Mini Review Open Access
Subtype Specific CSF Biomarkers in Sporadic Creutzfeldt-Jakob Disease
Saima Zafar*, Neelam Younas and Inga ZerrClinical Dementia Center and DZNE, University Medical Center Gottingen (UMG), Göttingen, Germany
- *Corresponding Author:
- Saima Zafar
Department of Neurology, Clinical Dementia Center and DZNE
University Medical Centre Gottingen, Gottingen, Germany
Tel: 00495513914962
E-mail: saima.zafar@med.uni-goettingen.de
Received date: May 02, 2017; Accepted date: May 31, 2017; Published date: June 07, 2017
Citation: Zafar S, Younas N, Zerr I (2017) Subtype Specific CSF Biomarkers in Sporadic Creutzfeldt-Jakob Disease. J Alzheimers Dis Parkinsonism 7:332. doi:10.4172/2161-0460.1000332
Copyright: © 2017 Zafar S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Sporadic Creutzfeldt-Jakob disease (sCJD) is a rare but fatal type of spongiform encephalopathy with unidentified origin. The conjoining methionine-valine polymorphism of PRNP gene at codon 129 and two types of prion protein (PrPsc types 1 and 2) described the six different molecular subtypes (MM1, MM2, MV1, MV2, VV1 and VV2) of sCJD. Presumptive subtype specific diagnosis showed differential clinical manifestations and levels of CSF 14-3-3 protein. Even with the above mentioned differential diagnostic guidelines, pre-mortem subtype specific diagnosis of sCJD can be unreliable with high rates of misdiagnosis. The need for more reliable biomarkers for improving the diagnosis as well as understanding the pathogenesis of this mysterious ailment is amplified. This review compiles the levels of CSF proteins, i.e., PrPC, PrPSC 14-3-3, tau, phosphorylated tau, S100B, neuron-specific enolase (NSE) alpha-synuclein and beta-amyloid to differential diagnosis subtype specific sCJD cases. The detection of pre-mortem distinction targets might be useful diagnostic tool for sCJD in subtype specific manner and might lead towards differential treatment approaches.
Keywords
Creutzfeldt-Jakob disease; CJD; PrP; Prion; 14-3-3; Tau; NSE; Alpha-synuclein
sCJD Subtype Specific CSF Proteins Signature
Sporadic Creutzfeldt–Jakob disease (sCJD) is an inveterate disease caused by the misfolding of the cellular prion protein. The two major different PrP conformations (type 1 and type 2) in combination with methionine/valine polymorphic at codon 129 contributes to the manifestation of six different molecular subtypes (MM1, MM2, MV1, MV2, VV1 and VV2) leading to differential clinical pathological phenotypes [1,2].
However, the diagnosis of sCJD can only be made with certainty after a patient has deceased, by histological examination of brain tissue at autopsy or, rarely, following brain biopsy. Histopathology should reveal evidence of the distinctive hallmarks of CJD, which are extracellular accumulations of PrPsc aggregates.
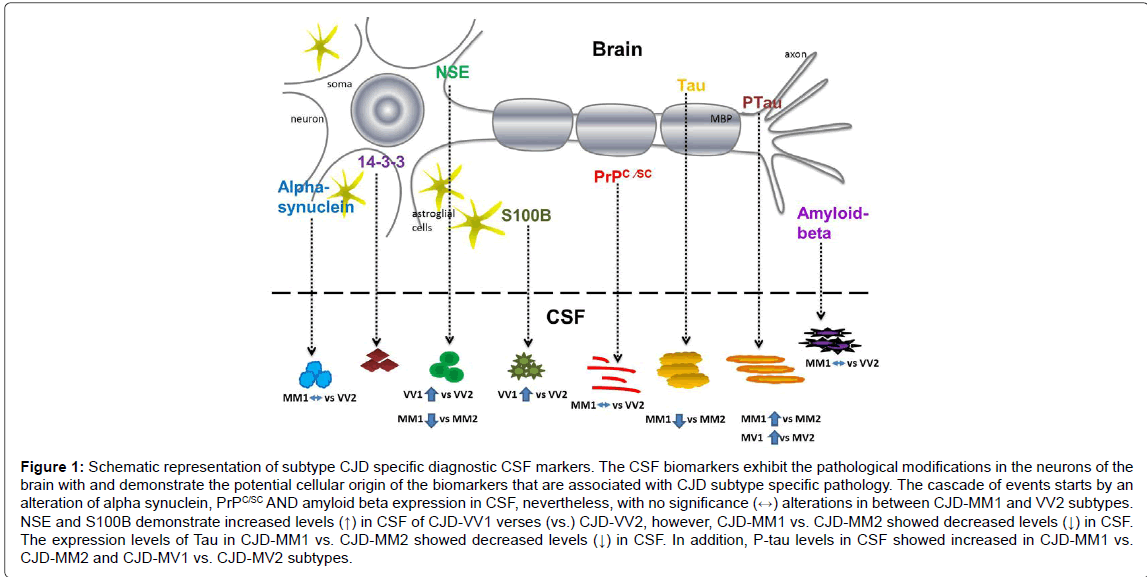
Due to diverse phenotypic heterogeneity of Creutzfeldt-Jakob disease (CJD) and atypical variants [3-5] differential diagnosis of CJD from other neurological diseases (sometimes reversible and treatable) is very challenging Initially diagnostic criteria of CJD were based on combination of clinical symptoms and biomarkers such as MRI, EEG and classical CSF protein marker 14-3-3 [6-9] protein. 14-3-3, which is released into interstitial fluid on death of neurons, indicates rapid neurodegeneration [6]. But this protein is not only present in CSF of CJD but also in other neurodegenerative diseases including some cases of rapidly progressing Alzheimer disease [10-14]. Later studies reported improved differential diagnosis between typical CJD and AD or between CJD and large clinically unselected dementia populations by using t-tau, t-tau to phosphorylatedtau ratio in combination to 14-3-3 [15-20]. Many studies have reported overall high diagnostic accuracy for CSF total-tau as compared to 14-3-3 or S100B [21-24]. Recent study has shown that level of total prion protein (t-PrP) in cerebrospinal fluid (CSF) is very useful marker to differentiate CJD from AD. Further a combination of t-PrP with CSF tau even provides more accuracy as compared to using 14-3-3 alone [25]. This review compiles the levels of CSF proteins i.e. PrPC, PrPSC, tau, phosphorylated tau, S100, NSE and alpha-synuclein to differential diagnosis of subtype specific sCJD cases (Figure 1).
PrPC and PrPSC
Over the current years, total PrP (t-PrP) level in CSF have been pronounced as a novel biomarker [25]. For clinicians the level of t-PrP in CSF might support the differential diagnosis of atypical cases in between the CJD and Alzheimer diseases. Additionally, the t-PrP ratio of CSF with tau proteins (Creutzfeldt-Jakob factor=Total tau/ (Phospho-tau × Total-PrP) lead to differentiate between CJD and atypical AD cases with 100% sensitivity and 95.7% specificity [25]. Many studies reported differential levels of t-PrP in in CJD (subtypes), Alzheimer disease, Parkinson disease, and dementia with Lewy Bodies disease and found slightly but significant decrease in comparison to age matched controls [26,27]. We also reported CJD subtype (MM1 and VV2) t-PrP level correlation in between CSF and brain at mRNA level and protein levels [26,28-31]. Recent development and consolidation of innovative techniques lead to improve the detection system with more sensitivity and specificity by using RT-QuIC ultrasensitive in vitro assay lead to diagnose CJD patients. The diagnostic sensitivity of RT-QuIC is between 82-96% and practically full specificity. Nonetheless, technique still not that improved to discriminate CJD subtypes in comparison to age matched healthy and non-demented controls [32-36]. However, recent reports also showed lower detection sensitivity in CJD subtypes interrelated to type 2 abnormal prion protein (PrPSc) (VV2, MV2 and MM2) than in typical MM1 subtype of CJD.
Altogether, these studies indicate that the level of PrP in CSF are on average lower level in individuals with prion disease and could be a specific marker in symptomatic prion disease patients than in controls [25-28,37] but not at the subtype levels.
Tau and Phosphorylated Tau
Currently, CSF protein analysis in combination to advanced MRI techniques such as DWI and FLAIR are the most prominent in vivo diagnostic markers for sCJD [24,38]. Most widely used surrogate protein markers are brain derived CSF proteins (14-3-3, t-PrP, total (t)-tau and t-tau/phosphorylated (p)-tau ratio) with different level of specificity and sensitivity for differential diagnosis of CJD from other rapidly progressive dementias [8,18,20,39-43]. While many studies have analysed utility of these biomarkers for differential diagnosis, only few studies have particularly analysed the effect of the disease-subtypes on the specificity and sensitivity of the biomarkers [23,24,44]. Recently, we showed that CSF protein analysis (14-3-3, tau protein, phosphorylated tau (181P) (p-tau) protein, amyloid β1-42, S100B and NSE can be used as a marker for pre-mortem diagnosis of sCJD subtypes when genotype of codon 129 is known [44,45]. Authors have reported significantly high tau levels in PrP type 1 patient with MM and MV genotype but lower in VV cases. Authors claim that a combination of genotyping and CSF tau assay can be authentic approach for subtype differentiation of the disease [23,45]. A very recent study has provided novel evidence for a significantly improved value of CSF biomarkers for the clinical diagnosis of CJD subtypes [24]. This study has shown moderate superiority of t-tau levels in terms of both specificity and sensitivity for all sCJD subtypes as compared to conventional CJD marker 14-3-3-protein. More precisely authors have shown that t-tau resulted in a lesser number of false positive results, particularly in cases having inflammatory related disorders and sub–acute dementias; and it has higher sensitivity than 14-3-3 for the sCJD MV2K type [24]. Altogether, data suggest that the t-tau assay has technical advantages as compared to the standard western blot 14-3-3 assay, providing an evidence for a change in the current approvals to prioritise t-tau analysis over 14-3-3 although in case of differential diagnosis with Alzheimer disease, t-tau is less accurate than 14-3-3 [24,37,46]. For differential diagnosis with AD, combination of Aβ42, p-tau and total-PrP levels with the calculation of t-tau/p-tau and other ratios based on different combinations of these four biomarkers has considerably improved diagnostic accuracy [25,27,42].
Figure 1: Schematic representation of subtype CJD specific diagnostic CSF markers. The CSF biomarkers exhibit the pathological modifications in the neurons of the brain with and demonstrate the potential cellular origin of the biomarkers that are associated with CJD subtype specific pathology. The cascade of events starts by an alteration of alpha synuclein, PrPC/SC AND amyloid beta expression in CSF, nevertheless, with no significance (↔) alterations in between CJD-MM1 and VV2 subtypes. NSE and S100B demonstrate increased levels (↑) in CSF of CJD-VV1 verses (vs.) CJD-VV2, however, CJD-MM1 vs. CJD-MM2 showed decreased levels (↓) in CSF. The expression levels of Tau in CJD-MM1 vs. CJD-MM2 showed decreased levels (↓) in CSF. In addition, P-tau levels in CSF showed increased in CJD-MM1 vs. CJD-MM2 and CJD-MV1 vs. CJD-MV2 subtypes.
NSE and S100B
Neuron-specific enolase (NSE) is reported to be elevated and was one of the first protein with potential for differential diagnosis of Creutzfeldt-Jakob disease from other dementing illnesses [24,47-50]. In our sCJD patient cohort homozygous group (MM and VV) showed elevated levels of NSE as compared to the heterozygous [45]. S100B protein is a glial associated, calcium binding, cytoplasmic neurotrophic factor linked to neuronal survival and brain damage [51]. S100B showed similar trend like NSE levels in sCJD homozygous and heterozygous groups. Sensitivities and specificities of CSF S100b range from 65 to 98% and from 29 to 90%, with an area under the ROC of 0.98% [21,45,52,53]. Alone it does not have better predictive potential than the already used clinical markers or CSF 14�?�3�?�3, but the combination of S100b with other markers including CSF 14�?�3�?�3 may improve diagnostic capability. However, NSE, or S-100 contributed substantially to correctly classify into ‘CJD’ or ‘non-CJD’ but is worth to screen patients with dementia and not as first screening test. Furthermore, the levels of NSE and S100B enabled differentiation between VV1 and VV2 subtypes, with elevation being predictive for the VV2 subtype [45,54].
The differential pathological profile of the particular sCJD subtypes contributed to differential NSE and S100B levels in CSF. The differential brain region and subtype specific inflammatory response might contribute to the different S100B CSF profile [29].
Alpha-Synuclein and Beta-Amyloid
The common pathogenic signs such as deposition or aggregation of the proteins, plaque or fibril formation demonstrated in more than twenty degenerative diseases [55]. Alpha-synuclein is an emerging, well conserved target which has been detected in biological fluids such as CSF and serum [56,57]. Recently, many reports demonstrate the elevated levels of alpha-synuclein in the CSF of CJD patients [58-60]. However, the underline mechanisms leading to elevated CSF levels of alpha-synuclein in CJD cases are still not clear, nevertheless so far no synuclein-related pathology in sCJD brain tissue has been reported. In advancement of CSF alpha-synuclein based RT-QuiC analysis in Lewy bodies and Parkinson's disease patients showed overall specificity of 100% in comparison with Alzheimer and control [61,62]. However, the CSF biomarkers of sCJD and dementia with Lew body sometime concomitant an overlap, with reduced levels of amyloid beta 42 and induced levels of tau [60,63-65]. Therefore, supplementation of alpha synuclein levels may be helpful for the perspective of differential diagnostics.
The expressional levels of beta-amyloid peptide in CSF are an extensive pragmatic diagnostic tool in Alzheimer’s disease [66,67]. A reduced level of Beta-amyloid has also been reported in the CSF of patients with sporadic CJD when compared to control samples [68]. These CSF biomarkers have proven to be an extremely valuable in the confirmatory diagnosis of CJD cases. However, all the known biomarkers are sensitive only when the disease is already at an advanced or terminal stage and there is no data available at preclinical stages of the prion disease.
Conclusion
Three core CSF CJD biomarkers have been evaluated in a great number of studies and may provide valuable information for differential CJD diagnostic from other rapidly progressive dementias. Though, diagnostic potential of CSF biomarkers and imaging techniques is very low for differentiation of CJD subtypes particularly for atypical variants of the disease. Efforts should be made to develop new biomarkers for pre-mortem differentiation of molecular subtypes in an attempt to treat the disease in a particular way depending on the disease subtype and underlying pathology related to that disease subtype.
However, all the known biomarkers are sensitive only when the disease is already at an advanced or terminal stage and there is no data available at preclinical stages of the prion disease.
References
- Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, et al. (2006) Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain 129: 2278-2287.
- Parchi P, de Boni L, Saverioni D, Cohen ML, Ferrer I, et al. (2012) Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: An inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol 124: 517-529.
- Chapuis J, Moudjou M, Reine F, Herzog L, Jaumain E, et al. (2016) Emergence of two prion subtypes in ovine PrP transgenic mice infected with human MM2-cortical Creutzfeldt-Jakob disease prions. Acta Neuropathol Commun 4: 10.
- Diack AB, Ritchie D, Bishop M, Pinion V, Brandel JP, et al. (2012) Constant transmission properties of variant Creutzfeldt-Jakob disease in 5 countries. Emerg Infect Dis 18: 1574-1579.
- Huang TY, Minamide LS, Bamburg JR, Bokoch GM (2008) Chronophin mediates an ATP-sensing mechanism for cofilin dephosphorylation and neuronal cofilin-actin rod formation. Dev Cell 15: 691-703.
- Hsich G, Kenney K, Gibbs CJ, Lee KH, Harrington MG (1996) The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Engl J Med 335: 924-930.
- Zerr I, Bodemer M, Weber T (1997) The 14-3-3 brain protein and transmissible spongiform encephalopathy. N Engl J Med 336: 874-875.
- Zerr I, Bodemer M, Gefeller O, Otto M, Poser S, et al. (1998) Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol 43: 32-40.
- Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro CJ, et al. (2000) Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology 55: 811-815.
- Burkhard PR, Sanchez JC, Landis T, Hochstrasser DF (2001) CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology 56: 1528-1533.
- Huang N, Marie SK, Livramento JA, Chammas R, Nitrini R (2003) 14-3-3 protein in the CSF of patients with rapidly progressive dementia. Neurology 61: 354-357.
- Toyo-oka K, Shionoya A, Gambello MJ, Cardoso C, Leventer R, et al. (2003) 14-3-3epsilon is important for neuronal migration by binding to NUDEL: A molecular explanation for Miller-Dieker syndrome. Nat Genet 34: 274-285.
- Schmidt C, Redyk K, Meissner B, Krack L, von Ahsen N, et al. (2010) Clinical features of rapidly progressive Alzheimer's disease. Dement Geriatr Cogn Disord 29: 371-378.
- Tagliapietra M, Zanusso G, Fiorini M, Bonetto N, Zarantonello G, et al. (2013) Accuracy of diagnostic criteria for sporadic Creutzfeldt-Jakob disease among rapidly progressive dementia. J Alzheimers Dis 34: 231-238.
- Riemenschneider M, Wagenpfeil S, Vanderstichele H, Otto M, Wiltfang J, et al. (2003) Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry 8: 343-347.
- Blennow K, Johansson A, Zetterberg H (2005) Diagnostic value of 14-3-3beta immunoblot and T-tau/P-tau ratio in clinically suspected Creutzfeldt-Jakob disease. Int J Mol Med 16: 1147-1149.
- Buerger K, Otto M, Teipel SJ, Zinkowski R, Blennow K, et al. (2006) Dissociation between CSF total tau and tau protein phosphorylated at threonine 231 in Creutzfeldt-Jakob disease. Neurobiol Aging 27: 10-15.
- Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, et al. (2014) Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: Results from the Swedish Mortality Registry. JAMA Neurol 71: 476-483.
- Forner SA, Takada LT, Bettcher BM, Lobach IV, Tartaglia MC, et al. (2015) Comparing CSF biomarkers and brain MRI in the diagnosis of sporadic Creutzfeldt-Jakob disease. Neurol Clin Pract 5: 116-125.
- Koscova S, Zakova SD, Tomeckova I, Melicherova K, Stelzer M, et al. (2016) Cerebrospinal fluid biomarkers in the diagnosis of Creutzfeldt-Jakob disease in Slovak patients: Over 10 year period review. Mol Neurobiol.
- Coulthart MB, Jansen GH, Olsen E, Godal DL, Connolly T, et al. (2011) Diagnostic accuracy of cerebrospinal fluid protein markers for sporadic Creutzfeldt-Jakob disease in Canada: A 6 year prospective study. BMC Neurol 11: 133.
- Cohen OS, Chapman J, Korczyn AD, Siaw OL, Warman-Alaluf N, et al. (2016) CSF tau correlates with the degree of cortical involvement in E200K familial Creutzfeldt-Jakob disease. Neurosci Lett 634: 76-78.
- Karch A, Llorens F, Schmitz M, Arora AS, Zafar S, et al. (2016) Stratification by genetic and demographic characteristics improves diagnostic accuracy of cerebrospinal fluid biomarkers in rapidly progressive dementia. J Alzheimers Dis 54: 1385-1393.
- Lattanzio F, Abu-Rumeileh S, Franceschini A, Kai H, et al. (2017) Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: Diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol 133: 559-578.
- Dorey A, Tholance Y, Vighetto A, Perret-Liaudet A, Lachman I, et al. (2015) Association of cerebrospinal fluid prion protein levels and the distinction between Alzheimer disease and Creutzfeldt-Jakob disease. JAMA Neurol 72: 267-275.
- Meyne F, Gloeckner SF, Ciesielczyk B, Heinemann U, Krasnianski A, et al. (2009) Total prion protein levels in the cerebrospinal fluid are reduced in patients with various neurological disorders. J Alzheimers Dis 17: 863-873.
- Abu RS, Lattanzio F, Stanzani MM, Rizzi R, Capellari S, et al. (2017) Diagnostic accuracy of a combined analysis of cerebrospinal fluid t-PrP, t-tau, p-tau and a beta42 in the differential diagnosis of Creutzfeldt-Jakob disease from Alzheimer’s disease with emphasis on atypical disease variants. J Alzheimers Dis 55: 1471-1480.
- Torres M, Cartier L, Matamala JM, Hernández N, Woehlbier U, et al. (2012) Altered prion protein expression pattern in CSF as a biomarker for Creutzfeldt-Jakob disease. PLoS ONE 7: e36159.
- Llorens F, Ansoleaga B, Garcia-Esparcia P, Zafar S, Grau-Rivera O, et al. (2013) PrP mRNA and protein expression in brain and PrP(c) in CSF in Creutzfeldt-Jakob disease MM1 and VV2. Prion 7: 383-393.
- Zafar S, Asif AR, Ramljak S, Tahir W, Schmitz M, et al. (2014) Anchorless 23-230 PrPC interactomics for elucidation of PrPC protective role. Mol Neurobiol 49: 1385-1399.
- Zafar S, Younas N, Correia S, Shafiq M, Tahir W, et al. (2017) Strain-specific altered regulatory response of Rab7a and tau in Creutzfeldt-Jakob disease and Alzheimer's disease. Mol Neurobiol 54: 697-709.
- Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, et al. (2011) Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 17: 175-178.
- McGuire LI, Peden AH, Orru CD, Wilham JM, Appleford NE, et al. (2012) Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol 72: 278-285.
- Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, et al. (2015) Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio 6: e02451-14.
- Cramm M, Schmitz M, Karch A, Mitrova E, Kuhn F, et al. (2016) Stability and reproducibility underscore utility of RT-QuIC for diagnosis of Creutzfeldt-Jakob disease. Mol Neurobiol 53: 1896-1904.
- Groveman BR, Orrú CD, Hughson AG, Bongianni M, Fiorini M, et al. (2016) Extended and direct evaluation of RT-QuIC assays for Creutzfeldt-Jakob disease diagnosis. Ann Clin Transl Neurol 4: 139-144.
- Foutz A, Appleby BS, Hamlin C, Liu X, Yang S, et al. (2017) Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol 81: 79-92.
- Zanusso G, Monaco S, Pocchiari M, Caughey B (2016) Advanced tests for early and accurate diagnosis of Creutzfeldt-Jakob disease. Nat Rev Neurol 12: 325-333.
- Blennow K, Johansson A, Zetterberg H (2005) Diagnostic value of 14-3-3beta immunoblot and T-tau/P-tau ratio in clinically suspected Creutzfeldt-Jakob disease. Int J Mol Med 16: 1147-1149.
- Baldeiras IE, Ribeiro MH, Pacheco P, Machado A, Santana I, et al. (2009) Diagnostic value of CSF protein profile in a Portuguese population of sCJD patients. J Neurol 256: 1540-1550.
- Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, et al. (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132: 2659-2668.
- Stoeck K, Sanchez-Juan P, Gawinecka J, Green A, Ladogana A, et al. (2012) Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: A longitudinal multicentre study over 10 years. Brain 135: 3051-3061.
- Leitão MJ, Baldeiras I, Almeida MR, Ribeiro MH, Santos AC, et al. (2016) Sporadic Creutzfeldt-Jakob disease diagnostic accuracy is improved by a new CSF ELISA 14-3-3γ assay. Neuroscience 322: 398-407.
- Gmitterova K, Heinemann U, Bodemer M, Krasnianski A, Meissner B, et al. (2009) 14-3-3 CSF levels in sporadic Creutzfeldt-Jakob disease differ across molecular subtypes. Neurobiol Aging 30: 1842-1850.
- Gmitterova K, Heinemann U, Krasnianski A, Gawinecka J, Zerr I (2016) Cerebrospinal fluid markers in the differentiation of molecular subtypes of sporadic Creutzfeldt-Jakob disease. Eur J Neurol 23: 1126-1133.
- Hamlin C, Puoti G, Berri S, Sting E, Harris C, et al. (2012) A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt-Jakob disease. Neurology 79: 547-552.
- Jimi T, Wakayama Y, Shibuya S, Hara H, Sagawa F, et al. (1989) Two cases of Creutzfeldt-Jakob disease with high neuron-specific enolase level in cerebrospinal fluid. Rinsho Shinkeigaku 29: 118-121.
- Zerr I, Bodemer M, Racker S, Grosche S, Poser S, et al. (1995) Cerebrospinal fluid concentration of neuron-specific enolase in diagnosis of Creutzfeldt-Jakob disease. Lancet 345: 1609-1610.
- Beaudry P, Cohen P, Brandel JP, Delasnerie-Laupretre N, Richard S, et al. (1999) 14-3-3 protein, neuron-specific enolase and S-100 protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Dement Geriatr Cogn Disord 10: 40-46.
- Kohira I, Tsuji T, Ishizu H, Takao Y, Wake A, et al. (2000) Elevation of neuron-specific enolase in serum and cerebrospinal fluid of early stage Creutzfeldt-Jakob disease. Acta Neurol Scand 102: 385-387.
- Rothermundt M, Peters M, Prehn JH, Arolt V (2003) S100B in brain damage and neurodegeneration. Microsc Res Tech 60: 614-632.
- Chohan G, Pennington C, Mackenzie JM, Andrews M, Everington D, et al. (2010) The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: A 10 year review. J Neurol Neurosurg Psychiatry 81: 1243-1248.
- Ladogana A, Sanchez-Juan P, Mitrova E, Green A, Cuadrado-Corrales N, et al. (2009) Cerebrospinal fluid biomarkers in human genetic transmissible spongiform encephalopathies. J Neurol 256: 1620-1628.
- Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Saanchez-Valle R, et al. (2006) CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology 67: 637-643.
- Dupiereux I, Zorzi W, Quadrio I, Perret-Liaudet A, Kovacs GG, et al. (2009) Creutzfeldt-Jakob, Parkinson, lewy body dementia and Alzheimer diseases: From diagnosis to therapy. Cent Nerv Syst Agents Med Chem 9: 2-11.
- Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, et al. (2008) Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol 213: 315-325.
- Williams SM, Schulz P, Sierks MR (2016) Oligomeric α-synuclein and β-amyloid variants as potential biomarkers for Parkinson's and Alzheimer's diseases. Eur J Neurosci 43: 3-16.
- Kasai T, Tokuda T, Ishii R, Ishigami N, Tsuboi Y, et al. (2014) Increased alpha-synuclein levels in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. J Neurol 261: 1203-1209.
- Llorens F, Zafar S, Ansoleaga B, Shafiq M, Blanco R, et al. (2015) Subtype and regional regulation of prion biomarkers in sporadic Creutzfeldt-Jakob disease. Neuropathol Appl Neurobiol 41: 631-645.
- Llorens F, Kruse N, Schmitz M, Gotzmann N, Golanska E, et al. (2016) Evaluation of alpha-synuclein as a novel cerebrospinal fluid biomarker in different forms of prion diseases. Alzheimers Dement S1552-5260: 33055-33062.
- Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, et al. (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3: 812-818.
- Førland MG, Öhrfelt A, Oftedal LS, Tysnes OB, Larsen JP, et al. (2017) Validation of a new assay for α-synuclein detection in cerebrospinal fluid.Clin Chem Lab Med 55: 254-260.
- Parnetti L, Tiraboschi P, Lanari A, Peducci M, Padiglioni C, et al. (2008) Cerebrospinal fluid biomarkers in Parkinson's disease with dementia and dementia with Lewy bodies. Biol Psychiatry 64: 850-855.
- Andersson M, Zetterberg H, Minthon L, Blennow K, Londos E (2011) The cognitive profile and CSF biomarkers in dementia with Lewy bodies and Parkinson's disease dementia. Int J Geriatr Psychiatry 26: 100-105.
- Parnetti L, Farotti L, Eusebi P, Chiasserini D, De Carlo C, et al. (2014) Differential role of CSF alpha-synuclein species, tau and Aβ42 in Parkinson's Disease. Front Aging Neurosci 6: 53.
- Blennow K, Zetterberg H (2009) Cerebrospinal fluid biomarkers for Alzheimer's disease. J Alzheimers Dis 18: 413-417.
- Zetterberg H (2015) Cerebrospinal fluid biomarkers for Alzheimer's disease: Current limitations and recent developments. Curr Opin Psychiatry 28: 402-409.
- Wiltfang J, Esselmann H, Bibl M, Hüll M, Hampel H, et al. (2007) Amyloid beta peptide ratio 42/40 but not A beta 42 correlates with phospho-tau in patients with low- and high-CSF A beta 40 load. J Neurochem 101: 1053-1059.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 3091
- [From(publication date):
June-2017 - Jul 15, 2025] - Breakdown by view type
- HTML page views : 2285
- PDF downloads : 806