Surface Immobilization of Antibacterial Quorum Sensing Inhibitors by Photochemical Activation
Received: 21-Jul-2016 / Accepted Date: 30-Jul-2016 / Published Date: 06-Aug-2016 DOI: 10.4172/2155-952X.1000238
Abstract
Infection of implanted medical devices is one of the major causes of nosocomial infections. A significant proportion of the devices become colonized by bacterial biofilms, thus resulting in high morbidity and risk of mortality. This study focuses on the non-specific covalent attachment of potent quorum sensing (QS) and biofilm inhibiting compounds, furanones (FUs) and dihydropyrrol-2-ones (DHPs), onto glass surfaces by azide/nitrene chemistry. The attachment of FUs and DHPs was confirmed by X-ray photoelectron spectroscopy (XPS) and contact angle measurements. The modified surfaces were then assessed for their antibacterial efficacy against Staphylococcus aureus and Pseudomonas aeruginosa using confocal laser scanning microscopy (CLSM). Both FU and DHP coated surfaces were able to significantly reduce bacterial adhesion (p<0.001) with p-bromophenyl substituted DHP giving maximum reductions of up to 93% and 71% against S. aureus and P. aeruginosa, respectively. Therefore, photoimmobilization of QS inhibitors is an effective technique to produce novel antibacterial biomaterial surfaces.
Keywords: Biomaterial, Antibacterial, Quorum sensing, Surface modification, Furanone, Dihydropyrrolone, Pseudomonas aeruginosa, Staphylococcus aureus
249265Abbreviations
HAI: Hospital-Acquired Infection; DHP: Dihydropyrrol-2-one; FU: Furanone; QSI: Quorum Sensing Inhibitor; AI: Autoinducer; APTS: 3-Aminopropyltriethoxysilane; ABA: 4-Azidobenzoic Acid; EDC: 1-Ethyl-3-(3-Dimethyl Aminopropyl)Carbodiimide Hydrochloride; NHS: N-Hydroxysuccinimide; XPS: X-Ray Photoelectron Spectroscopy; TSB: Tryptone Soya Broth; PBS: Phosphate Buffered Saline; OD: Optical Density; AHL: N-Acyl Homoserine Lactone; 3-Oxo-C12-HSL: N-(3-Oxododecanoyl)-L-Homoserine Lactone
Introduction
Infection of commonly used medical devices such as catheters, cardiac pacemakers, intraocular lenses, dental implants, accounts for 60-70% of all hospital acquired infections (HAIs) [1]. The cost for treatment ranges between $28-45 billion per annum in United States alone [2]. Duration of hospital stay, mortality and morbidity are also increased when infections are caused by multi-drug resistant bacteria [3-5]. With no effective therapies currently available, device-related infections are extremely difficult to treat. It has been estimated that about 80% of these infections are associated with biofilm formation on medical devices [2]. Biofilms on implants are upto 1000-fold more resistant to antibiotics when compared to their planktonic counterpart [6,7]. Therefore, the prevention of biofilm formation on biomaterials is a preferable strategy than treatment.
Various strategies to control the formation of biofilm on medical devices have been examined, including coating antibiotics such as norfloxacin [8], minocycline-rifampin [9], impregnating chlorhexidine [10], silver [11,12], and gendine [13] on the surface of the implants. However, these coatings may reduce infections only over a relatively short time frame as they are commonly dependent on the release of the antimicrobial for activity. A comparative study revealed that chlorhexidine, silver and minocycline-rifampin coated catheters lost their antibacterial activity within 28 days [14]. In a clinic trial, central venous catheters impregnated with silver had no significant effect in controlling bacterial colonization, bloodstream infections and ICU mortality [15]. Thus, there is an urgent need to develop new strategies to prevent bacterial infection on biomedical devices.
A marine alga, Delisea pulchra, from Australia produces halogenated furanone (FU) compounds [16,17] that have the ability to inhibit fouling by other marine organisms by blocking the bacterial communication pathway known as quorum sensing (QS). QS is a process where bacteria use various autoinducers (AI) or small signalling molecules to communicate with each other. This process plays an important role in controlling behavioural activities of bacteria such as the formation of biofilm and virulence factors. Halogenated FUs competitively bind to receptor proteins and displace the signalling molecules [18-20]. This can result in inhibition of biofilm formation of Gram-negative bacteria. However, most of the natural FUs are toxic to human cells, thus limiting their use [21]. Therefore, a range of FU analogues having low cytotoxicity have been synthesized which maintain excellent activity against Gram-negative and Gram-positive bacteria [22,23]. A few synthetic FUs have also been immobilized via covalent attachment on biomaterial surfaces and which showed good biofilm inhibitory activities in vitro and in vivo [24,25]. Halogenated FUs have also been attached to surfaces by a non-specific covalent attachment strategy; however, the activity of the compounds after attachment was not reported [26,27].
Structural analogues of FUs, dihydropyrrol-2-ones (DHPs) [28], also displayed excellent QS inhibiting activity with low cytotoxicity in solution as well as after specific covalent attachment on surfaces [29- 31]. However, a direct comparison of the activity of FUs and DHPs is lacking. Therefore, to better compare the effectiveness of FU and DHP immobilization strategies, the same techniques should be used to evaluate their activities.
In this study, DHPs and FUs were immobilized on azidefunctionalized surfaces by photoactivation under UV light. The antibacterial efficacy of the coated surfaces was assessed against two common pathogenic bacteria, Pseudomonas aeruginosa and Staphylococcus aureus. The attachment efficiency and antibacterial activity of the resulting surfaces were also compared with previously developed coatings where DHPs were attached via Michael addition reaction.
Materials and Methods
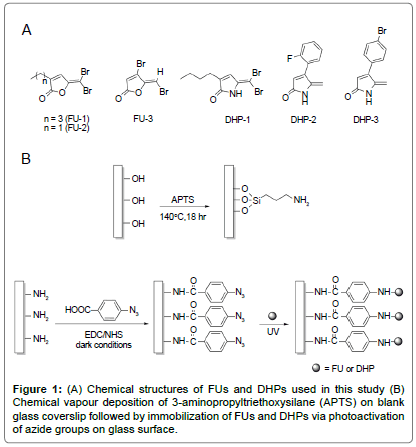
Attachment of 4-azidobenzoic acid (ABA)
Glass coverslips (No. 1, diameter 13 mm D 263 M glass, ProSciTech, Australia) were first cleaned in freshly prepared piranha solution (3:1 v/v concentrated sulphuric acid to 30% hydrogen peroxide) at 100°C for 1 h. After thorough rinsing with distilled water, the clean coverslips were rinsed once with absolute ethanol and air-dried. The substrates were then silanized according to the previously developed method [30]. Briefly, the clean substrates were placed on steel mesh within a glass vessel that contained a 3-aminopropyltriethoxysilane (APTS) solution (10% v/v in dry toluene; 1 ml). The glass vessel was sealed and heated at 140°C for 18 h. The coverslips were rinsed with dry toluene (x2), absolute ethanol and air-dried. The APTS-coated coverslips were then immersed in a solution of 4-azidobenzoic acid (ABA; 49.0 μM), 1-ethyl- 3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC, 245.2 μM) and N-hydroxysuccinimide (NHS, 98.0 μM) in absolute ethanol (1.5 ml), and agitated overnight at room temperature under dark room conditions (Figure 1B). The ABA-functionalized surfaces were rinsed twice with absolute ethanol and once with MilliQ water, air-dried and stored under dark conditions before use.
Attachment of FU and DHP
The synthetic halogenated FU compounds (FU-1, 2 and 3; Figure 1A) were synthesized as described [32]. Similarly, DHP compounds (DHP-1, 2 and 3; Figure 1A) were synthesized following the method developed previously by Kumar and Iskander [29].
Stock solutions of FU (25 mg/ml in dichloromethane) and DHP (25 mg/ml in acetone) were prepared and 200 μl of the FU or DHP solution was placed onto the ABA glass surface. After complete evaporation of the solvent, the surfaces were irradiated under UV at 320 nm for 10 min in a CL-1000 Cross-linker (Ultra-Violet Products Ltd, Upland, CA, USA) (Figure 1B). The unreacted FU and DHP were removed by extensively washing the samples with dichloromethane and acetone respectively, MilliQ water and absolute ethanol, then air dried and stored in clean sterile container.
X-ray photoelectron spectroscopy (XPS)
The surfaces were characterized using X-ray photoelectron spectroscopy (XPS; ESCALAB220-iXL, VG Scientific, West Sussex, England). The X-ray source was monochromated Al Kα and the photoenergy was 1486.6 eV with a source power of 120 W. The vacuum pressure was ≤ 10-8 mbar.
Contact angle measurements
Contact angles were determined using a contact angle goniometer (Rame-Hart, Inc. NRL USA, Model no. 100-00). Multiple drops of deionized water were placed on each surface using a micro-syringe. The angle between the droplet and the surface was measured using a 50 mm Cosmicar Television Lens (Japan). Rame-Hart Imaging software was used to calculate the contact angle. A minimum of fifteen measurements were made on five samples of each FU and DHP.
Antibacterial activity
Bacteria (Staphylococcus aureus SA38 and Pseudomonas aeruginosa PA01) from frozen stock (-80°C) were streaked on chocolate agar (Oxoid, UK) and incubated at 37°C overnight. A colony of the bacteria was taken from the plate and cultured overnight at 37°C in 15 ml tryptone soya broth (TSB; Oxoid, UK). The bacteria were washed twice with fresh TSB by centrifugation. The optical density (OD) of the culture was adjusted to OD660=0.1 which corresponds to 1 × 108 cfu/ml.
In a 12-well plate, the surfaces to be tested were first sterilized with 70% w/v ethanol for 30 min, then thoroughly washed with sterile phosphate buffered saline (PBS) three times and finally placed in 4 ml of the adjusted bacterial culture. The surfaces were incubated at 37°C for 24 h. The media was then replaced by fresh TSB (4 ml) and further incubated for 24 h at 37°C. Subsequently, the samples were washed twice with PBS before examination by fluorescence microscopy.
Bacterial adhesion analysis
The glass samples with adherent bacterial cells were stained with Live/Dead BacLight Bacterial Viability Kit (Molecular Probes, Inc., OR, USA) according to the manufacturers’ procedure and as described in the literature for analysis of biofilms on surfaces [30,31]. Bacteria were then fixed by adding 100 μl of 4% formaldehyde on each sample. Microscopic observation and image acquisition were performed with Olympus FV1200 Confocal Microscope. Images from 10 representative areas on each of triplicate samples for each surface were taken and analysed using ImageJ software [33]. The image analysis results were reported as the average percentage coverage of live and dead cells in the fields of view.
Statistical analysis of data
Further analysis of the data was done by the one-way analysis of variance (ANOVA) using GraphPad Prism 6.05 software. Post hoc multiple comparisons were done using Tukey correction. Statistical significance was set at 5%.
Results
XPS characterization of the coated surfaces
The surfaces were characterized by XPS at each step of the immobilization sequence to ensure surfaces were successfully modified. The XPS data collected for the blank (untreated), APTS, ABA, FU and DHP coated surfaces are summarized in Table 1.
| % C | % N | % Halogen | Contact Angle (°) | |
|---|---|---|---|---|
| Blank | 7.6 | 0.5 | - | 20 |
| APTS | 43.9 | 7.1 | - | 73 |
| ABA | 46.2 | 8.1 | - | 67 |
| FU-1 | 48.8 | 7.3 | 0.74% Br | 70 |
| FU-2 | 49.0 | 7.7 | 0.41% Br | 60 |
| FU-3 | 49.7 | 7.1 | 0.65% Br | 65 |
| DHP-1 | 47.9 | 7.7 | 0.17% Br | 71 |
| DHP-2 | 48.5 | 8.7 | 0.32% F | 69 |
| DHP-3 | 48.7 | 8.5 | 0.35% Br | 74 |
Table 1: XPS analysis and contact angle measurements of blank, ABA, FU and DHP coated surfaces.
Changes in the elemental composition of carbon, nitrogen and halogen indicate successful immobilization of FU and DHP on the surfaces (Table 1). After functionalization with APTS, the carbon and nitrogen percentages increased to 43.9% and 7.1% respectively when compared to the blank glass (4.9% C, 0.5% N). Both the carbon and nitrogen content increased even further to 46.2% and 8.1% respectively when ABA was coupled with the amine surface. The subsequent attachment of FU or DHP was confirmed by a further increase in carbon percentage. Finally, the detection of halogens from the FU and DHP compounds further confirmed the attachment of FUs and DHPs (0.74-0.41% Br for FUs, 0.17% Br for DHP-1, 0.32% F for DHP-2 and 0.35% Br for DHP-3).
Analysis of the high-resolution C1s spectra of the APTS surface demonstrated the presence of three distinct components C-H/C-C, C-N and C=O at binding energies 284.9 eV, 286.1 eV and 288.2 eV respectively. The N1s spectrum of the APTS surface showed two peaks at 399.6 eV and 401.4 eV corresponding to –NH2 and –NH3 + respectively. After the subsequent attachment of ABA, two new additional peaks in a 2:1 ratio emerged at 400.2 eV and 404.6 eV, which is a set of characteristic peaks attribute to the azide functionality [34–36]. The peak at 400.2 eV was assigned to the two terminal nitrogen atoms of the azide and the peak at 404.6 eV was assigned to the central nitrogen atom because of its low electron density compared to the terminal nitrogen atoms. Furthermore, an additional peak at 289 eV (N-C=O) in the carbon narrow scan was observed indicating that the coupling reaction between the amine-terminated surface and carboxylic acid of ABA successfully formed an amide bond. The characteristic azide peaks were not observed after the subsequent treatment of the ABA surface with FU or DHP, suggesting the azide functional groups were consumed for the covalent linkage of FUs and DHPs. Instead, the N1s spectra showed a peak corresponding to N-H at 399.5 eV which is consistent with the formation of an –NH2 group on photo-activating the azide, and also due to various side reactions of arylazides under UV light [37]. Furthermore, a shift in the peak for N-C=O (from 289.0 eV to 288.7 eV) in the C1s spectra was also observed for all FU and DHP coated surfaces along with broadening of the band, possibly due to addition of C-Br or C-F, indicating successful attachment of FU and DHP.
Contact angle measurements
The modified surfaces were also characterized by determining the contact angle after every modification step (Table 1). A significant change in contact angle was observed for APTS surface (from 20° to 73°), indicating an increase in surface hydrophobicity due to the aliphatic carbon chain of APTS which is hydrophobic in nature. The contact angle remained approximately the same (67°) after surface attachment of ABA, which is expected due to the presence of the hydrophobic aromatic ring in ABA. Subsequent attachment of FU and DHP resulted in similar contact angles to the ABA surfaces, due to the presence of different hydrophobic moieties (alkyl chain, phenyl ring and halogen atoms) on the FU and DHP compounds.
Antibacterial activity
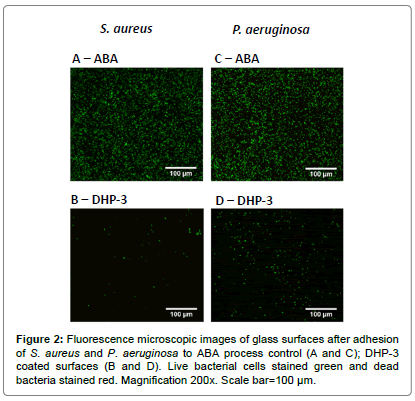
The adhesion of S. aureus and P. aeruginosa on the modified surfaces were evaluated using fluorescence microscopy, and representative images are shown in Figure 2. The total surface area covered by bacteria and the relative proportion of live and dead bacteria (stained green and red respectively) were evaluated by image analysis and the results are shown in Figure 3.
Figures 2A and 2B show microscopy images of live (green) and dead (red) S. aureus cells adhered to coated and control surfaces, where extensive colonization and biofilm formation can be seen on the ABA control (Figure 2A). Figures 2C and 2D show images for adhesion of P. aeruginosa on the surfaces. Both strains of bacteria displayed similar level of bacterial colonization on the ABA control surface (Figures 2A and 2C). The adhesion of both strains of bacteria on blank and APTS glass (data not shown) was similar to that reported in literature [30]. The bacterial coverage of both strains on all the FU and DHP coated surfaces was significantly lower than the process control.
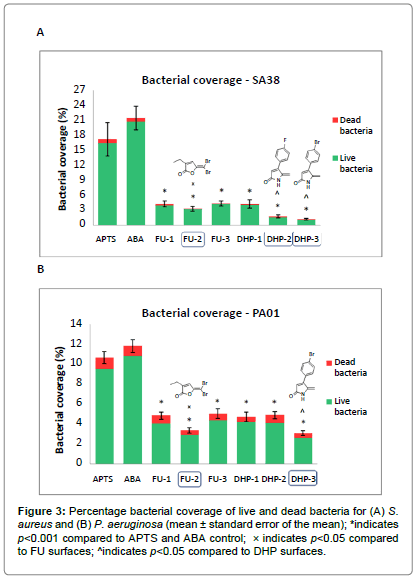
The image analysis results for S. aureus showed significant reductions in overall bacterial coverage of 75.4 ± 5.0%, 80.9 ± 4.1%, 74.8 ± 4.2% for FUs 1-3 and 75.6 ± 5.8%, 89.9 ± 1.6%, 93.4 ± 1.1% for DHPs 1-3 respectively compared to the ABA control surface (p<0.001) (Figure 3A). Amongst these, the most effective compounds were FU- 2, DHP-2 and DHP-3, which displayed comparatively lower bacterial coverage than other coated surfaces (p<0.05). There was no significant difference in the percentage of bacterial cells stained red (dead bacteria) between the controls and modified surfaces.
The attachment of P. aeruginosa on the FU and DHP coated surfaces was found to be significantly lower than the control, with reductions of 54.8 ± 2.2%, 68.7 ± 1.7%, 52.9 ± 3.0% for FU 1-3 and 55.9 ± 2.8%, 54.3 ± 2.1%, 71.23 ± 1.4% for DHP 1-3 respectively (p<0.001) (Figure 3B). In this case, FU-2 and DHP-3 gave maximum reduction in bacterial attachment compared to other FU and DHP surfaces (p<0.05). Similar to S. aureus, no significant difference was observed in the percentage of dead cells between the control and modified surfaces.
Discussion
Tens of millions of medical devices are used each year, and in spite of advances in biomaterial technologies, a significant proportion of the devices are colonized by bacterial biofilms, resulting in device failure and infections. The formation of biofilms on biomedical devices is therefore a serious problem that is very difficult to treat.
In the present study, various potent QS inhibiting compounds, FUs and DHPs, were covalently immobilized on glass surfaces by a nonspecific attachment strategy and the antibacterial efficacy of the resultant surfaces was assessed. XPS analysis indicated the successful attachment of FUs and DHPs via the described photoactivation strategy with FUs having slightly higher attachment efficiency compared to the DHPs. All the covalently bound FUs and DHPs were able to significantly reduce colonization of both Gram-positive (S. aureus) and Gram-negative (P. aeruginosa) bacteria on the surfaces. Surfaces immobilized with FU-2, DHP-2 and DHP-3 were found to be the most potent against bacterial adhesion of S. aureus, whereas for P. aeruginosa, the most active surfaces were coated with FU-2 and DHP-3. While all compounds were effective in reducing bacterial adhesion, FU-2 and DHP-3 displayed the best broad spectrum antibacterial activity. The high level of reduction in adherent bacteria displayed by DHP-3 (93% and 71% of reduction against S. aureus and P. aeruginosa respectively) is consistent with reports in the literature [29,30]. A previous study has also examined the efficacy of DHP-3 by covalently grafting it on the surface via a Michael addition reaction [30]. A higher surface concentration of DHP-3 was achieved via the non-specific azide reaction (0.35% Br) described in this study than by the Michael addition reaction (0.21% Br).
Numerous studies have demonstrated that an increase in surface concentration of an active compound leads to better antibacterial activity [25,38]. Surprisingly, in this study DHP surfaces have displayed potent activity even at low concentration. Among all the compounds used in this study, DHP-1 gave the least attachment (0.17% Br) to the surface but displayed a similar level of activity as FU-1 (p<0.05) which gave a maximum attachment (0.74% Br). This discrepancy could be due to the orientation of DHP on the surface, making it more available for antimicrobial activity compared to a similar concentration of FU. Similarly, FU-3 was expected to display maximum efficacy amongst all the FUs due to its high activity in solution and also high attachment efficiency (0.65% Br) [22,39]. Instead, FU-2 displayed the best activity out of all FUs at lower surface concentration (0.41% Br) with reductions of 81% and 69% of adherent S. aureus and P. aeruginosa respectively, while FU-3 displayed reductions of 74% and 52% for S. aureus and P. aeruginosa respectively.
Several strategies have been explored in the past to immobilize QS inhibiting compounds on the surfaces to inhibit biofilm formation [31,40,41]. For example, a furanone derivative has been physically adsorbed on various polymer surfaces commonly used for medical devices [40]. However, such a non-uniform coating is highly prone to leaching and gradual loss of the active compound. In another approach, FUs and DHPs were coated on surfaces via specific attachment strategies [30,31,41]. Although this attachment strategy overcomes the limitations of uneven coating and leaching, it requires extensive modification of the compound for surface attachment. Any structural change or modification of the active compound may also result in decrease in activity. The non-specific attachment strategy employed in this study does not require structural modification or functionalization of the compound. Also, unlike the previous attachment strategies, the azide reaction described in this study is much faster, making it easier and more convenient to implement. This study is the first to investigate the antimicrobial activity of photo-immobilized DHPs on surfaces.
DHPs act by interfering with the bacterial QS system. In particular, DHPs are able to disrupt the N-acyl homoserine lactones (AHL) regulated QS system in Gram-negative bacteria [29]. The mechanism through which DHPs inhibit QS is postulated to be similar to that of FUs, that is, via displacing the AHL signal from the receptor site without affecting bacterial growth [19,39,42,43]. Surface immobilized DHPs were capable of interfering with the AHL regulated las QS system in P. aeruginosa, thereby inhibiting biofilm formation [31].
In the current study, about 97% of adherent bacteria on the coated surfaces were alive, supporting the previous data that FUs and DHPs act without killing the bacteria and exerting no selective pressure on the bacteria to develop resistance [24,30]. Therefore, it is likely that the FU and DHP surfaces generated in this study act through the same mechanism of action for Gram-negative bacteria. On the other hand, for Gram-positive bacteria, the mode of action of FU and DHP is still not fully understood. Research investigating the effect of the AHL, N-(3-oxododecanoyl)-L-homoserine lactone (3-oxo-C12- HSL), on S. aureus showed that the mode of action of 3-oxo-C12-HSL involves inhibition of the agr-dependent QS system by binding to the cytoplasmic membrane of S. aureus [44]. Similarly, two new classes of compounds recently identified, one derived from 3-oxo AHLs and other from 3-acyl tetronic acids, have displayed agr QS inhibitory activity in S. aureus [45]. In another study, the mechanism of action of a derivative of AHL was found to be through the dissipation of the membrane potential and pH gradient of S. aureus and Bacillus cereus [46]. Therefore, it is possible that FUs and DHPs, which are structurally related to AHLs, inhibit QS of Gram-positive bacteria via an indirect approach through the interaction with the bacterial cell membrane.
In the current study, we have demonstrated an effective and versatile technique for the immobilization of QS inhibitors as antibacterial coatings. All the FU and DHP coated surfaces were able to reduce adhesion of S. aureus and P. aeruginosa, the most common pathogens associated with biomaterial infections. This suggests that the non-specific attachment of FUs and DHPs to the highly reactive azide groups does not impair the antibacterial activity of the compounds, indicating that the compounds retain their activity even after attachment. Since prior functionalization of compounds was not needed, it is a fast and easy technique for developing novel coatings for prevention of infections of biomedical devices.
Acknowledgement
This work was supported by a Discovery Project from Australian Research Council grant (DP 140102195). AT was supported by an Australian Postgraduate award. The authors would like to thank Dr. Bill Gong at the University of New South Wales Mark Wainwright Analytical Centre (UNSW MWAC) for the XPS measurements, and the Biomedical Imaging Facility at UNSW MWAC for assistance with the confocal laser scanning microscopy.
Disclosure Statement
Authors have no financial interest or benefit arising from the direct applications of their research.
References
- Scott II RD (2009) The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention.
- Cosgrove SE (2006) The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay and health care costs. Clin Infect Dis 42 Suppl 2: S82-89.
- Cruickshank M, Ferguson J (2008) Reducing harm to patients from health care associated infection? The role of surveillance. Australian Commision on Safety and Quality in Health Care.
- Australian Goverment-National Health and Medical Research Council (2010) Australian Guidlines for the prevention and control of infection in healthcare-executive summary.
- Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2: 114-122.
- Smith AW (2005) Biofilms and antibiotic therapy: Is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev 57: 1539-1550.
- Garty S, Shirakawa R, Warsen A, Anderson EM, Noble ML, et al. (2011) Sustained antibiotic release from an intraocular lens-hydrogel assembly for cataract surgery. InvestigOphthalmol Vis Sci 52: 6109–6116.
- Raad I,Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, et al. (1997) Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. The Texas Medical Center Catheter Study Group. Ann Intern Med 127: 267-274.
- Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, et al. (2005) Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials 26: 7145-7153.
- FazlyBazzaz BS,Khameneh B,Jalili-Behabadi MM,Malaekeh-Nikouei B,Mohajeri SA (2014) Preparation, characterization and antimicrobial study of a hydrogel (soft contact lens) material impregnated with silver nanoparticles. Cont Lens Anterior Eye 37: 149-152.
- Wang R,Neoh KG, Kang ET, Tambyah PA, Chiong E (2015) Antifouling coating with controllable and sustained silver release for long-term inhibition of infection and encrustation in urinary catheters. J Biomed Mater Res B ApplBiomater 103: 519-528.
- Bahna P, Dvorak T, Hanna H, Yasko AW, Hachem R, et al. (2007) Orthopaedic metal devices coated with a novel antiseptic dye for the prevention of bacterial infections. Int J Antimicrob Agents 29: 593-596.
- Hanna H,Bahna P, Reitzel R, Dvorak T, Chaiban G, et al. (2006) Comparative in vitro efficacies and antimicrobial durabilities of novel antimicrobial central venous catheters. Antimicrob Agents Chemother 50: 3283-3288.
- Antonelli M, De Pascale G, Ranieri VM, Pelaia P, Tufano R, et al. (2012) Comparison of triple-lumen central venous catheters impregnated with silver nanoparticles (AgTive®) vs. conventional catheters in intensive care unit patients. J Hosp Infect 82: 101-107.
- deNys R, Wright AD, König GM, Sticher O (1993) New halogenated furanones from the marine alga Deliseapulchra (cf. fimbriata). Tetrahedron 49: 11213–11220.
- de Nys R, Steinberg PD, Willemsen P, Dworjanyn SA, Gabelish CL, et al. (1995) Broad spectrum effects of secondary metabolites from the red alga Deliseapulchra in antifouling assays. Biofouling 8: 259–271.
- Kjelleberg S, Steinberg P, Givskov M, Gram L, Manefield M, et al. (1997) Do marine natural products interfere with prokaryotic AHL regulatory systems? AquatMicrobEcol 13: 85–93.
- Manefield M, de Nys R, Kumar N, Read R, Givskov M, et al. (1999) Evidence that halogenated furanones from Deliseapulchra inhibit acylatedhomoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145: 283-291.
- Ren D, Sims JJ, Wood TK (2001) Inhibition of biofilm formation and swarming of Escherichia coli by (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone. Environ Microbiol 3: 731-736.
- Reichelt JL, Borowitzka MA (1984) Antimicrobial activity from marine algae: Results of a large-scale screening programme. Hydrobiologia 22: 158–168.
- Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, et al. (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22: 3803-3815.
- Kjelleberg S, Steinberg PD, Holmstrom C, Back A (2006) Inhibition of gram positive bacteria. United States patent US 7,026,353.
- Baveja JK, Li G, Nordon RE, Hume EBH, Kumar N, et al. (2004) Biological performance of a novel synthetic furanone-based antimicrobial. Biomaterials 25: 5013–5021.
- Hume EBH, Baveja J, Muir B, Schubert TL, Kumar N, et al. (2004) The control of Staphylococcus epidermidisbiofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials 25: 5023–5030.
- Al-Bataineh SA, Britcher LG, Griesser HJ (2006) XPS characterization of the surface immobilization of antibacterial furanones. Surf Sci 600: 952–962.
- Al-Bataineh SA,Luginbuehl R, Textor M, Yan M (2009) Covalent immobilization of antibacterial furanones via photochemical activation of perfluorophenylazide. Langmuir 25: 7432-7437.
- Goh WK, Iskander G, Black DS, Kumar N (2007) An efficient lactamization of fimbrolides to novel ,5-dihydropyrrol-2-ones. Tetrahedron Lett 48: 2287–2290.
- Ho KK, Cole N, Chen R, Willcox MD, Rice SA, et al. (2010) Characterisation and in vitro activities of surface attached dihydropyrrol-2-ones against gram-negative and gram-positive bacteria. Biofouling 26: 913-921.
- Ho KKK, Chen R, Willcox MDP, Rice SA, Cole N, et al. (2014) Quorum sensing inhibitory activities of surface immobilized antibacterial dihydropyrrolones via click chemistry. Biomaterials 35: 2336–2345.
- Manny AJ, Kjelleberg S, Kumar N, de Nys R, Read RW, et al (1997) Reinvestigation of the sulfuric acid-catalysed cyclisation of brominated 2-alkyllevulinic acids to 3-alkyl-5-methylene-2(5H)-furanones. Tetrahedron 53: 15813–15826.
- Abrà moff MD, Magalhães PJ, Ram SJ (2004) Image processing with imageJ. BiophotonicsInt 11: 36–41.
- Wollman EW, Kang D, Frisbie CD, Lorkovic IM, Wrighton MS (1994) Photosensitive self-assembled monolayers on gold: Photochemistry of surface-confined aryl azide and cyclopentadienylmanganesetricarbonyl. AmChemSoc 116: 4395–4404.
- Sano M, Wada M, Miyamoto A, Yoshimura S (1996) Substitution reactions on a block copolymer film tethered to a solid surface by epitaxy. Thin Solid Films 284-285: 249–251.
- Prakash S, Long TM, Selby JC, Moore JS, Shannon MA (2007) "Click" modification of silica surfaces and glass microfluidic channels. Anal Chem 79: 1661-1667.
- Gritsan NP,Platz MS (2006) Kinetics, spectroscopy and computational chemistry of arylnitrenes. Chem Rev 106: 3844-3867.
- Chen R, Cole N, Willcox MDP, Park J, Rasul R, et al. (2009) Synthesis, characterization and in vitro activity of a surface-attached antimicrobial cationic peptide. Biofouling 25: 517–524.
- Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, et al. (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148: 1119-1127.
- Baveja JK,Willcox MD, Hume EB, Kumar N, Odell R, et al. (2004) Furanones as potential anti-bacterial coatings on biomaterials. Biomaterials 25: 5003-5012.
- Zhu H, Kumar A, Ozkan J, Bandara R, Ding A, et al. (2008) Fimbrolide-coated antimicrobial lenses: Their in vitro and in vivo effects. Optom Vis Sci 85: 292-300.
- Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, et al. (1996) Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol 178: 6618-6622.
- Rasmussen TB,Manefield M, Andersen JB, Eberl L, Anthoni U, et al. (2000) How Deliseapulchrafuranones affect quorum sensing and swarming motility in Serratialiquefaciens MG1. Microbiology 146 Pt 12: 3237-3244.
- Qazi S, Barry M, Muharram SH, Cockayne A, Hill P, et al. (2006) N-Acylhomoserine lactones antagonize virulence gene expression and quorum sensing in Staphylococcus aureus. Infect Immun 74: 910–919.
- Murray E, Crowley R, Truman A, Clarke S, Cottam J, et al. (2014) Targeting Staphylococcusaureus quorum sensing with non-peptidic small molecule inhibitors. J Med Chem 57: 2813–2819.
- Lowery CA, Park J, Gloeckner C, Meijler MM, Mueller RS, et al. (2009) Defining the mode of action of tetramic acid antibacterials derived from Pseudomonas aeruginosa quorum sensing signals. J Am ChemSoc 131: 14473-14479.
Citation: Taunk A, Ho KKK, Iskander G, Willcox MDP, Kumar N (2016) Surface Immobilization of Antibacterial Quorum Sensing Inhibitors by Photochemical Activation. J Biotechnol Biomater 6:238. DOI: 10.4172/2155-952X.1000238
Copyright: © 2016 Taunk A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 13880
- [From(publication date): 9-2016 - Jul 06, 2025]
- Breakdown by view type
- HTML page views: 12792
- PDF downloads: 1088