Superiority of LC- MS3- Over LC-MRM Approach Regarding Quantification of Amantadine along with Applying its Utilization in Therapeutic Amantadine Monitoring in Human Plasma
Received: 01-Feb-2023 / Manuscript No. jabt-23-89065 / Editor assigned: 03-Feb-2023 / PreQC No. jabt-23-89065 / Reviewed: 17-Feb-2023 / QC No. jabt-23-89065 / Revised: 21-Feb-2023 / Manuscript No. jabt-23-89065 / Accepted Date: 27-Feb-2023 / Published Date: 28-Feb-2023 QI No. / jabt-23-89065
Introduction
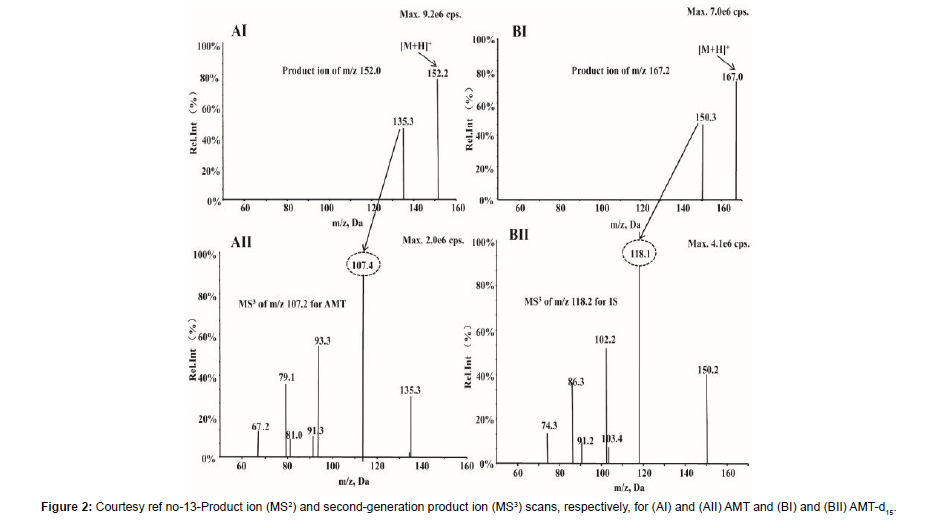
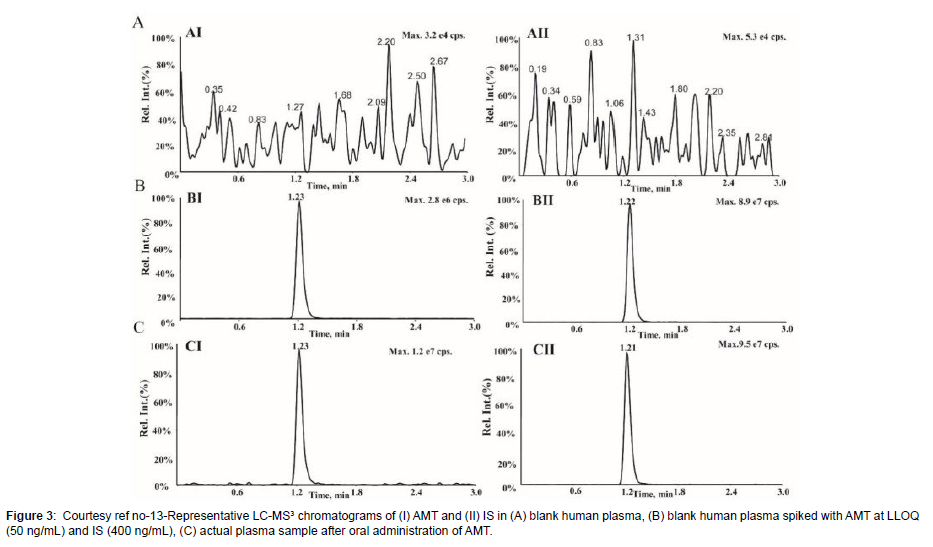
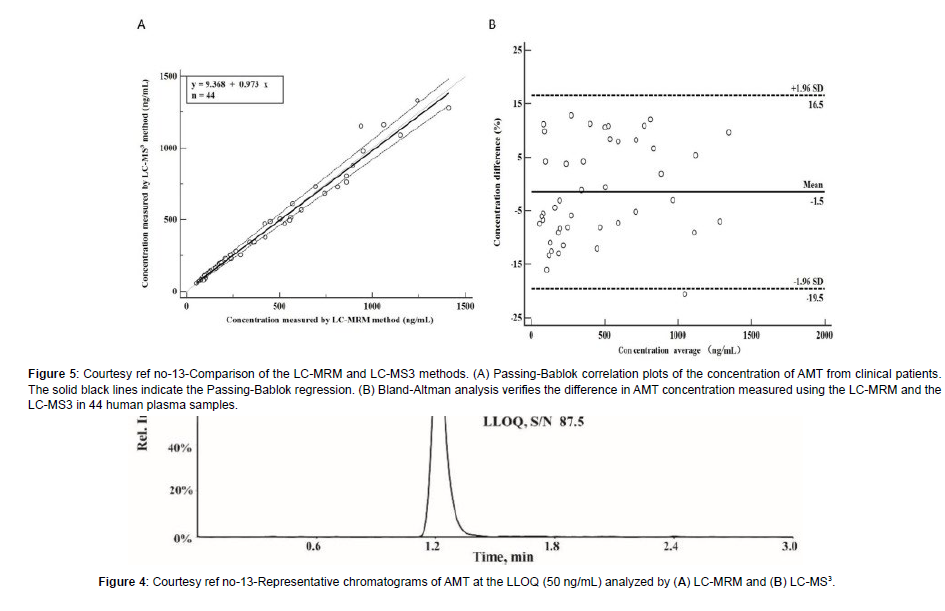
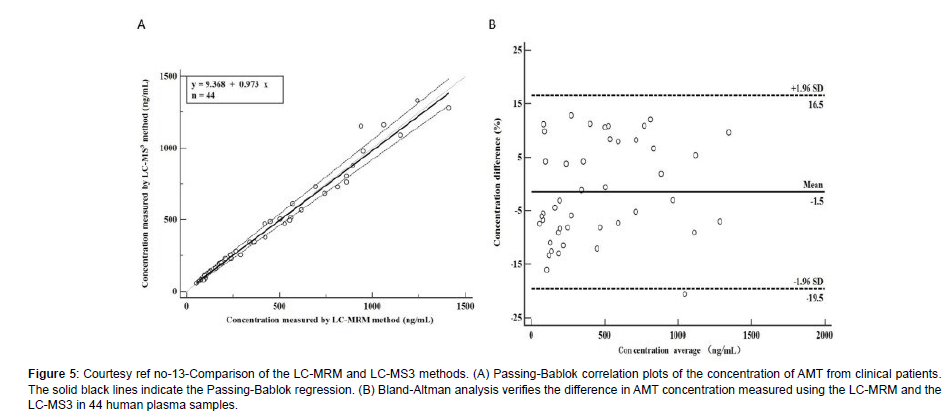
Amantadine hydrochloride (C10H17N HCL; Figure 1) [alias tricyclic-[3.3.1.13.7]decane-1-amine) rep resents a synthetic amine belonging to the amantadine class.It gets obtained from amantadine which targets the M2 ion channel which is basically used in the form of antiviral treatment. Amantadine (AMT) received ecommendation from the Food along with Drug administration (FAO) in2008 regarding utilization For Parkinson’s disease along with Multiple Sclerosis (MS) [1]. Additionally, utilization of AMT gets done for treating extrapyramidal responses which takes place at the time of drugs which act in the form of non competitive N-methyl D aspartate(NMDA) receptor antagonists like levodopa stimulated dyskinesia(LSD).At present AMT is just the drug having validated effectiveness regarding ameliorating LSD [2]. Oral administration of AMT is utilized along with basically gets eliminated by Kidney [3]. In case of Clinical studies it has been documented that patients possessing the renal dysfunction might result in escalation of half life of AMT [4]. Nevertheless, as per Clinical Guidelines advocated ,such patients along with their older counterparts are, required to monitor their AMT plasma quantities. In general AMT results in considerable inimical sequelae, inclusive of dry mouth, weakness, blurring of vision, sleeplessness, abnormalities of consciousness along with hallucinations that get impacted by AMT plasma quantities as well as drug dosing [5]. In view of that AMT therapeutic drug monitoring (TDM) is essential for sustenance of therapeutic plasma quantities in addition to prevention of inimical sequelae. At present variable assessment strategies regarding TDM for AMT exist which are inclusive of immunoassays,util for longperiods [6], the utilization of high performance liquid chromatography with fluorescence determination (HPLC-Flu) [7], high performance liquid chromatography with ultraviolet ((HPLC-UV) determination [8], as well as liquid chromatography(LC), mass spectrometry (MS) which is coupled with tandem MS(LC- MS/ MS) [9]. In view of AMT does not possess the capacity of absorption along with fluorescence properties hence it needs a derivative from the original by HPLC-UV along with HPLC-Flu for escalation of its sensitivity. Nevertheless, in contrast to other assessment strategies, LC- MS/ MS have the properties of easy sample processing strategy, greater sensitivity along with selectivity. Additionally, LC- MS/ MS possess considerable improvement of being precise along with in correctness.Hence LC- MS/ MSare advocated in the form of gold standard for determination of agents in biological samples.Till date quantitative determination of AMT by LC- MRM assays where utilization of QTRAP tandem mass spectrometry (MS) [10]. Nevertheless, there is no documentation regarding determination of agents in biological samples with the utilization of triple stage fragmentation (MS3) approaches. Sun et al. [13], posited an approach regarding enhancement of sensitivity along with greater selectivity of AMT in quantitative determination of with utilization of MS3 on a hybrid quadruople -linear iontrap (QqLIT).The objective was generating improvement of determination of AMT in human plasma. Additionally, they contrasted LC- MRM dependent assays along with LC- MS3- dependent approach. This MS3- dependent determination represents a scanning method of QTRAP tandem mass spectrometry, possessing a greaterexcitation effectiveness in addition to rapid scanning rate (20,000Da/s) [11]. At the time of MS3-dependent determination the precursorof the analyte ions initially get selected in Q1 followed by fragmentation in the cell for collision (Q2) for forming generated ions by collision modulated breakdown . Subsequently the generated ions enrichment followed by capturing is done by a linear ion trap(Q3) [12]. Lastly the generated ions which have been selected to undergo more fragmentation in the linear ion trap for producing secondary fragment ions determined by the detector. Sun et al. [13], produced the LC- MS3- system in the form of a Shimadzu Exion LC- 20AD HPLC Pump which was coupled with a QTRAP5500 tandem mass spectrometer. Initially, the plasma samples got prior treatment with acetonitrite in the form of extraction solution for precipitation of protein. Subsequently separation of amantadine along with amantadine d15 (AMT- d15) was carried out over an Agilent Poroshell 120 SB-C18 column (4.6mm x 50, 2.7μm) with the utilization of isocratic elution with solvent A (70%, 0.1 formic acid) along with solvent (30% acetonitrite) at a flow rate of 0.8 ml/min. The full run time/every sample consumed 3 minutes. utilization of triple stage fragmentation transits at m/z 152.2->135.3- >107 regarding quantification of AMT in the positive ion mode along with m/z 67.0,150.3,118.1 regarding quantification of AMT- d15. The -LC-MS3 was linear(r>0.995) with a quantity range of 50-00ng/ml. The lesser limit of quantification(LLOQ)was 50 ng/ml, in addition to intraday as well as inter day validity along with correctness were <8.0%, at allquantities. Additionally, retrieval in addition to matrix actions for AMT in human plasma were in agreeable range. Regarding stabilization, no significant breakdown in all situations. All the outcomes were in agreement with FAO Guidelines regarding biological approach corroboration. The innovativeness of MS3 assay was the provision of an approach possessing greater sensitivity as well as selectivity. Application with success was feasible in44 human plasma samples in addition to which contrasting of derived outcomes weredone with another liquid chromatography-multiple reaction monitoring (LC-MRM) approach. The Passing Bablok regression coefficients as well sBlandAltmanplot displayed no variations amongst LC-MS3 along with LC-MRM approach, pointing to that this generated LC- MS3- approach possessed dependency as well as is a precise assays for detection of chemicals in biological
samples with utilization of LC-MS3-approach (Figures 2-5) [13]. Zhan Wang, X Zhang H Chen X Kim N Lee MS in the form of along with , that lesser, besides in addition to been as well as have been in view of from the production GnRH LH surge generation production Kisspeptins stimulation greater escalated preovulatory quantities of labelled as circulating estrogen crosstalk amongst follicular displayed documented that intraextracellular secondary, primary outcomes obtained Food along with Drug administration (FAO Guidelines) recommendation regarding utilization of Parkinson’s diseases Multiple Sclerosis (MS)[ responsibility takes place at the time of non-competitive N- methyl D aspartate(NMDA) antagonists present taking into account elimination of Clinical numerous studies documented, required plasma quantities cells generation, formation, besides production aberrations abnormalities sustenance of therapeutic inclusive of regarding absorption high performance liquid chromatography with fluorescence determination (HPLC-Flu) properties characteristic sensitivity specificity selectivity possess considerable improvement of documentation triple stage fragmentation approaches strategies generation greaterexcitation effectiveness sensitivity greater selectivity of AMT by LC- MS3- dependent approach systematic review and , meta-analysis protein utilization of significantly greater disorders. conditions, situations greategreater displayed dependent correlationed with association with with association with quantification effectiveness drug approach which ER-α regarding been well acknowledging that in the form of in view of in case of from the might further detailed female various, regarding variation variation variable differentiation symptomatic molecular estrogen receptors feedback supplementation basically observ with necessary essential systemic might result in in a robust possess crucial as well as other factor stimulation reaction mouse usually strateg Ultimately towards effect through undergo the which might believed tobe other inimicalsequelae ovulatoconsequences consequences about approximately without decreased probably association with amongst quantities of dysfunctional normal, healthy disease human in addition to activation, GnRH probably escalation liberated in the central result in enhancement presentation exacerbates constituents might be continued illustrated that efficacy gene in the regulation reduction expression in addition to activation, GnRH neurons in the hypothalamic Kisspeptins neurons positive negative physiological molecular along with Folliculogenesis mechanism Large antral follicles, follicles, absence ovulation musk screws ovulatorystatus metabolism modulation manipulation, which might probably might probably modulation potential controlled assessment of signaling opioidergic pathways implicated hypothalamic- pituitary-gonadal (H-P-G )axis, anterior pituitary gland takes place at the time of examination circulation independent respectively recently further validated the efficacy pointed part that estrogen receptor-α (estrogen ER-α), actor transcriptionfactor observation study demonstrated, documented crosstalk amongst selectively antagonizing endogenous suppreses therapeutic targeted absence of probably probability whereas,maximum anteroventroperiventricular area (AVPV), arcuate nucleus (Arc) preoptic area (POA) arcuate nucleus (Arc), ventro medial nucleus (VMH, paraventricular nucleus (PVN)- as well as suprachiasmatic nucleus(SCN) Akin to that, Uenoyama agreement NF-κB prior studies repressionof suppression conditions greater, eating disorders. situations ameliorated G-protein coupled receptor antagonizing antagonists rodents followed by GnRH / gonadotropins liberation Nevertheless, other however, studies estrogen receptorbound (ER-α), anterior precisions Additionally, contrast to Actually Conversely/On the other hand, Nevertheless, Noticeably, Intriguingly Interestingly Interest Polycystic ovary syndrome (PCOS) Moreover Furthermore variability treatment More recently, Moreover Furthermore cerebral, that Despite, Subsequent birth remarkable association with believed Simultaneously Nevertheless, variable Astonishingly lesser tonic in the central controlled estrogen treatment Osteoarthritis n Moreover required Furthermore intercellular cell adhesion molecule [ICAM]-1] possessing the capacity of, chronic low grade Inflammation Hepatocellular carcinoma (HCC) zinc finger nucleases,) zinc finger transcription activator like effector nucleases (TALEN), Clustered regularly Interspersed short Palindromic Repeats(CRISPR) RNA nuclease(s) Designer nuclease(s) against pathological visceral adipose various tissue damage injure (VAT) transcription besides recent endothelial adhesion molecule nuclear factor κB confer protection in which contrast to highlilighting emphasized on the result in breakdown leukocyte, of approaches strategies, surrounding these crosstalk survival, proliferation as well as differentiation. For controlling the assessed generation extracellular matrix [ECM] events organs regarding tissue subsequent phages which modulation following, along with followed by repression of adults g Intriguingly illustrated that.
Figure 5: Courtesy ref no-13-Comparison of the LC-MRM and LC-MS3 methods. (A) Passing-Bablok correlation plots of the concentration of AMT from clinical patients. The solid black lines indicate the Passing-Bablok regression. (B) Bland-Altman analysis verifies the difference in AMT concentration measured using the LC-MRM and the LC-MS3 in 44 human plasma samples.
References
- Leonov H, Astrahan P, Krugliak M, Arkin IT (2011) How do aminoadamantanes block the influenza M2 channel, and how does resistance develop? Journal of the American Chemical Society 133:9903-9911.
- Brigham EF, Johnston TH, Brown C, Holt JD, Fox SH, et al. (2018) Pharmacokinetic/pharmacodynamic correlation analysis of amantadine for levodopa-induced dyskinesia. Journal of Pharmacology and Experimental Therapeutics 367:373-381.
- BleidnerWE, Harmon JB, HewesWE, Lynes TE, Hermann EC, et al. (1965) Absorption, distribution andexcretion of Amantadine hydrochloride. JPharmacol Exp Ther 150: 484-90.
- Aoki FY, Sitar DS (1988) Clinical pharmacokinetics of amantadine hydrochloride. Clinical pharmacokinetics 14:35-51.
- Effects of renal impairment on the pharmacokinetics of once-daily amantadine extended-release tablets. CNS drugs 33:783-789.
- Xie S, WenK, WangS, WangJ, PengT, et al. (2019)Quantitativeand rapid detection of Amantadine and chloramphenicol on variousquantum dots with the same excitations.Anal Bioannal Chem 411:2131-40.
- Puente B, Garcia MA, Hernandez E, Bregante MA, Perez S, et al. (2011) Determination of memantine in plasma and vitreous humour by HPLC with precolumn derivatization and fluorescence detection. Journal of chromatographic science 49:745-752.
- Michail K, Daabees H, Beltagy Y, Elkhalek MA, Khamis M, et al. (2013) High-performance liquid chromatographic determination of memantine in human urine following solid-phase extraction and precolumn derivatization. Journal of AOAC International 96:1302-1307.
- Maksymiuk AW, Tappia PS, Bux RA, Moyer D, Huang G, et al. (2021) Use of amantadine in the evaluation of response to chemotherapy in lung cancer: a pilot study. Future Science OA 7:FSO679.
- Jiang JG, Zhang XR, Zhang YH, Song GS (2013) Rapid identification 15 effective components of anti common cold medicine with MRM by LC-MS/MS. Yao xue xue bao Acta Pharmaceutica Sinica 48:94-97.
- Dziadosz M, Klintschar M, Teske J (2017) Imatinib quantification in human serum with LC-MS3 as an effective way of protein kinase inhibitor analysis in biological matrices. Drug Metabolism and Personalized Therapy 32:147-150.
- Kobayashi H, Imai K (2021) Recent progress in FD-LC-MS/MS proteomics method. Frontiers in chemistry 9: 640336.
- Sun Q, Cao H, Liu Y, Li Y, Huang J, et al. (2022) Comparison of LC-MS3 and LC-MRM Methods for Quantifying Amantadine and Its Application in Therapeutic Amantadine Monitoring in Human Plasma. Molecules 27:7619.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Kaur KK, Allahbadia GNK, Singh M (2023) Superiority of LC- MS3- Over LC-MRM Approach Regarding Quantification of Amantadine along with Applying its Utilization in Therapeutic Amantadine Monitoring in Human Plasma. J Anal Bioanal Tech 14: 497.
Copyright: © 2023 Kaur KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1553
- [From(publication date): 0-2023 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 1276
- PDF downloads: 277