Research Article Open Access
Supplemental Red Alga, Gracilaria vermiculophylla, from a Brackish Japanese Lake, Strengthens Egg shells and Improves the Haugh unit of Eggs in Laying Hens
Hiroaki Ozaki1, Masanori Kawahara2, Ryuichiro Nogami2, YuzoYamada2 and Hideaki Takahashi3*
1Tottori Swine and Poultry Experiment Station, Nanbu-cho, Tottori 683-0361, Japan
2Oils and Fats Fundamental Technology Laboratory, J-Oil Mills, Inc., Yokohama 230-0053, Japan
3Animal Breeding and Reproduction Research Division, National Institute of Livestock and Grassland Science, Tsukuba 305-0901, Japan
- *Corresponding Author:
- Hideaki Takahashi
Animal Breeding and Reproduction Research Division
National Institute of Livestock and Grassland Science
Tsukuba 305-0901, Japan
Tel: +81298388623
Fax: +81298388606
E-mail: naoe@affrc.go.jp
Received Date: October 15, 2013; Accepted Date: December 18, 2013; Published Date: December 23, 2013
Citation: Ozaki H, Kawahara M, Nogami R, Yamada Y, Takahashi H (2013) Supplemental Red Alga, Gracilaria vermiculophylla, from a Brackish Japanese Lake, Strengthens Egg shells and Improves the Haugh unit of Eggs in Laying Hens. J Fisheries Livest Prod 2:110. doi: 10.4172/2332-2608.1000110
Copyright: © 2013 Ozaki H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Fisheries & Livestock Production
Abstract
The red alga, Gracilaria vermiculophylla (called Ogonori in Japanese), is overabundant in the brackish Nakaumi- Lake, fifth-largest lake for surface area in Japan. The algal decomposing caused the lake water pollution. The aim of the present study was to examine if the Ogonori can be used as food additive for laying hens, for this reason, a crossbreed between Japanese Game sires and Rhode Island Red dams was used. Thirty hens were randomly chosen from the cross bred birds and evenly distributed into two groups: control and Ogonori group at 63 weeks of age. The control group was alimented with a diet formulated to satisfy the nutrients requirements of the Japanese Feeding Standard for Poultry. For the Ogonori group, the algae were harvested from Nakaumi Lake, washed in fresh water, sun-dried, freezedried, and ground to a meal. Ogonori meal was added to the control diet as 2% of fresh matter. Each chicken was fed with 140 g/day from 63 to 65 weeks (wks.) of age. Egg traits were measured, including egg weight, eggshell weight, eggshell thickness, yolk weight, albumen height, eggshell strength, and yolk color, for the first three eggs obtained each individual from 64 to 65 wks. Haugh unit, egg specific gravity, and albumen weight were also calculated. Eggshell strength and thickness were significantly higher in the Ogonori group than in the control. Albumen height and Haugh unit were significantly higher in the Ogonori group than in the control, although there was no significant difference in albumen weight between the two groups. These data suggest that Ogonori can be used as a feed additive for laying hens to improve the economically important egg traits. The mineral profile of Ogonori meal suggested an association between eggshell and albumen traits and high contents of minerals (manganese, iron, chromium, and aluminum).
Keywords
Chicken; Laying hens; Feed additive; Gracilaria vermiculophylla; Eggshell; Haugh unit
Introduction
The Chugoku Region is a wide area extending westward from Osaka in Honshu Island, Japan. It is further divided into the northern Sanin and southern Sanyo Regions by the Chugoku mountain range that runs horizontally from east to west. Nakaumi Lake, a brackish water body, is the fifth largest lake (86.8 km2 area, mean depth 5.4 m) in Japan and is located at the boundary of Tottori and Shimane Prefectures in the central part of the Sanin Region [1]. Since Nakaumi Lake is separated from the Japan Sea by the Yumigahama sandbar, the lake is classified as coastal lagoons. Nakaumi Lake is connected to the Japan Sea through the Sakai Channel and is connected to Shinji Lake through the Ohashi Channel. The Nakaura water gate in the Sakai Channel is always open [1]. The average concentrations of nitrogen and phosphorus in the surface water of Nakaumi Lake are 444 and 44 μg/L [2]. Around Nakaumi Lake, 260 species of wild birds have been observed, making it one of largest wintering spots for ducks and geese, hosting more than 75,000 birds every year. In particular, Nakaumi supports more than 1% of the East Asian population of Tundra Swans, Common Pochards, Tufted Ducks, and Scaups. Furthermore, it serves as the crucial southernmost wintering spot for Tundra Swans. Nakaumi was designated as a Ramsar site in 2005thanks to the rich biodiversity [3].
The red alga, Gracilaria vermiculophylla, (called “Ogonori” in Japanese), grows luxuriantly in Nakaumi Lake [4]. Ogonori is used as an organic fertilizer. Ogonori was prosperously cultivated as a source of agar in the 1960s, but its cultivation was reduced by increased imports of cheap agar from foreign countries and went extinct in the 1970s [5]. Today the overgrowth of in the lake and the subsequently decomposing algae has caused water pollution. Therefore, a solution could be to use industrially Ogonori to reduce the pollution of the lake.
The use of microalgae with high protein content, such as Arthrospira (Spirulina) [6,7] and Chlorella [8,9], in poultry diets has been extensively studied; however, studies on the potential use of macro algae in poultry diets are limited [10].The purpose of this study was to examine whether Ogonori could be used as a potential feed additive, and to evaluate the effects of Ogonori supplementation on egg-quality traits in laying hens.
Materials and Methods
Preparation of Ogonori meal
Ogonori was harvested from Nakaumi Lake, on the coast of Sakaiminato, Tottori Prefecture, Japan, in July 2012. The Ogonori was washed in fresh water, sun-dried, freeze-dried, shredded coarsely by a hammer, crushed through a food cutter (DX-61, DREMAX, Saitama, Japan),and ground to a meal using a mill (KIIW-1, Fuji Paudal, Osaka, Japan).
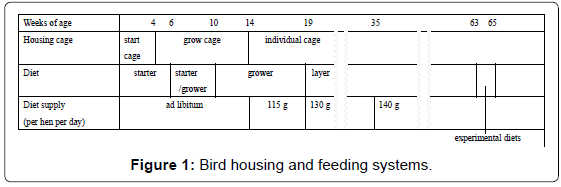
Bird housing and treatment
Experimental chickens, a cross between Japanese Game sires and Rhode Island Red dams, were raised in the Tottori Swine and Poultry Experiment Station. The crossbred birds have been used as sires of a chicken brand called “Tottori-jidori Piyo” [1].
Bird housing and feeding systems are summarized in Figure 1. The female chicks hatched on the same day were housed in a start cage from 0 to 4 wks. of age; in a grow cage from 4 to 14 wks. of age; and in individual cages from 14 wks. of age, in an open-side house. Chicks were fed a starter diet [metabolizable energy (ME), 2,950 kcal/kg; crude protein (CP), 21% (w/w)] from 0 to 6 wks.; a starter/grower diet [ME, 2,850 kcal/kg; CP, 17%] from 6 to 10 wks. of age; a grower diet [ME, 2,800 kcal/kg; CP, 15%] from 10 to 19wks of age; and a layer diet [ME, 2,780 kcal/kg; CP, 15%] from 19wksof age and thereafter. All diets were obtained from JA Nishinihon Kumiai Feed Co. (Kobe, Japan). The company guaranteed that all diets were formulated to satisfy the nutrient requirements of the Japanese Feeding Standard for Poultry [11]. Chicks were fed ad libitum from 0 to 14 wks. of age, 115 g diet/ day from 14 to 19wks of age, 130 g diet/day from 19 to 35wks of age, and 140 g diet/day 35wks of age and thereafter. Water was provided ad libitum. At 63 wks. of age, 30 laying hens were randomly chosen from the crossbred females and evenly allotted to two dietary treatment groups: control and Ogonori supplemental groups. For the Ogonori group, Ogonori meal was added to the layer diet as 2% (w/w) of fresh matter. Each chicken was fed140 g/day diet from 63 to 65 wks. Of age. All animals received humane care as outlined in the Guidelines for Proper Conduct of Animal Experiments [12].
Egg-trait measurement
For proximate analysis of experimental feed, dry matter, crude fat, crude protein, crude fiber, and crude ash of the layer diet were determined according to the technical manual for feed analysis of Japan Livestock Technology Association (JLTA) [13]. Moisture (%) was obtained by subtracting dry matter from 100%. Nitrogen-free extract was obtained by subtracting the sum of the moisture, ether extract, crude protein, crude fiber, and crude ash content from 100%. All samples were tested in duplicate. Gross energy (GE) was calculated using the following formula:
GE = 5.7 × CP+9.4 × Cfat+4.1 × (CF+NFE)
Where CP = crude protein; C Fat = crude fat; CF = crude fiber; NFE = nitrogen-free extract
Calcium and magnesium were measured using atomic absorption spectroscopy, and phosphorus was measured using the vanadmolybdic acid yellow color method described by the technical manual for feed analysis of JLTA [13]. Seventeen metals: manganese, iron aluminum, magnesium, copper, zinc, boron, barium, chromium, nickel, lead, thallium, silver, cobalt, indium, gallium, cadmium, bismuth, were measured by inductively coupled plasma mass spectrometry by the Japan Functional Food Analysis and Research Center (Fukuoka, Japan). All samples were tested in duplicate.
After 1 week of adaptation to the Ogonori diet, the following egg traits were measured: egg weight (g), eggshell weight (g), eggshell thickness (mm), yolk weight (g), albumen height (mm), and eggshell strength (breaking strength, kg) for the first three eggs obtained from each individual from 64 to 65 wks. of age. Eggshell thickness was measured using a micrometer (FN595, Fujihira Industry, Tokyo, Japan); eggshell strength was measured using an Eggshell Force Reader (FN597, Fujihira); albumen height was measured by a tripod micrometer (NFR3, Fujihira); albumen weight (g) was obtained by subtracting the sum of the eggshell and yolk weights from egg weight.
The Haugh unit (HU) value was calculated for individual eggs using the following formula:
HU = 100 log (H–1.7 × W0.37+7.6)
Where: H = albumen height (mm); W = egg weight (g)
The egg specific gravity (ESG) was calculated for individual eggs using the following formula:
ESG = EW/[0.9680 × (EW – SW) + 0.4921 × SW]
Where: EW = egg weight; SW = eggshell weight
Yolk color was measured on a colorimeter (CR-410, Konica Minolta, Tokyo, Japan) using the L*a*b*color system, in which the L*(lightness) value is a luminance or lightness component, the a*(redness) value is a chromatic component from green to red, and the b*(yellowness) value is the chromatic component from blue to yellow. The color of the egg yolk was measured with the Roche color fan (Hoffman- La Roche, Basel, Switzerland).
Determination of fatty acid composition in egg yolk
To determine fatty acid profiles, lipids from 0.1 g of each sample were extracted using 3 mL of chloroform: methanol (2:1, v/v) according to the method described by Iverson et al. [14]. The extract was thoroughly mixed with 1.5 mL of hexane. Following the addition of 200 μL of 2 M potassium hydroxide in methanol, the contents were vortex-mixed for 30 s. Next, 2 mL of saturated sodium chloride solution was added and mixed thoroughly. The sample was then centrifuged at 1000 × g for 5 min, and the supernatant containing fatty acid methyl esters was recovered. The fatty acid methyl esters were separated using a GC2010 Gas Chromatograph (Shimadzu Co., Kyoto, Japan) and a capillary column (DB-23, Shimadzu) (length, 30 m; internal diameter, 0.25 mm; film thickness, 0.25 μm). Helium carrier gas was used at a linear flow velocity of 35.4 cm/sec. The column was set at an initial temperature of 80°C for 2 min after which it was increased to 160°C by 35°C/min, and then to 185°C by 2°C/min, followed by an increase of 10°C/min to a maximum temperature of 230°Cat which it was maintained for 9 min. Other conditions were as follows: injection port temperature, 250°C; flame ionization detector temperature, 250°C; helium flow rate, 1.49 mL/min. Fatty acids were identified by comparison of retention times with those of the FAME Mix Equity-1 standard (Sigma-Aldrich Co., St. Louis, MO). All samples were tested in duplicate.
Statistical analysis
Comparisons between the treatment means were assessed by performing Student’s t-tests at a significance level of P<0.05using the Excel-Statistics 2010 software (Social Survey Research Information, Tokyo, Japan).
Results
The proximate analysis values for the experimental diets, metals in Ogonori meal, and composition of fatty acids in the experimental diets are shown in Tables 1, 2 and 3, respectively. The proximate analysis values in the two diet groups were similar, although calcium and magnesium content and crude ash in Ogonori diet were relatively high in comparison with those of the control diet (Table 1), reflecting the high mineral content in Ogonori meal (Table 2).Ogonori meal contained a relatively high proportion of palmitic acid (35.8%) and arachidonic acid (16.3%) (Table 3); however, the composition of fatty acids in the control and Ogonori diets was similar.
| Item | Control diet | Ogonori diet |
|---|---|---|
| Moisture (%) | 11.5 | 11.2 |
| Crude protein (%) | 15.7 | 17.0 |
| Ether extract (%) | 4.0 | 4.2 |
| Crude fiber (%) | 3.4 | 3.6 |
| Nitrogen free extract (%) | 54.2 | 51.6 |
| Crude ash (%) | 11.1 | 12.4 |
| Gross energy (kcal/kg) | 3632.5 | 3627.0 |
| Calcium (%) | 5.3 | 6.6 |
| Phosphorus (%) | 0.4 | 0.5 |
| Magnesium (%) | 0.19 | 0.21 |
Table 1: Proximate analysis values in experimental diets.
| Mineral | Mineral contents in Ogonori meal (mg/kg) | Mineral contents provided by supplemental Ogonori (mg/kg) | Requirement of NAROa (mg/kg) | Requirement of NRCb (mg/kg) |
|---|---|---|---|---|
| Calcium (Ca) | 89,100 | 1,782 | 32,000 | 32,500 |
| Manganese (Mn) | 43,000 | 860 | 25 | 20 |
| Iron (Fe) | 19,000 | 380 | 50 | 45 |
| Aluminium (Al) | 9,500 | 190 | n.d. | n.d. |
| Magnesium (Mg) | 3,260 | 65 | 500 | 500 |
| Copper (Cu) | 850 | 17 | n.d. | n.d. |
| Zinc (Zn) | 790 | 16 | 35 | 35 |
| Boron (B) | 500 | 10 | n.d. | n.d. |
| Barium (Ba) | 360 | 7 | n.d. | n.d. |
| Chromium (Cr) | 300 | 6 | n.d. | n.d. |
| Nickel (Ni) | 100 | 2 | n.d. | n.d. |
| Lead (Pb) | 69 | 1 | n.d. | n.d. |
| Thallium (Tl) | 36 | 1 | n.d. | n.d. |
| Silver (Ag) | 25 | 1 | n.d. | n.d. |
| Cobalt (Co) | 19 | 0 | n.d. | n.d. |
| Indium (In) | 12 | 0 | n.d. | n.d. |
| Gallium (Ga) | 11 | 0 | n.d. | n.d. |
| Cadmium (Cd) | 4 | 0 | n.d. | n.d. |
| Bismuth (Bi) | ―c | ―c | n.d. | n.d. |
a Japanese Feeding Standard for Poultry (National Agriculture and Food Research
Organization (NARO), 2011)
b Nutrient Requirements of Poultry (National Research Council (NRC), 1994)
c below measurable limit
n.d. = no data
Table 2: Minerals in Ogonori meal and mineral contents provided by supplemental Ogonori in Ogonori diet.
| Item | Ogonori meal | Control diet | Ogonori diet |
|---|---|---|---|
| Moisture (%) | 9.2 | 10.8 | 10.5 |
| Crude fat (%) | 0.8 | 5.2 | 4.8 |
| Fatty acid (%) | |||
| Myristic acid (C14:0) | 2.7 | 0.4 | 0.5 |
| Myristoleic acid (C14:1) | 0.8 | 0.1 | 0.1 |
| Pentadecylic acid (C15:0) | 0.4 | 0.1 | 0.1 |
| Pentadecenoic acid (C15:1) | 0.2 | 0.0 | 0.0 |
| Palmitic acid (C16:0) | 35.8 | 13.7 | 15.0 |
| Palmitoleic acid (C16:1) | 2.6 | 1.1 | 1.3 |
| Heptadecanoic acid (C17:0) | 0.5 | 0.2 | 0.2 |
| Heptadecenoic acid (C17:1) | 0.3 | 0.2 | 0.2 |
| Stearic acid (C18:0) | 2.6 | 4.1 | 4.5 |
| Oleic acid (C18:1) | 10.0 | 35.3 | 35.8 |
| Linoleic acid (C18:2) | 2.2 | 40.6 | 37.8 |
| Linolenic acid (C18:3) | 0.8 | 2.0 | 2.1 |
| Arachidic acid (C20:0) | 0.2 | 0.3 | 0.3 |
| Eicosenoic acid (C20:1) | 0.6 | 0.4 | 0.4 |
| Eicosadienoic acid (C20:2) | 0.3 | 0.1 | 0.1 |
| Eicosatrienoic acid (C20:3) | 0.3 | 0.0 | 0.0 |
| Arachidonic acid (C20:4) | 16.3 | 0.1 | 0.1 |
| Ecosapentaenoic acid (C20:5) | 0.8 | 0.1 | 0.1 |
| Behenic acid (C22:0) | 0.3 | 0.2 | 0.2 |
| Docosahexaenoic acid (C22:6) | 0.5 | 0.1 | 0.1 |
| Lignoceric acid (C24:0) | 0.4 | 0.2 | 0.2 |
| Unidentified fatty acids | 21.4 | 0.8 | 0.8 |
Table 3: Moisture, crude fat, and composition of fatty acids in Ogonori meal, Contral diet, and Ogonori diet.
Egg traits in the control and Ogonori diet groups were compared (Table 4). Eggshell strength was significantly higher in the Ogonori group (3.82 ± 0.92 kg/cm2) than in the control (3.36 ± 0.82 kg/cm2). Eggshell thickness was significantly higher in the Ogonori group (0.363 ± 0.03 mm) than in the control (0.350 ± 0.03 mm). Albumen height were significantly higher in the Ogonori group (6.60 ± 1.23 mm) than in the control (5.89 ± 1.42 mm), although there was no significant difference in albumen weight between the two groups. Haugh units were significantly higher in the Ogonori group (79.19 ± 8.87) than in the control (73.39 ± 12.34). Yolk color on the Roche scale was significantly paler in the Ogonori group (12.51 ± 0.87) than in the control (13.21 ± 0.69). Redness (a*) was significantly lower in the Ogonori group (22.48 ± 2.59) than in the control (24.14 ± 1.89).
| Trait | Control diet | Ogonori diet |
|---|---|---|
| Number of eggs | 45 | 45 |
| Egg weight (g) | 62.99 ± 3.27 | 63.33±5.46 |
| Eggshell strength (kg/cm2) | 3.36 ± 0.82b | 3.82±0.92a |
| Yolk weight (g) | 19.88 ± 1.55 | 19.55±1.54 |
| Eggshell weight (g) | 8.42 ± 0.75 | 8.44±0.85 |
| Eggshell thickness (mm) | 0.350 ± 0.03b | 0.363±0.03a |
| Albumen weight (g) | 34.69 ± 2.67 | 35.34±4.56 |
| Albumen height (mm) | 5.89 ± 1.42b | 6.60±1.23a |
| Haugh unit | 73.39 ± 12.34b | 79.19±8.87a |
| Yolk color (Roche scale) | 13.21 ± 0.69a | 12.51±0.87b |
| L | 68.60 ± 2.98 | 68.74±2.14 |
| a | 24.14 ± 1.89a | 22.48±2.59b |
| b | 57.61 ± 2.69 | 58.56±3.25 |
| Egg specific gravity | 1.11 ± 0.01 | 1.11±0.01 |
Values are means ± SD.
a,b Means within a row with different superscript letters are significantly different
(P<0.05).
Table 4: Egg traits in control and Ogonori diet groups.
The fatty acid composition of eggs in the control and Ogonori diet groups is shown in Table 5. Linoleic acid composition was significantly higher in the Ogonori group (8.58 ± 1.34 %) than in the control (8.02 ± 0.79 %), while the eicosatrienoic acid composition was significantly higher in the Ogonori group (0.12 ± 0.01 %) than in the control (0.15 ± 0.24 %).
| Item | Control diet | Ogonori diet |
|---|---|---|
| Number of eggs | 45 | 45 |
| Moisture (%) | 47.84 ± 0.68 | 47.71±0.76 |
| Crude fat (%) | 35.65 ± 0.95 | 35.39±0.79 |
| Fatty acid (%) | ||
| Myristic acid (C14:0) | 0.33 ± 0.02 | 0.33±0.03 |
| Myristoleic acid (C14:1) | 0.07 ± 0.01 | 0.07±0.02 |
| Pentadecylic acid (C15:0) | 0.03 ± 0.01 | 0.04±0.01 |
| Pentadecenoic acid (C15:1) | 0.06 ± 0.01 | 0.06±0.01 |
| Palmitic acid (C16:0) | 24.67 ± 0.94 | 24.40±0.66 |
| Palmitoleic acid (C16:1) | 2.77 ± 0.52 | 2.70±0.44 |
| Heptadecanoic acid (C17:0) | 0.13 ± 0.02 | 0.15±0.02 |
| Heptadecenoic acid (C17:1) | 0.14 ± 0.02 | 0.15±0.02 |
| Stearic acid (C18:0) | 8.14 ± 0.62 | 8.08±0.72 |
| Oleic acid (C18:1) | 46.97 ± 2.14 | 47.29±1.19 |
| Linoleic acid (C18:2) | 8.02 ± 0.79b | 8.58±1.34a |
| Linolenic acid (C18:3) | 0.26 ± 0.04 | 0.27±0.05 |
| Arachidic acid (C20:0) | 0.04 ± 0.01 | 0.04±0.01 |
| Eicosenoic acid (C20:1) | 0.24 ± 0.03 | 0.24±0.03 |
| Eicosadienoic acid (C20:2) | 0.17 ± 0.05 | 0.16±0.03 |
| Eicosatrienoic acid (C20:3) | 0.15 ± 0.24a | 0.12±0.01b |
| Arachidonic acid (C20:4) | 2.14 ± 0.31 | 2.09±0.24 |
| Ecosapentaenoic acid (C20:5) | 0.001 ± 0.005 | 0.001±0.005 |
| Behenic acid (C22:0) | 0.001 ± 0.004 | 0.002±0.007 |
| Eicosadienoic acid (C20:2) | 0.016 ± 0.012 | 0.009±0.013 |
| Docosahexaenoic acid (C22:6) | 0.83 ± 0.17 | 0.81±0.14 |
| Lignoceric acid (C24:0) | 0.10 ± 0.02 | 0.11±0.12 |
| Unidentified fatty acids | 4.38 ± 0.38 | 4.29±0.57 |
Values are means ± SD.
a,b Means within a row with different superscript letters are significantly different
(P<0.05).
Table 5: Moisture, crude fat, and composition of fatty acids of eggs in Control and Ognori diet groups.
Discussion
Microalgae (e.g., Arthrospira and Chlorella) are widely used as feed supplements for animals. In meat-type chickens, these microalgae, which contain up to 50% crude protein, can be used as a replacement for 5–10% of conventional proteins [15]. In laying hens, enhanced yolk color due to carotenoid accumulation has been reported [6,9]. In contrast, the incorporation of macro algae (e.g., Ulva and Sargassum) into poultry diets is limited, as these algae have low protein and lipid contents and high contents of indigestible polysaccharides, and thus low gross energy yield [10]. Carrillo et al. [16] reported that supplementation with Sargassum increases yolk color, as reported for Arthrospira and Chlorella. To our knowledge, this is the first report to show that dietary supplementation with algae improved eggshell strength and thickness, albumen height, and Haugh unit, whereas redness of egg yolk was slightly reduced.
While the effect of a 2% Ogonori meal supplement on dietary nutritional value was limited, the mechanism by which supplemental Ogonori improved egg traits is unclear. The most likely possibility is that the high mineral content in Ogonori, especially manganese (Mn) and iron (Fe), may affect these traits. Mineral contents provided by supplemental Ogonori was high (Mn, 860 mg/kg; Fe, 380 mg/ kg) compared to the requirements of the Japanese Feeding Standard for Poultry (Mn, 25 mg/kg; Fe, 50 mg/kg) [11] and the Nutrient Requirements of Poultry [17]. Zamani et al. [18] reported that 30 to 60 mg/kg of supplemental Mn increased percentage, stiffness, breaking strength, and fracture toughness of eggshell and did not affect eggshell thickness or egg weight in laying hens. Paik et al. [19] reported that supplemental iron (200 mg/kg) increased egg weight and Haugh unit but did not have significant effect on eggshell strength or thickness.
Chromium and aluminum contents provided by supplemental Ogonori (Cr, 6 mg/kg; Al, 190 mg/kg) were considerably high in the present study. Sahin et al. [20] reported that increasing supplemental Cr from 0.4 to 1.2 mg/kg lead to a linear increase in egg weight, eggshell weight, eggshell thickness, albumen index, albumen weight, yolk index, yolk weight, and specific gravity in Japanese quail. Sahin et al. [21] reported that supplemental Cr (0.4 mg/kg) increased egg weight, egg specific gravity, eggshell thickness, eggshell weight, and Haugh unit of laying hens reared under low ambient temperature. Yildiz et al. [22] reported that yolk and albumen weight increased linearly when0.25 to 1.0 mg/kg organic Cr was included in the diet of laying hens. Meanwhile, Uyanik et al. [23] reported that supplemental Cr (20 mg/kg) increased albumen and yolk index values but did not have a significant effect on egg weight, specific gravity, shape index, shell thickness, or Haugh unit, suggesting that 20 mg/kg Cr in the poultry diet exceeded the amount by which positive effects on egg quality traits could be attained. To our knowledge, there is no report concerning a direct association between egg traits and dietary supplementation with Al. However, effects of zeolite, which contains Al, on egg traits in laying hens, have been studied [24–28]. For example, Fendri et al. [28] reported that supplementation with 1% zeolite (115–131 mg/kg of Al2O3 equivalent) increased eggshell strength, egg weight, and albumen weight. These data suggest that individual and/or synergistic effects of Mn, Fe, Cr, and Al can explain the improvement of eggshell strength, eggshell thickness, albumen height, and Haugh unit.
It is possible that other minerals, such as calcium (Ca), zinc (Zn), magnesium (Mg), boron (B), and copper (Cu), also affect egg traits. The control diet in the present study contained 5.3% Ca, which is above the requirements of the NARO and NRC standards for laying hens. Sahin et al. [29] reported that supplemental Zn (30 mg/kg) increased egg weight, egg specific gravity, eggshell thickness, eggshell weight, and Haugh unit of laying hens reared under low ambient temperature. Kim et al. [30] reported that feeding aged laying hens diets containing increased concentrations of Mg, up to 3.0 g/kg, improved eggshell strength. It should be mentioned that the contents of Zn and Mg used in the previous studies [29,30] were significantly higher than the requirements of the NARO and NRC standards. While there is no standard requirement for B or Cu for laying hens, Mizrak et al. [31] reported that supplementation with25 to 200 mg/kg B increased albumen height and Haugh unit, and Lim and Paik [32] reported that supplemental Cu (100 mg/kg as methionine chelate) increased specific gravity and eggshell strength.
When laying hens were fed diets containing oleic, linoleic, and α-linolenic acid, these fatty acids were observed to readily incorporate into the egg yolk [33,34].Van Elswyk et al. [35] reported that feeding hens with supplemental fish oil increased n-3 polyunsatured fatty acids in the yolk. Herber and Elswyk[36] reported that supplemental marine microalgae, which have high eicosapentaenoic acid content, promoted efficient deposition of docosahexaenoic acid and n-3 fatty acids in yolks. Ginzberg et al. [37] reported that supplemental red microalgae (Porphyridiumsp.), which has high linoleic and arachidonic acid content, promoted efficient deposition of these fatty acids in yolk. In the present study, the cause of the increased linoleic and eicosatrienoic acid contents in yolks of hens fed supplemental Ogonori remain unclear, since the fatty acid profiles of the control and Ogonori diets were similar.
It is also not clear why supplemental Ogonori reduced redness of the yolk color. As mentioned previously, supplementation with Sargassum macro algae increases yolk color [16]. Michalak et al. [38] reported that redness was reduced when the required concentrations of minerals (Cu, Mn, Zn, Cr, and cobalt) were supplied by a macro algae mixture of Enteromorphaprolifera and Cladophora sp. These data suggests that yolk color is also influenced by macro algae species.
In conclusion, this is the first report to show the possibility of using Ogonori (Gracilaria vermiculophylla) as a feed additive for laying hens to improve egg traits, especially eggshell strength and Haugh unit, and suggests an association between high mineral contents (Mn, Fe, Cr, and Al) in Ogonori and the egg traits. We think that Ogonori meal can be safely and effectively used as a feed additive for laying hens, since the mineral contents of Ogonori meal are much lower than those toxic levels as described in [11,17]. Since the contents of these minerals are much higher than those used in previous studies, investigation of the minimum required Ogonori supplementation will be needed in the future.
References
- Tottori Prefecture website (1996) Tottori Prefectural Office, Tottori, Japan. https://www.pref.tottori.lg.jp/.
- Goto M, Kamiya H, Ishibashi T (2004) Water quality of Lakes Shinji and Nakaumi. Rep Inst Public Health Environ Sci 45: 112–116 (in Japanese).
- https://www.nakaumi-shinjiko.jp/
- Miyamoto Y, Hatsuda A (2007) Horizontal and vertical distribution of macroalgal assemblages in modern Lake Nakaumi, a coastal lagoon. Laguna 14: 9-16.
- Moriwaki S, Michine A (2007) Catch fluctuations in Nakaumi, estuarine inland-sea, western Japan. Rep Shimane Pref Fish TechnolCtr 1: 41–48
- Ross E, Dominy W (1990) The nutritional value of dehydrated, blue-green algae (Spirulinaplantensis) for Poultry. PoultSci 69: 794-800.
- Mariey YA, Samak HR, Ibrahem MA (2012) Effect of using Spirulinaplatensis algae as a feed additive for poultry diets: 1- productive and reproductive performances of local laying hens. Egypt PoultSci 32: 201-215.
- Lipstein B, Hurwitz S (1980) The nutritional value of algae for poultry. Dried Chlorella in broiler diets. Br PoultSci 21: 9-21.
- Lipstein B, Hurwitz S, Bornstein S (1980) The nutritional value of algae for poultry. Dried Chlorella in layer diets. Br PoultSci 21: 23-27.
- Shields RJ, Lupatsch I (2012) Algae for aquaculture and animal feeds. Technikfolgenabschätzung- Theorie und Praxis 21: 23-37.
- National Agriculture and Food Research Organization (2011) Japanese Feeding Standard for Poultry. Tsukuba, Japan.
- Science Council of Japan (2006) Guidelines for Proper Conduct of Animal Experiments. Tokyo, Japan.
- Japan Livestock Technology Association (2000) Technical manual for feed analysis. Tokyo, Japan.
- Iverson SJ, Lang SLC, Cooper MH (2001) Comparison of the bligh and dyer and folch methods for total lipid determination in a broad range of marine tissue. Lipids 36: 1283-1287.
- Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J BiosciBioeng 101: 87-96.
- Carrillo S, Bahena A, Casas M, Carranco ME, Calvo CC, et al. (2012) The alga Sargassumspp. as alternative to reduce egg cholesterol content. Cuban J AgrSci 46: 181-186.
- National Research Council (1994) Nutrient Requirements of Poultry. (9thedn), National Academy Press, Washington DC, USA.
- Zamani A, Rahmani HR, Pourreza J (2005) Effect of different levels of manganese and zinc on performance traits and breaking eggs in laying hens. Pakistan J BiolSci 8: 1035-1040.
- Paik IK, Lee HK, Park SW (2009) Effects of organic iron supplementation on the performance and iron content in the egg yolk of laying hens. J PoultSci 46: 198-202.
- Sahin K, Kucuk O, Sahin N, Ozbey O (2001) Effects of dietary chromium picolinate supplementation on egg production, egg quality and serum concentrations of insulin, corticosterone and some metabolites of Japanese quails. Nutr Res 21: 1315-1321.
- Sahin K, Ozbey O, Onderci M, Cikim G, Aysondu MH (2002) Chromium supplementation can alleviate negative effects of heat stress on egg production, egg quality and some serum metabolites of laying Japanese quail. J Nutr 132: 1265-1268.
- Yildiz AO, Parlat SS, Yazgan O (2004) The effects of organic chromium supplementation on production traits and some serum parameters of laying quails. Revue Med Vet 155: 642-646.
- https://journals.tubitak.gov.tr/veterinary/issues/vet-02-26-2/vet-26-2-30-0105-18.pdf
- Roland DA, Laurent SM, Orloff HD (1985) Shell quality as influenced by zeolite with high ion-exchange capability. PoultSci 64: 1177-1187.
- Rabon HW, Roland DA, Bryant M, Barnes DG, Laurent SM (1991) Influence of sodium zeolite A with and without pullet-sized limestone oyster shell on eggshell quality. PoultSci 70: 1943-1947.
- Özturk E, Erener G, Sarica M (1998) Influence of natural zeolite on performance of laying hens and egg quality. Trend J Agric Forest 22: 623-628.
- Kermanshahi H, Haji Agha Jani E, Hashemipour H, Pilevar M (2011) Efficacy of natural zeolite and pigments on yolk color and performance of laying hens. Afr J Biotechnol 10: 3237-3242.
- Fendri I, Khannous L, Mallek Z, Traore AI, Gharsallah N, et al. (2012) Influence of zeolite on fatty acid composition and egg quality in Tunisian laying hens. Lipids Health Dis 11: 71.
- Sahin N, Onderci M, Sahin K (2002) Effects of dietary chromium and zinc on egg production, egg quality, and some blood metabolites of laying hens reared under low ambient temperature. Biol Trace Elem Res 15: 163-169.
- Kim CH, Paik IK, Kil DY (2013) Effects of increasing supplementation of magnesium in diets on productive performance and eggshell quality of aged laying hens. Biol Trace Elem Res 151: 38-42.
- Mizrak C, Yenice E, Can U, Yildirim U, Atik Z (2010) Effects of dietary boron on performance, egg production, egg quality and some bone parameters in layer hens. S AfrAnimSci 40: 257-264.
- Lim HS, Paik IK (2003) Effects of supplementary mineral methionine chelates (Zn, Cu, Mn) on the performance and eggshell quality of laying hens. Asian-Aust J AnimSci 16: 1804-1808.
- Murty NL, Reiser R (1961) Influence of graded levels of dietary linoleic and linolenic acids on the fatty acid composition of hen’s eggs. J Nutr 75: 287-294.
- Donaldson WE (1967) Lipid composition of chick embryo and yolk as affected by stage of incubation and maternal diet. PoultSci 46: 693-697.
- Van Elswyk ME, Sams AR, Hargis PS (1992) Composition, functionality, and sensory evaluation of eggs from hens fed dietary menhaden oil. J Food Sci 57: 342-344.
- Herber SM, Van Elswyk ME (1996) Dietary marine algae promotes efficient deposition of n-3 fatty acids for the production of enriched shell eggs. PoultSci 75: 1501-1507.
- Ginzberg A, Cohen M, Sod-Moriah UA, Shany S, Rosentrauch A, et al. (2000) Chickens fed with biomass of the red microalga Porphyridiumsp. have reduced blood cholesterol level and modified fatty acid composition in egg yolk. J ApplPhycol 2: 325-330.
- Michalak I, Chojnacka K, Dobrzanski Z, Górecki H, Zielinska A, et al. (2011) Effect of macroalgae enriched with microelements on egg quality parameters and mineral content of eggs, eggshell, blood, feathers and droppings. J AnimPhysiolAnimNutr 95: 374-387.
Relevant Topics
- Acoustic Survey
- Animal Husbandry
- Aquaculture Developement
- Bioacoustics
- Biological Diversity
- Dropline
- Fisheries
- Fisheries Management
- Fishing Vessel
- Gillnet
- Jigging
- Livestock Nutrition
- Livestock Production
- Marine
- Marine Fish
- Maritime Policy
- Pelagic Fish
- Poultry
- Sustainable fishery
- Sustainable Fishing
- Trawling
Recommended Journals
Article Tools
Article Usage
- Total views: 16517
- [From(publication date):
June-2014 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 11903
- PDF downloads : 4614