Supervised, Individualized Exercise Programs Help Mitigate Costs during Cancer Treatment
Received: 13-Jun-2018 / Accepted Date: 15-Jun-2018 / Published Date: 22-Jun-2018 DOI: 10.4172/2165-7386.1000338
Abstract
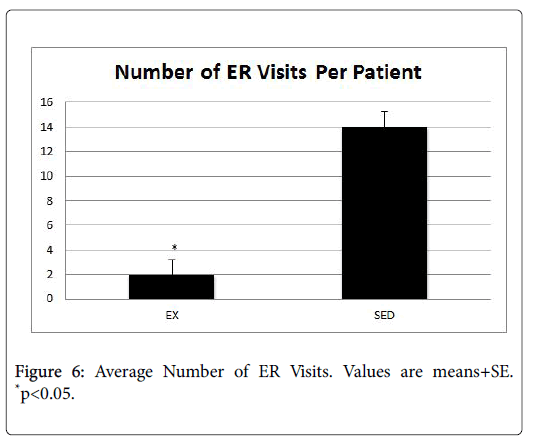
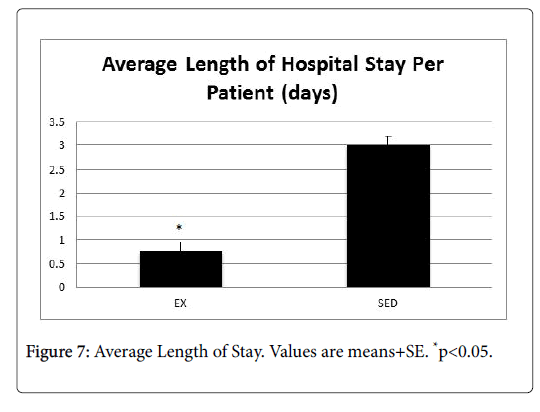
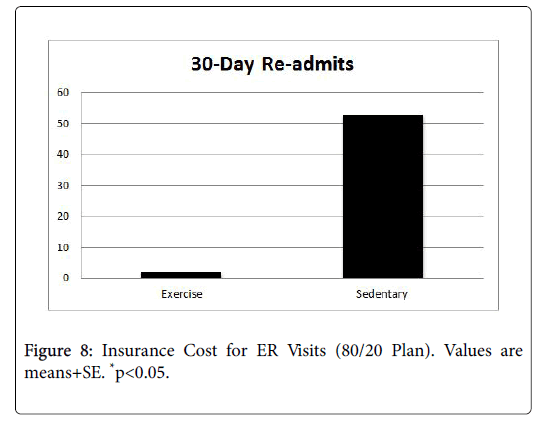
Background: Cancer and its associated treatments often result in long-term physical and psychological side effects that negatively impact the cancer survivor's quality of life. In addition, the financial costs of cancer are substantial and are projected to reach $158 billion by the year 2020. Research indicates that endurance exercise training is helpful in attenuating the deleterious effects of cancer treatments by increasing survival, attenuating myocardial lesions and myocyte disarray, increasing levels of antioxidants, decreasing lipid peroxidation induced by oxidative stress and markers of apoptosis, and preserving cardiac function. However, nationally less than 5% of patients are ever referred to a cancer rehabilitation exercise program. Cost is a barrier to these programs, as they often are not reimbursable under most insurance plans. Purpose: Therefore, the purpose of this investigation was to determine if exercise training during cancer treatment helped to minimize side effects and reduce health care costs. Specifically, treatment tolerance, length of hospital stay, hospital readmits, ER visits, and treatment compliance were measured. Methods: This was a retrospective, two-group study which ascertained the protective effect of an exercisetraining program during cancer treatment. All oncology patients who received cancer treatment at Kettering Medical Center in Dayton, Ohio between January-December 2016 were identified by office staff. Their medical records were pulled and patients were placed in one of two groups: those who exercised during treatment, and those who remained sedentary. The medical records were reviewed to determine outcome data for length of hospital stays, hospital readmits, ER visits, treatment compliance, fatigue, and anxiety/depression related to oncology conditions. The age range of the patients was 21-93 years. Patients were excluded if they had pre-existing cardiac, liver, and bone marrow conditions prior to treatment. Individuals in the exercise group (EX, n=672) completed 12 weeks of prescribed, individualized exercise that included cardiovascular, strength training, and flexibility components. The intensity level for the cardiovascular exercise ranged from 30%-45% of the individual’s predicted VO2max. The strength training involved a full body workout, with emphasis on all major muscle groups. Individuals in the sedentary group (SED, n=728) did not participate in an exercise program during treatment. Results: Patients in the EX group had significantly lower reports of fatigue, pain, and cardiac problems (p<0.05), as well as fewer notes of depression and anxiety than their SED group counterparts. In addition, the EX group tolerated their treatment significantly better than the SED group (p<0.05). Finally, the EX group had a significantly lower number of ER visits (EX=2, SED=14, p<0.05), 30-day readmits (EX=2, SED=53, p<0.05) as well as a shorter length of stay (EX=0.75, SED=3 p<0.05). Conclusion: Results from this investigation point to a protective effect of moderate-intensity exercise that translated to reductions in ER visits, 30-day readmits, and length of hospital stay, which translated into cost savings for the payer, provider, and patients, alike.
Keywords: Quality of life; Cancer treatment; Myocyte; Anxiety; Depression
Introduction
Cancer is a significant national health problem. The American Cancer Society (ACS) estimates that approximately 1.6 million Americans will develop cancer in 2017 and more than 600,000 will die of the disease [1]. The most common cancers in 2016 were lung, breast, prostate, colon and rectal, bladder, melanoma, non-Hodgkin lymphoma, thyroid, kidney, leukemia, endometrial, and pancreatic cancer [2]. In the US, cancer is second only to heart disease as the most common cause of death in adults of all ages. For women between the ages of 40-79 years, and men between 60-79 years, it is the leading cause of death [1]. Cancer mortality is higher among men than women [2].
Positively, the overall death rate has declined by 13% since the year 2004, primarily due to early detection and advances in treatment options [1]. However, more than 15.5 million men and women are living today as cancer survivors [3]. This indicates that although the overall cancer mortality rates have declined the number of cancer survivors have increased. Therefore, cancer is now identified as a chronic disease [4], and its associated treatments often result in long-term physical and psychological side effects that impact the cancer survivor's quality of life. The financial costs of cancer alone are substantial, and are projected to reach $158 billion by the year 2020 [2]. The challenge for health care providers today is to develop systems of long-term follow-up care, address the short and long-term effects of current cancer therapy, and develop new curative therapies with minimal toxicities [5,6].
The current armaments for treating cancer include surgery, chemotherapy, irradiation, and biological, hormonal and targeted therapies [4]. Cancer cells involve DNA mutations that often occur during DNA replication. In the normal cell cycle, checkpoints facilitate DNA repair; however, cancerous cells lose their checkpoint integrity and escape DNA repair [7]. The resulting mutations impact the regulatory mechanisms that restrict normal cell proliferation [7]. Treatment for cancer is individualized according to a number of factors, including type and duration. Using a combination of agents rather than just one provides a synergistic cell kill with the potential that less drug-resistant cells remain. The negative of antineoplastic treatments is that normal cells, as well as malignant cells, are often disrupted, leading to many side effects and long-term morbidities [8].
Treatment-related morbidities impact functional ability and quality of life. Multiple comorbidities are encountered by cancer survivors, including nausea, vomiting, alopecia, fatigue, constipation/diarrhea, bone marrow suppression, cardiovascular dysfunction, muscle weakness, pain, mucositis, sleep disturbances, and peripheral neuropathy [4,8]. Efforts have been made to explore alternative therapies to reduce toxicities, such as the use of slow infusions [9-11], antioxidants (probucol [12,13], ubiquinone [14], ambroxiron [15], alpha-lipoic acid [16], p-coumaric acid [17], and melatonin [18]), iron chelators [19,20], and drug encapsulated liposomes [21]. However, these strategies provide limited improvements, and in some instances may have additional negative side effects for cancer patients.
Research indicates that endurance exercise training is helpful in attenuating the deleterious effects of cancer treatments by increasing survival [22], attenuating myocardial lesions and myocyte disarray [23], increasing levels of antioxidants [24], decreasing lipid peroxidation induced by oxidative stress and markers of apoptosis [25,26] and preserving cardiac function [27,28]. However, nationally less than 5% of patients are ever referred to a cancer rehabilitation exercise program [29]. Therefore, the purpose of this investigation was to determine if exercise training during cancer treatment helped to minimize side effects. Specifically, length of hospital stays, hospital readmits, ER visits, and treatment compliance were measured. In addition, psychological factors including fatigue, depression, and anxiety were compared. These data were then used to determine a costsavings analysis for each patient who participated in an exercise program during cancer treatment.
Methods
Subjects
This was a retrospective, two-group study which ascertained the protective effect of an exercise training program during cancer treatment. The IRB of Kettering Health Network approved all study methods and procedures prior to the onset of data collection.
All oncology patients who received cancer treatment at Kettering Medical Center in Dayton, Ohio between January-December 2016 were identified by office staff. Their medical records were pulled and patients were placed in one of two groups: those who exercised during treatment, and those who remained sedentary.
The medical records were reviewed to determine outcome data for length of hospital stays, hospital readmits, ER visits, treatment compliance, fatigue, and anxiety/depression related to oncology conditions. The age range of the patients was 21-93 years. Patients were excluded if they had pre-existing cardiac, liver, and bone marrow conditions prior to treatment.
Exercise training protocol
Each patient was given the opportunity to participate in a cancer exercise program through Maple Tree Cancer Alliance, an organization that provides exercise training to individuals battling cancer. All interested patients were referred by hospital oncologists, and began participation in the exercise program upon referral. These individuals constituted the exercise training (EX) group. The EX group completed 12 weeks of prescribed, individualized exercise that included cardiovascular, strength training, and flexibility components. The intensity level for the cardiovascular exercise ranged from 30%-45% of the individual’s predicted VO2max. The strength training involved a full body workout, with emphasis on all major muscle groups. Machines, free weights, and tubing were all employed.
Patients completed 3 sets of 10 repetitions for each exercise. Flexibility training involved static stretching of all major muscle groups for 15-20 seconds at the completion of each workout. Patients met with a trainer once a week and were given instructions on how to remain active at home. Individuals in the sedentary (SED) group did not participate in the exercise program at Maple Tree Cancer Alliance. It is unknown if they exercised on their own.
Surveys
The medical record of each patient constituted the data source for this study. Specific variables that were collected include: cardiovascular changes (through echocardiogram, resting heart rate, and blood pressure response), treatment-related side effects, length of hospital stays, treatment compliance, and medications. In addition, demographic characteristics of subjects, including age, gender, type of cancer, BMI, comorbid conditions, and ethnicity were collected.
Data analysis
Data analysis was conducted using SPSS software. Descriptive statistics was conducted on the data variables according to convention. A two-factor analysis of variance (ANOVA) was used to determine differences due to the main effects (group, drug) and interaction of these factors. A multi-factor analysis of variance (MANOVA) was used to determine between and within group differences. A significance level of p<0.05 was used for all statistical analyses.
Results
All patient records from January 1, 2016-December 31, 2016 were analyzed. Patients were placed into one of two groups-those who participated in the exercise program of Maple Tree Cancer Alliance (EX, n=672), and those who did not (SED, n=728). Table 1 presents patient demographics. Patients who had pre-existing conditions were excluded from the investigation. Outcome variables, including length of hospital stays, ER visits, and treatment tolerance were measured. In addition, psychological factors including fatigue, depression, and anxiety and the physiological variables of pain and cardiac abnormalities were compared.
| Age (years)/Gender | EX (n=672) | SED (n=728) |
| 65 ± 0.6 | 63 ± 0.6 | |
| Male | 28% ± 0.08 | 48% ± 0.03 |
| Female | 72% ± 0.08 | 52% ± 0.03 |
| Ethnicity | BMI | |
| White | 69% ± 0.08 | 69% ± 0.03 |
| African American | 13% ± 0.06 | 8% ± 0.02 |
| Hispanic | 0% | 0.01% ± 0.01 |
| Asian | 0% | 0% |
| Unknown | 0.03% ± 0.03 | 20% ± 0.03 |
| Type of Cancer | BMI | |
| Breast | 44% ± 0.03 | 28% ± 0.03 |
| Colon | 9% ± 0.05 | 11% ± 0.02 |
| Prostate | 10% ± 0.05 | 5% ± 0.01 |
| Lung | 13% ± 0.06 | 16% ± 0.02 |
| Leukemia | 0.03% ± 0.03 | 0.03% ± 0.01 |
| Brain | 0% | 1% ± 0.01 |
| Hodgkin's | 0% | 4% ± 0.01 |
| Other | 22% ± 0.07 | 36% ± 0.03 |
| Stage | BMI | |
| I | 23% ± 0.06 | 19% ± 0.02 |
| II | 29% ± 0.05 | 27% ± 0.02 |
| III | 39% ± 0.05 | 37% ± 0.02 |
| IV | 3% ± 0.03 | 10% ± 0.02 |
| Unclear | 6% ± 0.08 | 7% ± 0.03 |
| Treatment Regimen | BMI | |
| Chemotherapy | 25% ± 0.08 | 12% ± 0.02 |
| Radiation | 0 | 0% |
| Surgery | 0 | 1% ± 0.01 |
| Hormonal | 19% ± 0.07 | 47% ± 0.03 |
| Unclear | 22% ± 0.07 | 8% ± 0.02 |
| Discontinued or completed | 34% ± 0.08 | 28% ± 0.03 |
Table 1: Demographics of the patients.
These data were then used to determine a cost-savings analysis for each patient who participated in an exercise program during cancer treatment. Cost savings was based on an 80/20 insurance payment plan, with the cost of an adult office visit ranging from $130-$180, and the cost of an ER visit ranging $580-$700.
Patient impact
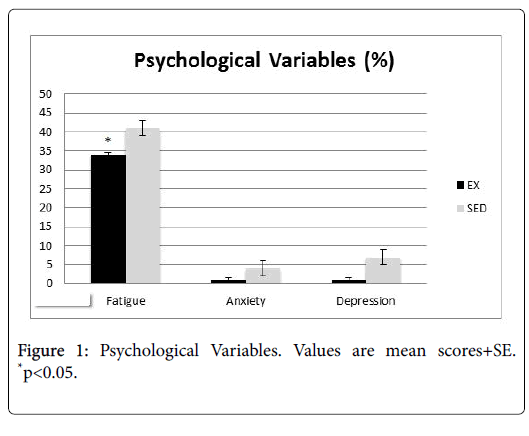
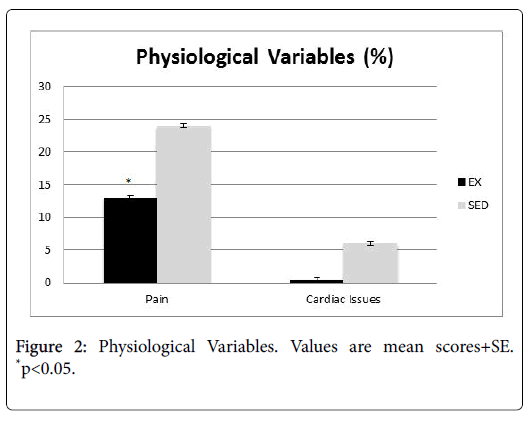
Patient charts were reviewed for physician notes pertaining to psychological variables (Figure 1, fatigue, anxiety, and depression), as well as physiological variables (Figure 2, pain, cardiac abnormalities).
Patients in the EX group had significantly lower reports of fatigue, pain, and cardiac problems (p<0.05). While the difference was not significant, they also had fewer notes of depression and anxiety than their SED group counterparts.
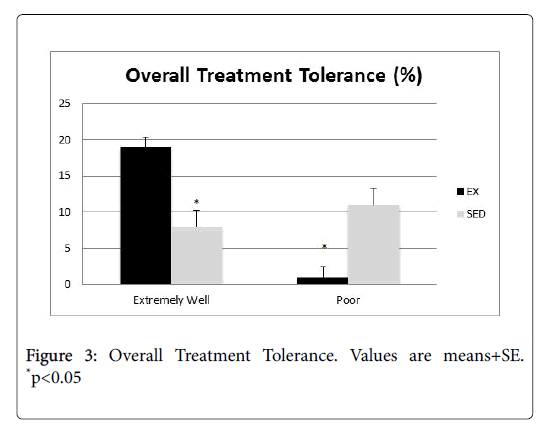
Physician notes pertaining to treatment tolerance (Figure 3) were also analyzed. It is worth noting that those in the EX group tolerated their treatment significantly better than the SED group, and those who were tolerating their treatment poorly was significantly higher in the SED group (p<0.05).
Cost savings for patients
Data related to number of office visits and ER visits were analyzed by an independent health economist, who conducted a cost-savings analysis for each group.
Cost savings was based on an 80/20 insurance payment plan, with the cost of an adult office visit ranging from $130-$180, and the cost of an ER visit ranging $580-$700.
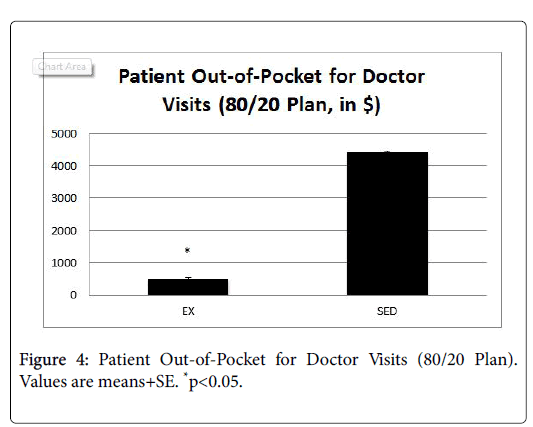
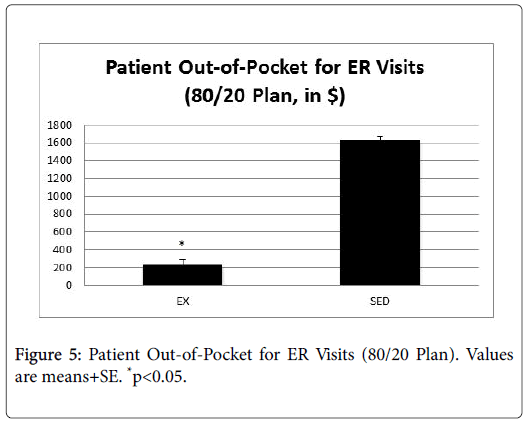
Figure 4 presents the average out-of-pocket savings for doctor visits, and Figure 5 presents the average out-of-pocket savings for ER visits.
In both cases, patients in the EX group had significantly lower outof- pocket costs compared to the SED group (p<0.05).
Impact to providers
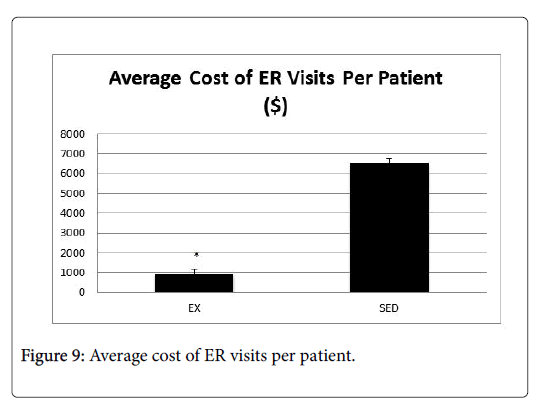
In order to determine the impact to the providers, we examined the average number of ER visits and length of stay for patients in both the EX and SED groups (Figures 6 and 7). In both instances, the EX group had a significantly lower number of ER visits as well as a shorter length of stay than the SED group (p<0.05).
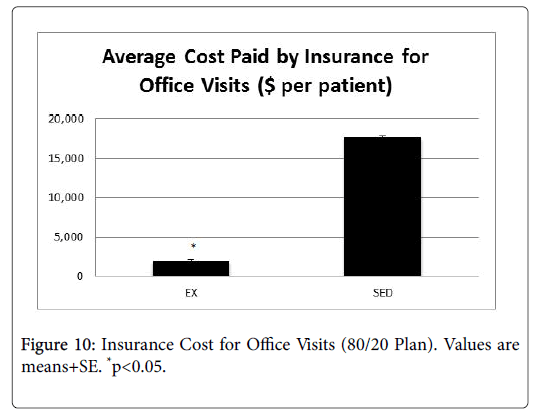
Impact to insurance
In order to determine the impact to insurance payers, data related to number of office visits and ER visits were analyzed by an independent health economist, who conducted a cost-savings analysis for each group. Cost savings was based on an 80/20 insurance payment plan, with the cost of an adult office visit ranging from $130-$180, and the cost of an ER visit ranging $580-$700. Figures 8-10 present the average cost of the insurance payers for both the EX and SED groups. In both cases, the cost for the EX group was significantly lower than the SED group (p<0.05).
Discussion
Maple Tree Cancer Alliance employs a unique system of exercise prescription through a phase system designed to protect immunity and maximize physical health during cancer treatment. The purpose of this investigation was to determine if this approach to exercise training during cancer treatment helped to minimize length of hospital stays, hospital readmits, ER visits, and treatment tolerance, in addition to feelings of fatigue, depression, and anxiety. Result from this investigation point to a protective effect of moderate-intensity exercise that translated to reductions in all of the aforementioned variables, as well as cost savings for the payer, provider, and patients, alike.
Exercise is a valid rehabilitative measure that can be introduced at various points along the cancer trajectory. The underlying mechanism contributing to the protective effect of exercise on cancer is unclear and likely to be multi-faceted. It is possible that there is a connection between body size and fat stores [30-33]. An increased body mass index has been linked to increased inflammation and levels of insulin and insulin-like growth factors, all of which are known to exacerbate the long-term and untoward effects of cancer [34].
In addition, lipid peroxidation and reactive oxygen species (ROS) may be a contributor to the protective effect of exercise. Aerobic metabolism is dependent on oxidative phosphorylation, whereby ATP is formed through mitochondrial electron transport. This involves the sequential transfer of electrons through a series of oxidation/reduction reactions. Cytochrome C oxidase serves as the final electron acceptor for this system. Under normal conditions, this reduces oxygen to water. However, occasionally intermediate proteins in the mitochondrial electron transport system may release electrons directly to oxygen, resulting in the formation of ROS. ROS therefore are partially reduced and highly reactive metabolites of oxygen [35], and cause damage by binding to proteins, lipids, and nucleic acids and altering their function [36,37].
The increased oxygen consumption that accompanies exercise often triggers a concurrent increase in ROS. The body is equipped with the enzymatic antioxidants superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) to minimize damage caused by ROS. However, if the amount of ROS exceeds the cell’s antioxidant capacity, a state of oxidative stress results. This leads to lipid peroxidation, DNA damage, and apoptosis [38,39]. It is possible that exercise may induce oxidative stress and subsequent apoptosis of pre-cancerous and cancer cells, and thus protect against the deleterious effects of cancer.
Economic impact
The economic impact of cancer is tremendous. In fact, the Agency for Healthcare Research (AHRQ) estimates that the direct medical cost of cancer in 2014 was $87.8 billion. Of this, 58% were for outpatient hospital visits and 27% was for inpatient hospital stays [40]. This number is expected to rise to more than $175 billion by the year 2020 [41]. The rising cost of cancer care has a negative impact on many stakeholders involved in the health care system, including insurance providers, hospitals, and patients, making cancer less affordable for a number of Americans. The term “financial toxicity” has been used to describe this growing concern, as medical costs are the leading cause of personal bankruptcy [42]. In fact, people living with cancer are three times more likely to file for bankruptcy than those without cancer [43]. Patients are sometimes forced to choose between cancer treatment and paying for food, shelter, and other necessities. One study reported that among 164 patients, 45% reported cost-related medication nonadherence [44]. A quarter of patients with insurance reported that they had used up all or most of their savings to deal with cancer [45].
The increasing cost of health care also affects health insurance companies, who have responded by decreasing the utilization of services and increases in patient financial responsibility through larger co-pays and high deductibles. Finally, the increasing cost of treatment influences care providers, whose desire is to provide treatment of the greatest benefit, without regard for cost.
In light of this growing demand to reduce health-care costs is the need for high-quality, evidence-based cancer care. To deliver the highest value of care, it must be patient-centered, integrated, and coordinated, achieving the most meaningful outcomes at a sustainable cost [46]. Individualized exercise prescription falls in line with an integrated system of medical care, and the present data indicate that it would increase cost savings for patients, payers, and providers alike. However, nationally, only two percent of patients are ever referred to oncology rehabilitation services [47]. Thus, addressing this gap in care must be addressed. It is our recommendation that the unique phased system of exercise employed by Maple Tree Cancer Alliance be part of the standard program of care for individuals battling cancer.
References
- https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html
- American Cancer Society. Cancer Statistics. National Cancer Institute, 2017.
- Simon S (2016) ACS Report: Number of US Cancer Survivors Expected to Exceed 20 million by 2026.
- Lind J (1992) Tumor cell growth and cell kinetics. Sem Oncol Nurs 8: 3-9.
- National Institutes of Health (2011) Cancer Costs Projected to reach at least $158 Billion in 2020.
- Rieger PT (2006) Cancer biology and implications for practice. Clin J Oncol Nurs 10: 457-460.
- Honea N, Brant J, Beck SL (2007) Treatment-related symptom clusters. Sem Oncol Nurs 23: 142-151.
- Berrak SG, Ewer MS, Jaffe N, Pearson P, Ried H, et al. (2001) Doxorubicin cardiotoxicity in children: Reduced incidence of cardiac dysfunction associated with continuous-infusion schedules. Oncol Rep 8: 611-614.
- Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, et al. (1982) Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Ann Intern Med 96: 133-139.
- Legha SS, Benjamin RS, Mackay B, Yap HY, Wallace S, et al. (1982) Adriamycin therapy by continuous intravenous infusion in patients with metastatic breast cancer. Cancer 49: 1762-1766.
- Li T, Danelisen I, Bello-Klein A, Singal PK (2000) Effects of probucol on changes of antioxidant enzymes in adriamycin- induced cardiomyopathy in rats. Cardiovasc Res 46: 523-530.
- Siveski-Iliskovic N, Hill M, Chow DA, Singal PK (1995) Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation  91: 10-15.
- Katsuhisa S, Takahashi M, Kitano M, Sugimura T, Wakabayash K (2006) Suppression of Azoxymethane-induced Colonic Premalignant Lesion Formation by Coenzyme Q10 in Rats. Asian Pacific J Cancer Prev 7: 599-603.
- Nowak D, Pierscinski G, Drzewoski J (1995) Ambroxol inhibits doxorubicin-induced lipid peroxidation in heart of mice. Free Radic Biol Med 19: 659-663.
- Al-Majed AA, Gdo AM, Al-Shabanah OA, Mansour MA (2002) Alpha-lipoic acid ameliorates myocardial toxicity induced by doxorubicin. Pharmacol Res 46: 499-503.
- Abdel-Wahab MH, El-Mahdy MA, Abd-Ellah MF, Helal GK, Khalifa F, et al. (2003) Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat’s heart. Pharmacol Res 48: 461-465.
- Dziegiel P, Murawska-Cialowicz E, Jethon Z, Januszewska L, Podhorska-Okolow M, et al. (2003) Melatonin stimulates the activity of protective antioxidative enzymes in myocardial cells of rats in the course of doxorubicin intoxication. J Pineal Res 35: 183-187.
- Cusack BJ, Gambliel H, Musser B, Hadjokas N, Shadle SE, et al. (2006) Prevention of chronic anthracycline cardiotoxicity in the adult Fischer 344 rat by dexrazoxane and effects on iron metabolism. Cancer Chemo ther Pharmacol 58: 517-526.
- Saad SY, Najjar TA, Al-Rikabi AC (2001) The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res 43:211-218.
- Mrozek E, Rhoades CA, Allen J, Hade EM, Shapiro CL (2005) Phase I trial of liposomal encapsulated doxorubicin (Myocet; D-99) and weekly docetaxel in advanced breast cancer patients. Ann Oncol 16: 1087-1093.
- Combs AB, Hudman SL, Bonner HW (1979) Effect of exercise stress upon the acute toxicity of adriamycin in mice. Res Comm Chem Pathol Pharmacol 23: 395-398.
- Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ (1985) Â Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol 59: 1298-1303.
- Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, et al. (2005) Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol 100: 451-460.
- Ascensao A, Magalhaes J, Soares JM, Ferreira R, Neuparth MJ, et al. (2005) Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopahty and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol 289: H722-H731.
- Chicco AJ, Hydock DS, Schneider CM, Â Hayward R (2006) Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol 100: 519-527.
- Chicco AJ, Schneider CM, Hayward R (2005) Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol 289: R424-R431.
- Chicco AJ, Schneider CM, Hayward R (2006) Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovas Pharmacol 47:182-189.
- Silver JK, Baima J, Mayer RS (2013) Impairment driven cancer rehabilitation: An essential component of quality care and survivorship. CA Cancer J Clin 63: 295-317.
- Broocks A, Pirke KM, Schweiger U, Tuschl RJ, Laessle RG, et al. (1990) Cyclic ovarian function in recreational athletes. J Appl Physiol 68: 2083-2086.
- Russell JB, Mitchell D, Musey PI, Collins DC (1984) The relationship of exercise to anovulatory cycles in female athletes: Hormonal and physical characteristics. Obstet Gynecol 63:452-456.
- McTiernan A, Kooperberg C, White E (2003) Is exercise effective in reducing the risk of breast cancer in postmenopausal women? JAMA 290: 1331-1336.
- Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG (1989) The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol 129: 1120-1131.
- Phillips  K, Lindeman GJ (2014) Breast Cancer Prevention for BRCA1 and BRCA2 Mutation Carriers. Future Oncol 104: 499-502.
- Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29: 222-230.
- Kanter MM, Hamlin RL, Unverferth DV, Davis HW, Merola AJ (1985) Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol 59: 1298-1303.
- Minotti G, Cairo G, Monti E (1999) Role of iron in anthracycline cardiotoxicity: New tunes for an old song? The FASEB Journal 13: 199-212.
- Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52: 711-760.
- Abraham R, Basser RL, Green MD (1996) A risk-benefit assessment of anthracycline antibiotics in antineoplastic therapy. Drug Saf 15: 406-429.
- Agency for Healthcare Research and Quality. Medical Expenditure Panel Survey 2017.
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML (2011) Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst 103: 117-128.
- Himmelsetin D (2009) Medical Bankruptcy in the United States, 2007: Results of a National Study. The Am J Med 122: 741-746.
- Felder TM, Bennett CL (2013) Special Series: State of Oncology Practice-Commentary: Can Patients Afford to Be Adherent to Expensive Oral Cancer Drugs?: Unintended Consequences of Pharmaceutical Development. J Oncol Prac 17 : 64s-66s.
- Zulllig LI, Peppercorn JM, Schrag D, Taylor DH, Lu Y, et al. (2013) Financial distress, use of cost-coping strategies, and adherence to prescription medication among patients with cancer. J Oncol Prac 20: 60s-63s
- National Survey of Households Affected by Cancer; USA Today, Kaiser Family Foundation, and Harvard School of Public Health 2006: Kaiser Family Foundation Publication #7590.
- Alvarnas J, Majkowski GR, Levine AM (2015) Moving toward economically sustainable value-based cancer care in the academic setting. JAMA Oncol 1: 1221-1222.
- Smith SR, Zheng JY (2017) The Intersection of Oncology Prognosis and Cancer Rehabilitation. Curr Phys Med Rehabil Rep 5: 46-54.
Citation: Wonders K (2018) Supervised, Individualized Exercise Programs Help Mitigate Costs during Cancer Treatment. J Palliat Care Med 8: 338. DOI: 10.4172/2165-7386.1000338
Copyright: © 2018 Wonders K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
9th World Conference on Nursing Education & Nursing Practice
Toronto, Canada
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3736
- [From(publication date): 0-2018 - Nov 24, 2024]
- Breakdown by view type
- HTML page views: 3085
- PDF downloads: 651