxin weapons. Toxicology 214: 210-220.
- Johnson EA(2003) Bacterial Pathogens and Toxins in Foodborne Disease. In Food Safety: Contaminants and Toxins pp:25-45.
- Yowler BC, Schengrund CL (2004) Botulinum neurotoxin A conformation upon binding to ganglioside GT1b. Biochemistry 43: 9725-9731.
- Pless DD, TorresER, ReinkeEK, BavariS (2001) High affinity, protective antibodies to the binding domain of botulinum neurotoxin type A, Infection and Immunity 69: 570-574.
- Ferracci G,Miquelis R, Kozaki S, Seagar M, Leveque C (2005) Synaptic vesicles chips to assay botulinum neurotoxins. J Biochem 391: 659-666.
- Marconi S, Ferracci G, Berthomieu M, Kozaki S, Miquelis R, et al. (2008) A protein chip membrane capture assay for botulinum neurotoxin activity. Toxicology and Applied Pharmacology 233: 439-446.
- Doellgast GJ, Triscott MX, Beard GA, Bottoms JD, Cheng T, et al. (1993) Sensitive enzyme-linked immunosorbent assay for detection of Clostridium botulinum neurotoxins A, B, and E using signal amplification via enzyme-linked coagulation assay. J ClinMicrobiol 3: 2402-2409.
- Ekong TA, McLellan K, Sesardic D(1995) Immunological detection of Clostridium botulinum toxin type A in therapeutic preparations. J Immunol Methods 180: 181-191.
- Szilagyi M, Rivera VR, Neal D, Merrill GA, Poli MA (2000) Development of sensitive colorimetric capture ELISAs for Clostridium botulinum neurotoxin serotypes A and B.Toxicon 38: 381-389.
- Schmidt JJ, Stafford RG, Millard CB, (2001) High throughputassays for botulinum neurotoxin proteolytic activity: serotypes A, B, D, and F, Anal. Biochem 296 130-137.
- Chiao DJ, Shyu RH, Hu CS, Chiang HY, Tang SS (2004) Colloidal gold-based immunochromatographic assay for detection ofbotulinum neurotoxin type B. J Chromatogr B AnalytTechnol Biomed Life Sci 809: 37-41.
- Barr JR, Moura H, Boyer AE, Woolfitt AR, Kalb SR, et al. (2005)Botulinum neurotoxin detection and differentiation by mass spectrometry Emerg Infect Dis 11: 1578-1583.
- Gessler F, Hampe K, Bohnel H(2005) Sensitive detection of botulinum neurotoxin types C and D with an immunoaffinity chromatographic column test.Appl Environ Microbiol 71:7897-7903.
- Sapsford KE, Taitt CR, Loo N, Ligler FS(2005) Biosensor detection of botulinum toxoid A and staphylococcal enterotoxin B in food. Appl Environ Microbiol 71: 5590-5992.
- Sharma SK, Eblen BS, Bull RL, Burr DH, Whiting RC (2005) Evaluation of lateral flow Clostridium botulinum neurotoxin detection kits for food analysis. Appl Environ Microbiol 71: 3935-3941.
- Rivera VR, Gamez FJ, Keener WK, White JA, PoliMA (2006) Rapid detection of Clostridium botulinum toxins A, B, E, and F in clinical samples, selected food matrices and buffer using paramagnetic bead-based electrochemiluminescence detection. Anal Biochem 353: 248-256.
- Cherif B, Roget A, Villiers CL,Calemczuk R, Leroy V, Marche PN, Livache T, Villiers MB(2006) Clinically related protein–peptide interactions monitored inreal time on novel peptide chips by surface plasmon resonance imaging. ClinChem52: 255-262.
- Fitzgerald SP, Lamont JV, McConnell RI, Benchikh O, et al.(2005) Development of a high throughput automated analyzer using biochip array technology. ClinChem 51: 1165-1176.
- LaddaJ, Taylor AD, HomolaJ, Jiang S (2008) Sens Actuators B. 130: 129-134.
- Gupta G, Singh PK, Boopathi M, Kamboj DV, Singh B, et al. (2010) Surface Plasmon resonance detection of biological warfare agent Staphylococcal enterotoxin B using high affinity monoclonal antibody. Thin Solid Films 519:1171-1177.
- Gupta G, Singh PK, Boopathi M, Kamboj DV, Singh B, et al. (2010) Molecularly imprinted polymer for the recognition of biological warfare agent Staphylococcal enterotoxin B based on surface Plasmon resonance, Thin Solid Films 519:1115-1121.
- Singh PK, Agrawal R, Kamboj DV, Gupta G, Boopathi M, et al. (2010) Construction of SCVF antibody against Staphylococcal superantigen SEB. Appl Environ Microbiol.
- Potter KJ, Bevins MA, Vassilieva EV, Chiruvolu VR, Smith TJ, et al. (1998) Production and purification of the heavy chain fragment C of botulinum neurotoxin serotype B expressed in the methylotropic yeast Pichiapastoris. Protein ExprPurif13: 357-65.
- Canziani GA, Klakamp S, Myszka DG(2004) Kinetic screening of antibodies fromcrude hybridoma samples using Biacore. AnalBiochem 325:301-307.
- Safsten P, Klakamp SL, Drake AW, Karlsson R, Myszka DG(2006) Screeningantibody–antigen interactions in parallel using Biacore A100. Anal Biochem353: 181-190.
- Tsai WC, LiIC (2009) SPR-based immunosensor for determining staphylococcal enterotoxin A.Sens Actuators B Chem 136: 8-12.
- Stenberg E, Persson B, Roos H, Urbaniczky C (1991) Quantitative determination of surface concentration of protein with surface plasmon resonance using radiolabeled proteins.J Coll Interf Sci 143: 513-526.
- LiuJT, Chen LY, Shih MC, Chang Y, Chen WY(2008) Anal Biochem 375: 90-96.
- Wassaf D, Kuang G, Kopacz K, Wu QL, Nguyen Q, et al. (2006) High-throughput affinity ranking of antibodies using surface Plasmon resonance microarrays. Anal Biochem 351: 241-253.
- Savara A, Schmidt CM, Geiger FM, Weitz E (2009) Adsorption Entropies and Enthalpies and Their Implications for Adsorbate Dynamics.J Phys Chem C 113: 2806-2815.
- GlasstoneSD (1947) Thermodynamics for chemists. Van Nostrand Company New Yorkpp: 288.
- Cabilio NR, Omanovic S, Roscoe SG (2000)Electrochemical Studies of the Effect of Temperature andpH on the Adsorption of rLactalbumin at Pt.Langumir 16:8480-8488.
- Gregory RB (1995) Protein Solvent Interactions. Marcel Dekker Inc New York.
- Bard AJ, Faulkner LR(1980) Electro-chemical methods: Fundamentals and Applications Wiley & Sons New Yorkpp: 368.
- Darain F, Park DS, Park JS, Shim YB(2004) Development of an immunosensor for the detection of vitellogenin using impedance spectroscopy.BiosensBioelectron 19: 1245-1252.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 11653
- [From(publication date):
June-2016 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10730
- PDF downloads : 923