Research Article Open Access
Sulfonylureas can Stimulate Insulin Release by Flip-Flop across Phospholipid Membranes
Jamal Alruwaili*Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, Northern Border University, Arar-91431, Saudi Arabia
- Corresponding Author:
- Jamal Alruwaili
Department of Medical Laboratory
Technology, Faculty of Applied Medical Sciences
Northern Border University, P.O. Box 1321
Northern Border 91431, Saudi Arabia
Tel: +966535186118
E-mail: jamalalruwaili786@gmail.com
Received date: November 30, 2015; Accepted date: January 28, 2016; Published date: February 08, 2016
Citation: Alruwaili J (2016) Sulfonylureas can Stimulate Insulin Release by Flip-Flop across Phospholipid Membranes. J Biotechnol Biomater 6:218. doi:10.4172/2155-952X.1000218
Copyright: © 2016 Alruwaili J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The design of new drugs and drug-delivery systems requires insight about how a drug interacts with the phospholipid bilayer. Sulfonylureas are used to treat type 2 diabetes mellitus because they stimulate insulin secretion from pancreatic β-cells. Two generations of Sulfonylurea drugs have been marketed to treat type II diabetes mellitus. Tolbutamide was chosen to represent the first generation of these drugs, whereas Glibenclamide was from the second generation. This study investigated how the Sulfonylureas cross the phospholipid membrane and it found that these drugs are transported by non-energy dependent flip-flop mechanism. A u-shaped conformation for both Tolbutamide and Glibenclamide is being proposed to explain the binding of these drugs to the phospholipid membrane.
Keywords
Sulfonylureas; Flip-flop; Type 2 diabetes mellitus
Introduction
Pancreatic β-cells synthesize insulin to maintain blood glucose levels within a relatively narrow range. Any change in their functioning has an effect on glucose homeostasis. This apparent simplicity of the β-cell’s role sharply contrasts with the astonishing complexity of its regulation, which is ensured by an array of metabolic (glucose and other nutrients), neural, hormonal, and pharmacological factors such as Sulfonylureas.
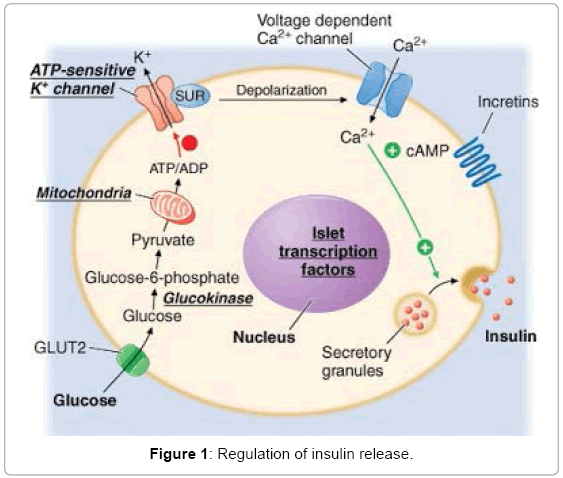
Sulfonylureas block the KATP channels of β-cell of pancreas by binding to Sulfonylurea receptor (SUR) which results in depolarization of the cell membrane. This also raises [Ca2+]i, and increases insulin secretion (Figure 1). In pancreatic β-cells, the ATP/ADP ratio determines KATP channel activity. Under normal conditions, the KATP channels in pancreatic β-cells are spontaneously active, allowing potassium ions to flow out the cell [1]. In the presence of higher glucose metabolism, and consequently increased levels of ATP, the KATP channels close, causing the membrane potential of the cell to depolarize, thus promoting insulin release [1]. The change from one state to the other takes place quickly due to multimerization at C-terminus of KATP channel molecules [2].
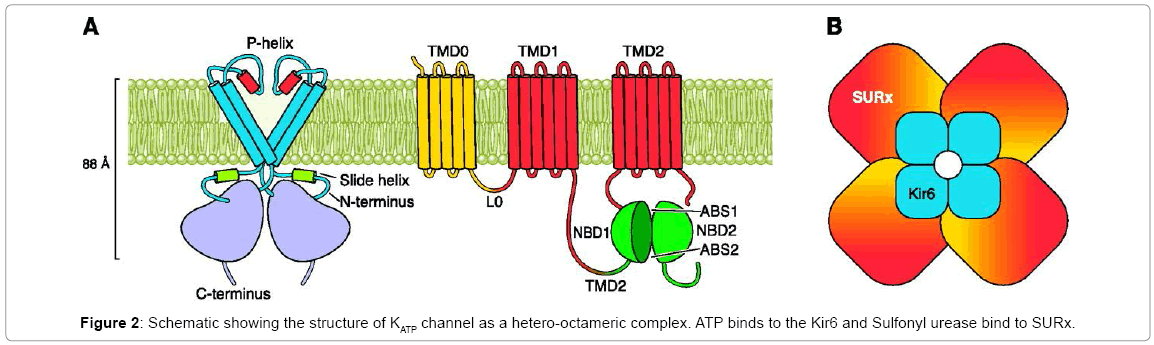
The KATP channel is a hetero-octameric complex of two different types of protein subunits: (a) an inwardly rectifying K+ channel called Kir6.x, and (b) a Sulfonylurea receptor (SUR) [3,4] (Figure 2). Kir6.x assembles as a tetramer to form the channel pore (Figure 2). Binding of ATP to its intracellular domains produces channel inhibition [5]. SUR is a member of the family of ATP-binding cassette transporters (ABC transporters). They typically contain 17 transmembrane helices (TMs), arranged as one group of 5 TMs, and two repeats each of 6 TMs and a cytosolic loop containing consensus sequences for nucleotide binding and hydrolysis (Figure 2) [6-9]. SUR makes Kir6.2 sensitive to certain drugs, such as the inhibition by Sulfonylureas [3,4].
SUR possesses a large drug-binding pocket that can bind many different kinds of compounds. The binding of Sulfonylureas to the cytoplasmic domains of SUR1 results in the closing of the Kir6.2 pore. The exact mechanism of this is unknown. However, it is believed that there must be a close physical association between the Glibenclamidebinding site on SUR and the Kir6.2 subunit, since both proteins can be photo-affinitylabeled by [125I]-Glibenclamide [10]. It has been shown that five residues within the first TM of Kir6.2 are essential for interaction with SUR [11]. Recent studies have also indicated that the first five TMs of SUR1 are involved [12].
Sulfonylureas are used to treat type 2 diabetes because they stimulate insulin secretion from pancreatic β-cells. They act by binding to the SUR subunit of the ATP-sensitive potassium (KATP) channel and inducing channel closure. It is also known that Tolbutamide blocks channels containing SUR1 (β-cell type), but not SUR2 (cardiac, smooth muscle types), whereas Glibenclamide can block both types of channels. This study investigated delivery of these compounds across the phospholipid membrane of small unilamellar vesicles (SUVs) by fluorimetric assays and proposed a model to explain the binding of these compounds to the phospholipid membrane.
Materials and Methods
Both Tolbutamide and Glibenclamide were purchased from Sigma Chemicals (St. Louis, USA). D2O was purchased from Cambridge Isotope Labs (Boston, USA), Egg PC was purchased from Avanti Polar Lipids (Pelham, USA). Pyranine (8-Hydroxy-1,3,6-pyrenetrisulfonate) from Eastman Kodak Co. (USA) and the G-25 Sephadex gel was bought from Amersham Pharmacia Biotech Inc. (Uppsala, Sweden). The 5 mm NMR tubes and 3-(Trimethylsilyl) propionic-2,2,3,3-d4 acid sodium salt (TSP) were from Wilmad Labs (Buena, USA). Bovine Serum Albumin (BSA) used in the fluorimetric experiments was bought from Sigma Chemicals (St. Louis, USA). NMR experiments were performed on a Bruker DMX 500 spectrometer. The fluorimetric experiments were performed using a FluoroMax 2 fluorimeter. The mass spectra were acquired on a Finnegan Electrospray Ionization Mass Spectrometer. The samples were sonicated by the use of a Branson 350 sonifier fitted with a micro-tip. The pH was measured by the use of a Beckman Φ 71 pH meter.
Control experiments
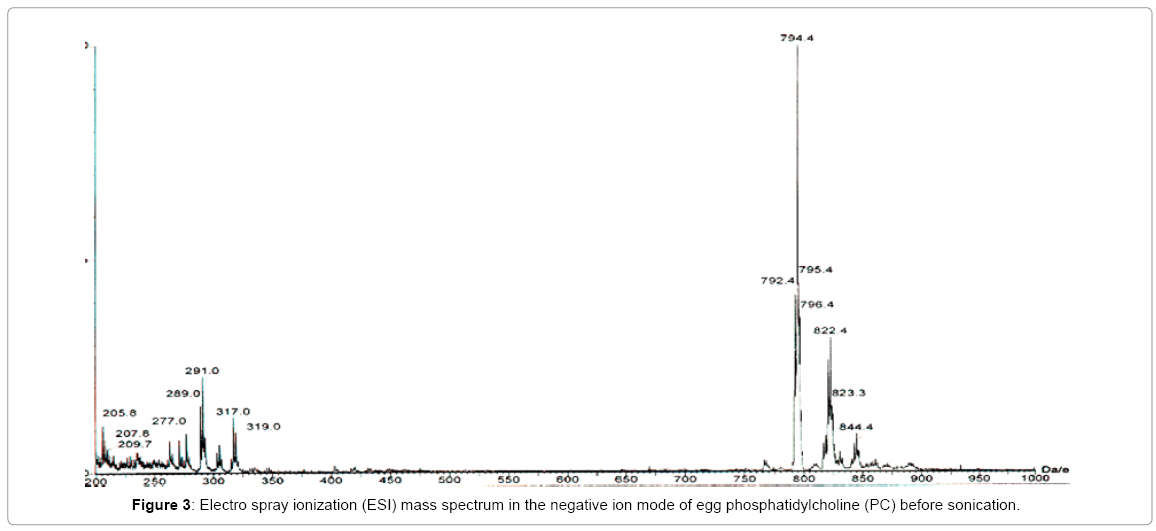
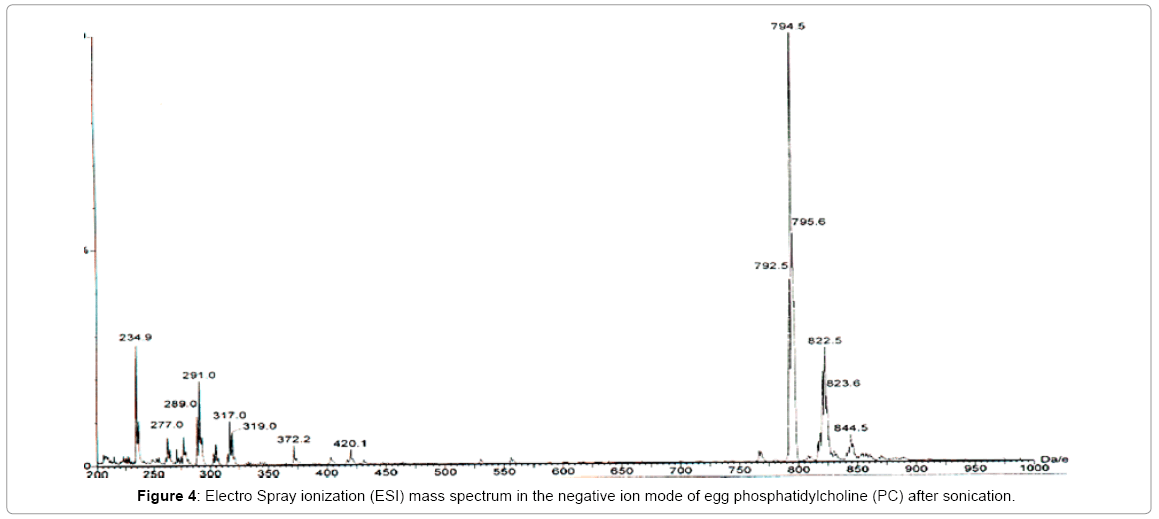
The effect of sonicationon egg Phosphatidylcholine (PC) during the preparation of small unilamellar vesicles (liposomes with a single lipid bilayer) was investigated by acquiring electrospray ionization mass spectra in the negative ion mode, before and after sonication of the sample (Figures 3 and 4). For this experiment, 2.25 mL of commercially available egg PC solution in chloroform (sold by Avanti) was drawn by a pipette. The mass of PC was estimated to be 45 mg. The chloroform was evaporated under nitrogen and the dried sample was lyophilized for one hour. The lyophilized PC was suspended in deionized water and was then sonicated for one hour in an ice/water bath by a Branson 350 sonifier using a 30% duty cycle and an output of 3. The 1.8 mL solution was extracted with an equal amount of Chloroform. The chloroform solution was then analyzed by electrospray ionization mass spec (ESI) in the negative ion mode. A similar sample was prepared for comparison but it was kept un-sonicated. Both the spectra (Figures 3 and 4) were acquired with identical acquisition parameters. These spectra show the presence of endogenous fatty acid in egg yolk PC. The overall profile of all the molecular species present in the sample is very similar except for the appearance of a signal at an m/z ratio of 234.9 which can be regarded as a contaminant.
NMR experiments
The sample of Tolbutamide in solution was prepared by adding 3 mg of Tolbutamide to 1.8 mL 0.5 w/v % KCl solutions. The sample of 20 mole % Tolbutamide incorporated in SUVs was prepared by co-sonicating 45 mg egg Phosphatidylcholine (PC) with 3.0 mg Tolbutamide in 0.5 w/v % KCl solution. 2.25 mL of commercially available egg PC solution in chloroform was drawn by a pipette. 3.0 mg of Tolbutamide was added to it. The chloroform was evaporated under nitrogen and the sample was lyophilized for one hour. 1.62 mL of 0.5 w/v % KCl and 0.18 mL D2O was then added to the lyophilized sample and it was allowed to hydrate for two hours at 4°C. The solution was then sonicated in an ice/water bath for one hour using a Micro-tip sonicator and a 30% duty cycle.
The sample was titrated by adding 1-2 μL of 1 M KOH at a time. The sample of 10 mole % Glibenclamide incorporated in SUVs was prepared by adding 146 μL of 21 mM solution of Glibenclamide in Dimethyl Sulfoxide (DMSO) to 0.9 mL of 0.5 w/v % KCl solution and 0.1 mL D2O containing 25 mg PC pre-sonicated to form SUVs. The SUVs were prepared by the method already described before. The pH of the SUV solution was 10.63. The solution turned milky on the addition of DMSO and its pH dropped to 7.92. The pH was raised to 10.28 and the solution became translucent immediately.
1-D 1H NMR experiments were performed at 20ºC in a Bruker DMX 500 NMR spectrometer. 3-(Trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TSP) was added to the samples prior to the NMR experiments as an internal standard. The 1H NMR chemical shifts of Tolbutamide are shown in Table 1. The 1H NMR chemical shifts of Glibenclamide were non-rigorously assigned. The downfield 1H chemical shift of the phenyl ring of both Tolbutamide and Glibenclamide was plotted against the pH of the solution. Curve-fitting was done using the “best fit”. The pH value corresponding to the midpoint of the sigmoidal curve was taken as the pKa of the sample. The pKas of Tolbutamide were determined both in solution and as 20 mole % additives to small unilameller vesicles (SUVs). The pKa of 10 mole % Glibenclamide was determined only in SUVs because of its insolubility in aqueous solution. All the pKa determinations were done by plotting chemical shift versus bulk pH [13].
| Aliphatic methyl group | 0.8-0.9 ppm |
| Aromatic methyl group | 2.3-2.4 ppm (solvent-dependent) |
| Methylene groups | 1.2-1.4 ppm |
| Aromatics ring 1H (ortho to SO2) |
7.72-7.86 ppm (solvent-dependent) |
| Aromatics ring 1H (meta to SO2) |
7.08-7.15 ppm (solvent-dependent) |
| NH (adj. to SO2) | exchangeable |
| Other NH | exchangeable |
Table 1: 1H Chemical shift assignments of Tolbutamide.
Fluorimetric experiments
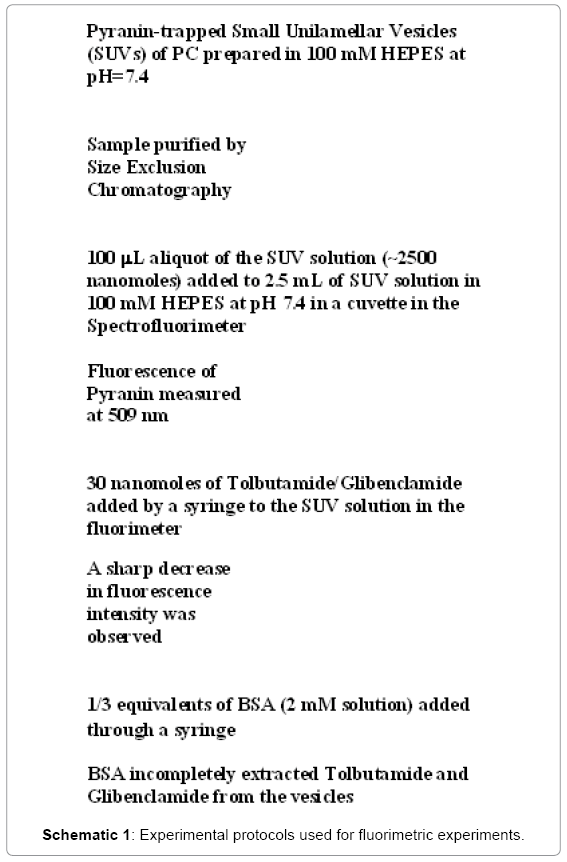
Pyranine, a pH-sensitive dye (Figure 5), was trapped inside small unilamellar vesicles (SUVs) that were prepared by established methods [14] (Schematic 1). 2.25 mL of commercially available egg Phosphatidylcholine (PC) solution in chloroform (sold by Avanti) was drawn by a pipette. The mass of PC was estimated to be 45 mg. The chloroform was evaporated under nitrogen and the dried sample was lyophilized for one hour. At the end of the hour, 1.71 ml of 100 mM HEPES at pH 7.4 was added to it along with 90 μL of 0.5 mM Pyranine solution (pH 8.2). The sample was centrifuged for twenty minutes in a low speed centrifuge. It was then sonicated for one hour in an ice/water bath by a Branson 350 sonifier using a 30% duty cycle and an output of 3. Pyranine was separated from the SUVs by the use of a G-25 Sephadex column containing 5 g of packing material.
Pyranine did not come off the column by elution but the SUV solution eluted off with 100 mM HEPES at pH 7.4. The purified SUV solution was stored at 4ºC overnight before use. A 100 mL aliquot of the SUV solution (~2500 nanomoles) was added to 2.5 mL of SUV solution in 100 mM HEPES at pH 7.4. The fluorescence of the trapped Pyranine was observed at 509 nm by the Spectrofluorimeter. Then 30 nanomoles of Tolbutamide/Glibenclamide were added by a syringe to the SUV solution and a sharp decrease in fluorescence intensity was observed (Schematic 1).
Results
In literature, the pKa values of Tolbutamide and Glibenclamide are reported as 5.43 and 5.30 respectively [15]. The sulfonamide group itself has a pKa of 10.1 in solution. When this group is next to a carbonyl in a molecule, its pKa is further lowered to a value between 5 and 6.5. It is known from previous studies that the effect of the ionization state of the molecule can be felt by a group close to the ionizable group [13]. For this reason, it was decided to measure the pH-dependence of the phenyl ring 1H chemical shift of both Tolbutamide and Glibenclamide which are ortho to the sulfonamide group. Curve-fitting the pH-dependent chemical shift of the phenyl ring 1H ortho to the sulfonyl group provided sigmoidal curves in each case. The middle points of the sigmoidal curves were taken as the pKa values. The pKa of Tolbutamide was found to be 7.0 and Glibenclamide to be 6.75 when bound to SUVs.
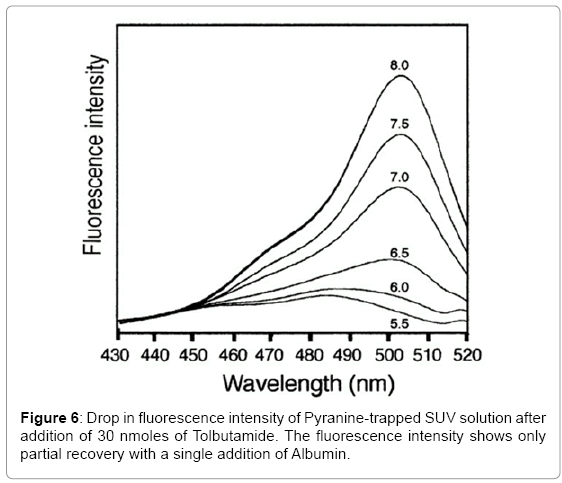
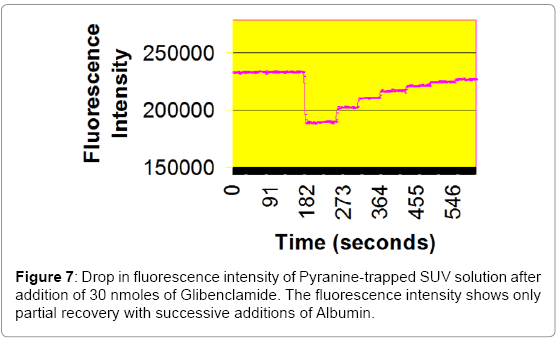
In these fluorescence experiments (Figures 6 and 7), 30 nanomole aliquots of sulfonylurea compounds were added to a buffered suspension of vesicles with trapped Pyranine. The observed drop in fluorescence intensity is due to a drop in pH inside the vesicles. When ten nanomoles of BSA (1/3 equivalents) was added to extract the Sulfonylureas from the vesicles, a partial recovery of the fluorescence intensity was observed. This is probably due to the fact that BSA does not have a very high affinity for these compounds [16]. It reportedly has three binding sites for Tolbutamide with a Kd of 21 μM. It is unlikely that these derivatives could form aggregates in solution at concentrations of 16.7 μM.
Discussion
Sulfonylureas are anti-diabetic drugs widely used in the management of type 2 diabetes mellitus [16]. The Sulfonylurea receptor is a part of the ATP-dependent potassium channel (KATP channel) in pancreatic β-cells [17]. Sulfonylurea binding leads to inhibition of the channel, which changes the resting potential of the cell, leading to an influx of calcium and insulin release. The net effect is increased responsiveness of β-cells to both glucose and non-glucose secretagogues, resulting in more insulin being released [16].
KATP channel openers, such as Sulfonylureas, which bind to SUR, promote ATPase activity in purified sarcolemma. At higher concentrations, openers reduce ATPase activity, possibly through stabilization of Mg-ADP complex at the channel site. K1348A and D1469N mutations attenuate the effect of openers on KATP channel activity. Sulfonylurea-induced channel activation is also inhibited by the creatine kinase/creatine phosphate system that removes ADP from the channel complex. Mutations in the SUR1 gene or the Kir6.2 gene lead to the loss of ATPase activity. The cell can thus become depolarized resulting in calcium influx. The high cytosolic calcium concentrations cause the release of insulin and produce a syndrome called persistent hyperinsulinemic hypoglycemiaof infancy [18].
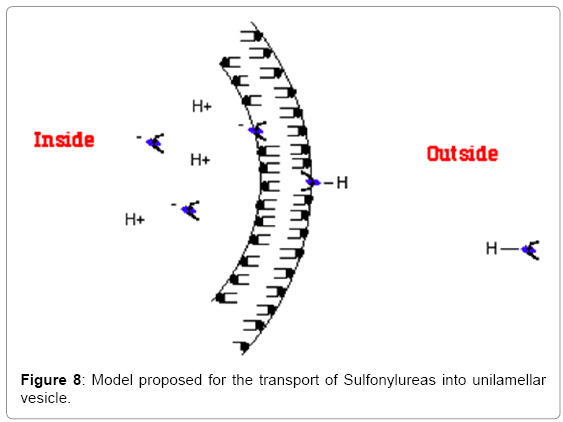
Both Tolbutamide and Glibenclamide, which represent 1st and 2nd generation Sulfonylureas respectively, can exist in three states in solution (Schematic 2): (i) they can solubilize (ii) they can form aggregates (iii) they can become incorporated into the phopholipid bilayer or (d) they can form a monolayer at the air-water interface. Sulfonylureas have pKas ranging from 5-6.5 in solution [16]. By conducting a pH-titration of Tolbutamide, its pKa was determined both in solution and when incorporated in small unilamellar vesicles (SUVs). It was found that the pKa of Tolbutamide undergoes an upward shift when it is present in the phospholipid bilayer. The amphiphilic nature of such compounds is considered a pre-requisite for binding to the cell membrane. It was previously shown that amphiphilic compounds experience an upward pKa shift upon binding to the phospholipid membranes (Table 2). It also known that such amphiphilic compounds are preferably lipidsoluble. Table 3 shows a comparison of partition coefficients of two long-chain fatty acids, Lauric acid (12:0) and Oleic acid (18:1) with Torasemide, which is a Sulfonylurea derivative. The comparison shows that Sulfonylureas can be incorporated in the phospholipid bilayer in such a manner that the ionizable group remains exposed to the lipid-water interface while the hydrophobic portion of the molecule remains buried in the bilayer. This information suggests a u-shaped conformation of Tolbutamide and Glibenclamide when bound to the phospholipid membrane (Figure 8).
| Amphiphilic Compound | Physical State | Apparent pKa |
| Acetic acid | 3% in H2O | 4.7 |
| Propionic acid | 4% in H2O | 4.9 |
| Butyric acid | 4% in H2O | 4.8 |
| Octanoic acid | Monomers in H2O | 4.8 |
| Decanoic acid | Lamellar liq. crystals (>CMC) |
6.8 |
| Oleic acid | 5 mole % in PC vesicles (100 mM) |
7.5 |
| Oleic acid | 1.5 weight% in PC-trioleinmicroemulsion |
7.3 |
| Oleic acid | Bound to albumin | 4.2 |
| Cholic acid | 2 mM in H2O (<CMC) |
4.98 |
| Cholic acid | 100 mM in H2O (>CMC) |
5.5 |
| Cholic acid | 3 weight% in PC vesicles | 6.8 (major) 7.3 (minor) |
Table 2: The apparent pKa values of amphiphilic compounds when bound to phospholipid membranes.
| Amphiphilic Compound | Solubilizing Medium | Partition Coefficient (w/w%) | Reference |
| Lauric Acid (C12:0) | membrane/water | 23.0 | [20] |
| Oleic Acid (18:1) | membrane/water | 11.7 | [21] |
| Torasemide (Sulfonylurea) | octanol/water | 2.9 | [22] |
Table 3: The values of partition coefficients of various amphiphilic compounds.
To monitor transport of Sulfonylureas across the bilayer of the vesicles, 30 nanomole aliquots of Tolbutamide and Glibenclamide were added to a buffered suspension of liposomes with trapped Pyranine dye. The observed drop in fluorescence intensity can be explained to be due to a drop in pH inside the vesicles due to the ionization of the sulfonamide group. Ten nanomoles of Albumin (BSA) were then added to extract the Sulfonylureas from the vesicles. A partial recovery of the fluorescence intensity was observed. This is probably due to the fact that BSA does not have a very high affinity for these compounds [19]. It possesses three binding sites for Tolbutamide with a Kd of 21 μM.
On the basis of these experiments it was proposed that the model originally proposed for transport of long chain fatty acids [14,20,21] can be used to explain the rapid transfer of similar hydrophobic compounds such as sulfonylureas across phospholipid membranes. The proposed mechanism is in conformity with the “Overton Rule” [22] which states that the ability of a molecule to cross the phospholipid bilayer and enter the cells depends upon its “selective solubility” in the phospholipid bilayer. Therefore the more hydrophobic a molecule, the greater is the probability that it’ll cross the cell membrane and enter inside the cell.
Conclusion
The control experiments prove that the experimental protocol is sound. The overall profile of the molecular species present in the sample is very similar before and after sonication. The pKa measurements of Tolbutamide and Glibenclamide show that they can bind to the phospholipid bilayer in a u-shaped confirmation so that the ionizable group remains exposed to the lipid-water interface while the hydrophobic portion of the molecule remains buried in the bilayer. The fluorimetric experiments show that both Tolbutamide and Glibenclamide are able to flip-flop across the phospholipid membrane. Transport of both these compounds into the lumen of small unilamellar vesicles(SUVs) is accompanied by a drop in pH that results in a decrease in the observed fluorescence intensity of the trapped Pyranine.
References
- Craig TJ, Ashcroft FM, Proks P (2008) How ATP inhibits the open K(ATP) channel. J Gen Physiol 132: 131-144.
- Markworth, Edelweiss, Christina Schwanstecher, Mathias Schwanstecher (2000) ATP4- mediates closure of pancreatic beta-cell ATP-sensitive potassium channels by interaction with 1 of 4 identical sites. Diabetes 49: 1413-1418.
- Ashcroft FM, Gribble FM (1999) ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia 42: 903-919.
- Lydia Aguilar-Bryan, Bryan, Joseph (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20:101-135.
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM (1997) Truncation of Kir6.2 produces ATP-sensitive K-channels in the absence of the sulphonylurea receptor. Nature 387: 179-181.
- Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP 4th,et al. (1996) Adenosine diphosphate as an intracellular regulator of insulin secretion.Science 272: 1785-1787.
- Gribble FM, Tucker SJ, Haug T, Ashcroft FM (1998) MgATP activates the beta cell KATP channel by interaction with its SUR1 subunit. ProcNatlAcadSci U S A 95: 7185-7190.
- Gribble FM, Tucker SJ, Ashcroft FM (1997) The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide.The EMBO Journal16: 1145-1152.
- Shyng S, Ferrigni T, Nichols CG (1997) Regulation of KATP channel activity by diazoxide and MgADP Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor.The Journal of general physiology110: 643-654.
- Aguilar-Bryan L, Bryan J (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels 1.Endocrine reviews 20: 101-135.
- Schwappach B, Zerangue N, Jan YN, Jan LY (2000) Molecular basis for K ATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter.Neuron26: 155-167.
- Chan KW, Logothetis DE (2001) The first transmembrane domain of SUR1 enhances functional expression of Kir6. 2. BiophysJ80:2820.
- Hamilton JA, Small DM (1981) Solubilization and localization of triolein in phosphatidylcholine bilayers: a 13C NMR study.Proceedings of the National Academy of Sciences 78: 6878-6882.
- Zhang F, Kamp F, Hamilton JA (1996) Dissociation of long and very long chain fatty acids from phospholipid bilayers.Biochemistry35: 16055-16060.
- Slater AM (2014) The IUPAC aqueous and non-aqueous experimental pKa data repositories of organic acids and bases.Journal of computer-aided molecular design 28: 1031-1034.
- Bressler R, Johnson DG (1997) Pharmacological regulation of blood glucose levels in non-insulin-dependent diabetes mellitus. Arch Intern Med 157: 836-848.
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP 4th, Boyd AE 3rd, et al. (1995) Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268:423.
- Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, et al. (2000) ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J 14: 1943-1952.
- Jakoby MG 4th, Covey DF, CistolaDP (1995) Localization of tolbutamide binding sites on human serum albumin using titration calorimetry and heteronuclear 2-D NMR.Biochemistry 34: 8780-8787.
- Hamilton JA (1998) Fatty acid transport: difficult or easy? J Lipid Res 39: 467-481.
- Hamilton JA, Kamp F (1999 ) How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes 48: 2255-2269.
- Overton CE (1895)Über die osmotischenEigenschaften der lebendenPflanzen-und Tierzelle. Fäsi& Beer, Germany.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14737
- [From(publication date):
March-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 13725
- PDF downloads : 1012