Subcortical Effects on Voice and Fluency in Dysarthria: Observations from Subthalamic Nucleus Stimulation
Received: 12-Oct-2017 / Accepted Date: 23-Oct-2017 / Published Date: 30-Oct-2017 DOI: 10.4172/2161-0460.1000392
Abstract
Objective: Parkinson’s disease (PD), caused by basal ganglia dysfunction, is associated with motor disturbances including dysarthria. Stimulation of the subthalamic nucleus, a preferred treatment targeting basal ganglia function, improves features of the motor disorder, but has uncertain effects on speech. We studied speech during contrasting stimulation states to reveal subcortical effects on voice and articulation. Measures were made on selected samples of spontaneous and repeated speech.
Methods: Persons with Parkinson’s disease (PWP) who had undergone bilateral deep brain stimulation of the subthalamic nucleus (DBS-STN) provided spontaneous speech samples and then repeated portions of their monologue both on and off stimulation. Excerpts were presented in a listening protocol probing intelligibility. Also analysed were a continuous phrase repetition task and a second spontaneous speech sample. Fundamental frequency (F0), harmonic-to-noise ratio (HNR), jitter, shimmer and fluency were measured in these three speech samples performed with DBS stimulation on and off.
Results: During subcortical stimulation, spontaneous excerpts were less intelligible than repeated excerpts. F0 and HNR were higher and shimmer was decreased in repetition and stimulation. Articulatory dysfluencies were increased for spontaneous speech and during stimulation in all three speech samples.
Conclusion: Deep brain stimulation disrupts fluency and improves voice in spontaneous speech, reflecting an inverse influence of subcortical systems on articulatory posturing and laryngeal mechanisms. Better voice and less dysfluency in repetition may occur because an external model reduces the speech planning burden, as seen for gait and arm reach. These orthogonal results for fluency versus phonatory competence may account for ambivalent reports from dysarthric speakers and reveal the complexity of subcortical control of motor speech.
Keywords: Parkinsonian dysarthria; Speech intelligibility; Deep brain stimulation; Speech task; Acoustic measures; Basal ganglia; External vs. internal models for motor behaviors
Introduction
Since deep brain stimulation of the subthalamic nucleus, first introduced as a medical treatment for Parkinson’s disease (PD) in 1987, was approved in 1997 by the FDA, it has become an established therapy of choice for tens of thousands of individuals world-wide with PD and other neurological or psychiatric disorders [1,2]. PD is caused by basal ganglia disease and leads to motor disturbance, including dysarthria, and deep brain stimulation has a dramatic effect on subcortical function. This scenario presents a natural experimental setting for studying subcortical effects on two important components of speech, voice and fluency. The present study examines the effects on speech of stimulatory intervention in subcortical disease. Both spontaneous and repeated speech, obtained under controlled conditions with and without stimulation states, were analysed using listening studies, acoustic measures, and fluency analyses. The focus is on the effects of subcortical stimulation therapy on voice and articulation [3].
The physiological basis for the positive effects of deep brain stimulation on motor systems is not well understood [4-8] but there is a general consensus in the PD community that the therapy reliably reduces tremor and bradykinesia and provides for more consistent symptom control [9-11]. The therapeutic effectiveness of this treatment may allow a dose reduction of PD medications or lessen the need for increased doses. The medications, with prolonged use, are often associated with dyskinesias [12-14], adventitious movements of face and arms that can interfere with activities of daily living.
Dissociation between corporeal motor behaviours and speech in subcortical disease has been reported. Sustained phonation and monologue speech were impaired during high frequency stimulation, while motor scores improved [15]. From some observations, it appears that laryngeal and articulatory functioning is outside of dopaminergic control in persons with Parkinson’s disease (PWP) [16]. This view is supported by a longitudinal study of prosodic impairment, in which the speech measures did not correlate with motor scores in the PWP study group [17,18], likely due to differences in the role of dopaminergic processes in the regulation of speech and limb movements [19,20]. Similarly, the improvement in motor difficulties afforded by deep brain stimulation does not predictably extend to speech.
Effects of Subcortical Stimulation in Speech: An Overview
The effects of stimulation therapy, as seen in quantitative and qualitative measures of speech and intelligibility, have often yielded post-surgical speech intelligibility deterioration [21-27]. Inconsistencies in published and patient reports, as well as a variety of approaches to the question, are prevalent [28]. In a meta-analysis of studies of speech following deep brain stimulation surgery using a variety of speech tasks and motor speech measures, four of seven studies revealed negative effects, but individual differences were noted [29]. No change or improvement in speech is also seen following stimulation therapy [30-32].
When improvements in association with stimulation are noted, they often reference or include voice quality. Laryngeal vocal fold mobility was affected by DBS in a single PWP [33]. A voice disorder forms part of the PD speech disability profile; variable fundamental frequency, hypophonia, monotone, and breathiness. Examining samples of steady phonation and repetition in one individual, stimulation resulted in improved measures of F0, jitter and shimmer, harmonics-tonoise ratio, speech rate, intensity and duration [34]. Wang et al. [35] observed increased intensity, a benefit to the hypophonic Parkinsonian speaker, in sustained vowel production. An increase in harmonic-tonoise ratio (HNR) in periodic portions of speech was said to reflect more efficient function of vocal fold vibration and articulatory setting with subcortical stimulation [20,36,37]. Reduced vocal tremor [38], increased maximum phonation time [32,39,40], better stability of pitch and or amplitude [32,39], and increases in F0 variability and intensity [34,41-43] have also been associated with stimulation therapy. Some studies reported improvements for prosody as well as articulation [39,44,45]. Vocal function utilizing voice-voiceless contrasts in speech was found to progressively deteriorate in a group of PWP who had undergone DBS, but this was attributed to disease progression and reduction of medication, not to the DBS therapy [46]. In contrast to these reports in improved or non-affected vocal function, vocal fold closure, accompanied by breathy and strained vocal quality, was reportedly negatively affected by DBS in a large group of PWPs [47].
The dissociation between speech and (other) motor measures is compounded by conflicting subjective reports from persons who have been treated with deep brain stimulation [48,49]. In an extensive study, acoustic measures showing improvement with deep brain stimulation were at odds with perceptual ratings by PWPs and their physicians [50]. In a study of personal impressions using the Voice Handicap Index (VHI) before and after surgery, self-reports describing speech were more variable in PD with than without stimulation [51].
To illustrate these considerations, we recount comments from one of our study participants, during his monologue produced as part of our clinical evaluation:
“Um, somehow my mouth feels like it’s, uh, in the way of my words rather than helping me speak ‘em. And, uh, I feel a little bit of slur, uh, which is, uh, interesting ‘cause it pretty much started this way after I had the DBS hooked up again.”
Speech Task Effects
Traditionally, the speech disturbance in PD was attributed to “neuromuscular abnormalities in much of the speech musculature, usually related to restriction in the range or speed of movement patterns” [52]. Although variability in movement and speech symptoms was noted [53], it was generally held that “the motor control problems are present regardless of tasks or context” [54]. Dysarthria was characterized by “highly consistent articulatory errors” [54]. Consistency of motor features across speech tasks was emphasized, so that persons with dysarthria show “very little difference in articulatory accuracy between automatic-reactive and volitional purposive speech” [55]. This assumption, that the characteristic signs of dysarthria occur uniformly across speaking conditions, influenced current assessment protocols, which utilize reading or repetition to estimate overall speech intelligibility [56,57].
Despite these perspectives published in the standard literature, acoustic differences as a function of speech task between spontaneous speech and reading were reported [58-60]. A similar role of repetition, contrasting in speech measures with spontaneous speech, was also noted [61-63]. In two individuals with severe dysarthria as a consequence of PD, repetition, reading and sung speech, when compared to spontaneous speech, yielded different outcomes in intelligibility and acoustic measures [64,65]. It appears that speech produced during reading or repetition differs systematically from spontaneous speech. These studies all point to the conclusion that perceptual and acoustic measures derived from repetition or reading [66-68] may not mirror spontaneous speech [69]. As reading and repetition have yielded similar results in comparison to spontaneous speech, the current study focused on compared measures for repetition and spontaneous speech.
Materials and Methods
Speech samples
Speech samples were obtained from six right-handed PWPs, all males, diagnosed with non-tremor predominant PD and treated with bilateral deep brain stimulation of the subthalamic nucleus (DBS-STN). Medication and PD rating data are presented in Table 1. Mean age was 58 (range 56-62), mean years of education was 16.1 (range 15-18); mean age of diagnosis was 47.0 (40-51), averaging 11.2 years post PD diagnosis [9- 15]. Less demographic uniformity within the group members is seen in one parameter, months since DBS, where the mean number of months was 20.0 and the range was 2-56. Participants were American English speakers with normal hearing by self-report with no other medical or neurological diagnoses and no previous history of speech or language disorders. All were diagnosed with mildly dysarthric, hypokinetic speech including hypophonia, imprecise articulation, dysfluencies, and rate abnormalities, consistent with the diagnosis of PD.
| PD(yrs) | DBS(yrs) | Levodopa | H&Y Off | H&Y On | UPDRS-III Off | UPDRS-III On |
|---|---|---|---|---|---|---|
| 10 | 9 | 600 | 2.5 | 2.5 | 27.5 | 19.0 |
| 15 | 2 | 600 | 2.5 | 2.0 | 26.0 | 23.0 |
| 11 | 4 | 600 | 2.0 | 2.0 | 14.0 | 1.0 |
| 9 | 4 | 300 | 2.5 | 2.0 | 23.5 | 21.5 |
| 11 | 56 | 600 | 4.0 | 3.0 | 52.5 | 31.0 |
| 11 | 37 | 400 | 2.0 | 1.0 | 11.5 | 4.0 |
ED: Education; PD: Parkinson’s Disease; DBS: Deep Brain Stimulation; H&Y: Hoehn
and Yahr rating scale for PD; UPDRS: Unified Parkinson’s Disease Rating Scale
The stimulator frequency was 185 Hz and the pulse width was 60 μs in all cases
Table 1: Overview of demographic data for study participants.
Procedure
Three sets of speech samples were obtained. First, PD participants provided five minutes of spontaneous speech, from which utterances were taken and randomized for a repetition task. This allowed for closely matched exemplars of spontaneous and repeated speech. Secondly, in a continuous repetition of an utterance, participants produced a challenging 4-word sentence (Pop the top cop) repeatedly for a period of 60 s at two separate sessions. Third, 60 s of spontaneous speech was obtained at two separate times, resulting in 2 min samples. All speech samples were digitally recorded and subjected to analysis. Recordings were obtained with the stimulators turned on and again with the stimulators turned off during separate testing sessions at least one week apart. All recordings were made at least 12 h following the last dose of PD medication.
Listening test
The listening test provided the first measure in this study: Intelligibility. The listening test, utilizing materials from the first speech sample (5 min of spontaneous speech), was designed so that repeated and spontaneous utterances did not both appear to the same listener. One hundred seventy utterances were randomized and presented in each version. Two significant departures from the previous study were introduced [37]: First, no linguistic support was provided in the transcription exercise; listeners were instructed to transcribe each entire excerpt. Secondly, after adjusting the headphone volume to a comfortable listening level for each listener, the playback was reduced by 7.2 dB to mimic the lower volume of PD speakers [70,71]. Thirty native English speakers (25 females, 5 males) served as listeners. Their mean age was 37.9 + 17 years and the mean education level was 16.1 + 2 years. All listeners were born and received their primary and secondary school education in the USA. All research subjects provided informed consent in accordance with the Helsinki declaration of 1975 (and as revised in 1983).
Measures
Intelligibility and difficulty
Listeners’ performance was scored as the percentage of correctly transcribed words. Difficulty ratings on a scale from 1-5 were also obtained for each speech sample.
Acoustic analysis
Measures included the fundamental frequency (F0) mean and coefficient of variation (CoVar; a measure of the variability in the intonation contours) and the voice harmonic-to-noise (HNR) ratio. HNR is a reflection of efficiency of vocal fold vibration as filtered by the vocal tract in the form of periodic and aperiodic signals [72], calculated over voiced portions of the excerpts. Other measures were two indicants of vocal fold vibratory stability, jitter (frequency perturbation) and shimmer (amplitude perturbation) using Praat [73]. These analyses were performed on spontaneous and repeated excerpts, the 60 s continuous phrase repetition task and the 2 min spontaneous speech samples.
Fluency ratings
Two independent raters identified vowel distortions, consonant substitutions and word and sound omissions derived from the spontaneous and repeated excerpts, from the utterance repetition (Popthe- top-cop) task and the 2 min of spontaneous speech. Discrepancies between raters were adjudicated by a third rater, all trained in speech science and acoustic phonetics.
Results
Spontaneous versus repeated utterances
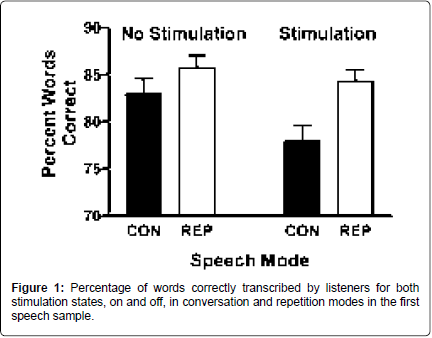
Intelligibility: In the listening study, fewer words were correctly transcribed when the basal ganglia were stimulated [F (1,29)=9.462; p=0.005]. When the two tasks, conversation and repetition, were compared, fewer words were correctly transcribed from spontaneous than repetition [F (1,29)=14.06; p=0.001]. These two conditions, stimulation state and task, interacted as well [F (1,29)=4.543; p=0.042]. Postdoc comparisons revealed that more words were correctly transcribed from repetition than from spontaneous speech during the stimulated state [t (29)=-2.342; p=0.026] and during the off state [t (29)=- 3.619; p=0.001]. In contrast, six percent fewer words were transcribed from spontaneous speech with subthalamic nucleus stimulation on compared to off [t (29)=3.098; p=0.007], but there was no stimulation effect on words transcribed during repetition (Figure 1). There was a 10% increase in the difficulty ratings for transcriptions with stimulation on compared to off [F (1,29)=4.374; p=0.045]. Transcriptions from spontaneous speech were rated 11% more difficult than those from repetitions [F (1,29)=23.416; p<0.001]. These two conditions interacted as well [F (1,29)=13.481; p=0.001]. Pairwise, the pattern of difficulty rating differences reflected the pattern for intelligibility. Transcriptions from spontaneous speech were rated more difficult than transcriptions from repetitions in the on [t (29)=5.937; p<0.001] and off [t (29)=2.435; p=0.021] states. Transcriptions from spontaneous speech were also rated as more difficult with stimulation on compared to off [t (29)=- 3.711; p=0.001], but this was not found for repetitions.
Acoustic measures: Mean F0 was higher during DBS stimulation [F (1,59)=4.361; p=0.041]. The same main effect was found for median F0 [F (1,59)=5.206; p=0.026] and minimum F0 [F (1,59)=4.591; p=0.036]. Spontaneous speech produced higher minimum [F (1,59)=42.577; p<0.001] and maximum F0 values [F (1,59)=9.811; p=0.003].
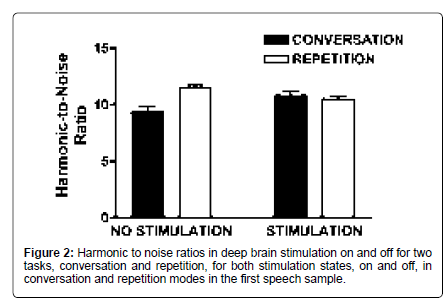
Harmonic-to-noise ratio (HNR) was higher on repeated stimuli compared to those taken from spontaneous speech [F (1,59)= 9.88; p<0.001] and task interacted with stimulation status [F (1,59)=19.05; p<0.001]. During spontaneous speech, HNR was higher with stimulation on compared to off [t (59)=-2.8; p=0.007]. In contrast, HNR during repetition was higher off compared to on [t (59)=3.11; p=0.003]. With stimulation off, HNR was higher during repetition compared to spontaneous speech [t (59)=-5.86; p<0.001]. When deep brain stimulation was on, this difference was eliminated with spontaneous speech HNR improving to the level of repetition. These measures reflect the consistent findings of a sturdier voice, first, during repetition and secondly, with stimulation (Figure 2).
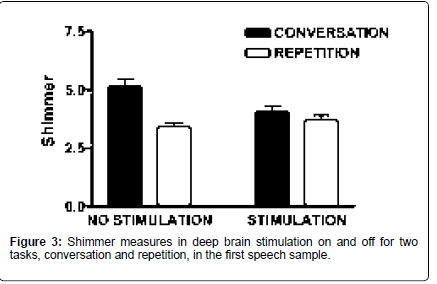
Shimmer was higher during spontaneous speech compared to repetition [F (1,59)=19.8; p<0.001], and task interacted with stimulation status [F (1,59)=8.52; p=0.005]. During conversation, shimmer was higher with off compared to on [t (59)=2.67; p=0.01]. Shimmer did not differ as a function of stimulation status during repetition. In the off state, shimmer was higher during spontaneous speech compared to repetition [t (59)=4.67; p<0.001]. These measures also suggest that the spontaneous speech mode and the absence of stimulation both contribute to a less stable vocal signal. Neither task nor stimulation state had significant effects on jitter measurements (Figure 3).
Fluency measures: The text excerpts utilized by the listeners for the intelligibility measure were examined for dysfluency. The numbers derived from these short speech samples were too sparse for statistical analysis. The overall raw tally of dysfluencies was, for stimulation on, repetition 6 and spontaneous speech 28; for off, repetition 8, spontaneous speech 20, mirroring other findings for increased dysfluency in spontaneous as compared with repeated samples, as well as increased dysfluency during stimulation.
Continuous Sentence Repetition (Pop the Top Cop)
Acoustic measures
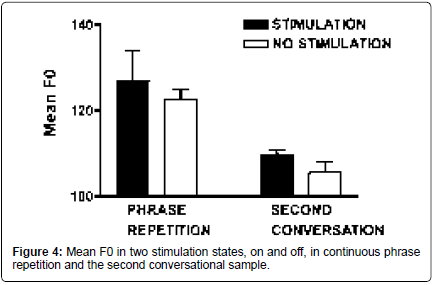
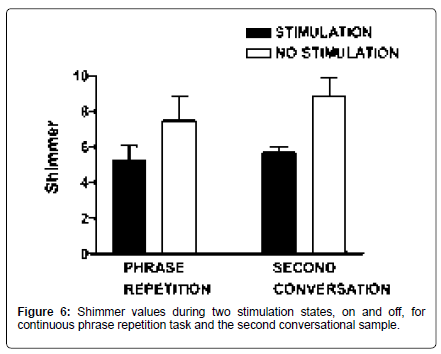
On the continuous sentence repetition task (/pop the top cop/), mean F0 increased 3.6% [t (9)=-3.31; p=0.009], and HNR increased 20.7% with stimulation [t (9)=-3.06; p=0.014]. Shimmer (11 point amplitude perturbation quotient) was reduced by 30% with stimulation [t (8)=2.98; p=0.018] (Figures 4-6).
Fluency measures
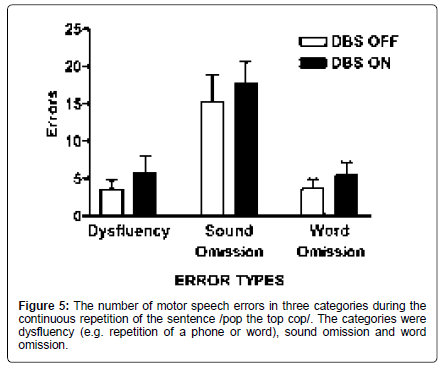
For the continuous utterance repetition task (pop the top cop), the numbers of speech errors in three categories dysfluencies, sound omission and word omission were assessed (Figure 5). Taking into account the duration of PD as a covariate, there was a significant effect of stimulation [F (1,12)=6.06; p=0.03], with 28.9% more errors in the on condition across categories (on: 9.64; off: 7.48). Pairwise, there was a significant interaction between stimulation state and error type, with the greater effect observed for dysfluencies [F (1,12)=5.85; p=0.032]. Further, there were a greater number of vowel distortions with stimulation off compared to the on condition [F (1,54)=5.437; p=0.023], implying insufficient articulatory posturing with stimulation on (Figure 5).
Min spontaneous speech sample
Acoustic measures: On the second spontaneous speech sample, mean F0 increased 3.9% with stimulation on, comparable to the increase observed in the repetition task [t (5)=-2.59; p=0.049]. HNR did not differ, but shimmer was reduced by 36% with stimulation [t (5)=2.73; p=0.041] (Figures 4 and 6).
Fluency measures: There were significantly more dysfluencies produced with stimulation on compared with off measured across two minutes of spontaneous speech [F (1,40)=6.586; p=0.014].
Discussion
Both stimulation state and speech task influenced intelligibility, difficulty ratings, acoustic measures, and fluency ratings. The most notable declines in intelligibility and fluency were observed for spontaneous speech during stimulation.
The effects of stimulation and task on listeners’ difficulty ratings were similar to those for intelligibility. Difficulty ratings were higher for spontaneous speech than for repeated speech in both stimulation states.
The dysarthria of PD speakers typically includes higher shimmer and jitter, lower HNR, lower fundamental frequency, and intensity variability [52,74,75]. An improvement in vocal fold functionality, including F0, jitter and tremor, has been described following levodopa therapy [76], often viewed as analogous, in its effects, to stimulation treatment. Results from the present and comparable studies suggest that stimulation treatment offers an amelioration of these vocal disabilities without necessarily improving intelligibility.
Improved vocal quality during stimulation was also demonstrated by increased harmonic-to-noise ratio, a measure of periodicity in voice quality and quantitative indicator of degree of hoarseness [77], for spontaneous speech. Similar effects have been reported previously. In a case study of a person with PD and severe dysarthria, a noisier, more aperiodic signal was seen in spontaneous speech than in reading, repeated speech or singing [64].
Voice measures improved in the same manner for the continuously repeated utterance, Pop the top cop, and for the second spontaneous speech sample. F0 was higher in the stimulated state, suggesting stiffening effects on vocal fold function. Shimmer was reduced, implying less variability in vibratory processes. Finally, harmonic-to-noise ratio was higher in the utterance repetition task with stimulation, implying greater periodicity in the signal arising from vocal fold vibration. This consistent array of improved voice measures in association with deep brain stimulation strongly suggests a subcortical influence on laryngeal deportment, possibly with the aid of more consistent respiratory control.
Despite improved measures for voice, intelligibility was overall mildly depressed following stimulation, especially for spontaneous speech. This dichotomy was also found in another study, which reported better vowel quality in the stimulated state without greater speech intelligibility [78]. It appears that stimulation improves vocal parameters, but it does so in the context of poorer intelligibility, for which compromised articulatory parameters provide a likely explanation.
In an earlier study, PD speakers were found to be more dysfluent with stimulation [79] and two cases of dysfluency following deep brain stimulation have been reported [80]. Stimulated speech contained more intraphrase pauses than non-stimulated speech [81]. In the current study, vowel distortions, attributable to articulatory insufficiency, and more lexical and phonological dyfluencies were documented under stimulation than off stimulation in all the samples examined. Previously, clinical ratings were higher on vocal parameters (better voice quality) and lower on dysfluencies (greater fluency) for repetition than spontaneous speech [37]. A concomitant discrepancy between positive effects on voice and negative effects on fluency has been reported elsewhere [3,24]. An overall profile suggests that voice characteristics, weak in untreated PD, are stronger during repetition and are enhanced with subcortical stimulation, while fluency is reduced in spontaneous speech. This combination of strong vocal production driving diminished articulatory control may account for the impression of difficulty with talking reported by persons with PD as well as for the lowered intelligibility in spontaneous speech and subcortically stimulated speech.
The motor planning framework may provide one of the keys to understanding intelligibility effects with respect to acoustic changes in speech following stimulation. Evidence comes from a study on the effect of stimulation on vowel space [82]. Individuals with PD on and off stimulation were compared with healthy control speakers producing sustained vowels. Vowel space was determined for the initial 250 ms as well as the midpoints of each vowel. There were no significant differences in the midpoint vowel space measurements between on and off states and control values. However, in the control and off conditions, the initial vowel spaces were significantly larger than the midpoint vowel spaces. In contrast, on stimulation, the initial vowel space was significantly smaller than in the off and control conditions. It appeared that deep brain stimulation may alter vocal tract posturing at the initiation of production. These results are consistent with the findings in the current study, in which articulatory imprecision is enhanced by deep brain stimulation.
The present results also demonstrate that in addition to stimulation status, task affects speech and that these two factors interact. Several studies have revealed significant differences in speech measures taken from spontaneous speech in comparison with reading or repetition in PD [65,83,84] in dysarthric [85] and cerebellar speech [61,63]. In a presentation of severe stuttering in a person diagnosed with Parkinsonian syndrome, repetitions and prolongations were 200% greater in spontaneous speech than in reading, repetition or singing [65]. Using four tasks in a previous study, automatic speech, elicited speech, spontaneous speech and reading aloud, significant differences in habitual loudness or pitch were found when performance measures were compared [86,87]. In another study, vowels were extracted from sustained phonation, sentence repetition, reading, and monologue and submitted to detailed acoustic analysis. Spontaneous speech was the most sensitive in differentiating between controls and PD patients, suggesting that articulatory difficulties are more likely to emerge in spontaneous speech than in structured tasks [88-90]. Acknowledging the significance of task effects in clinical evaluations, an algorithm to systematically relating measures from spontaneous speech with those from repetition, in terms of scaled estimates of intelligibility, was developed [69].
It has been proposed that cognitive-linguistic load is accountable for lower intelligibility and weaker articulatory performance in spontaneous speech, but this view has not been verified by empirical study. Attempts to document a distinction in reading versus spontaneous speech measures based on a controlled cognitive-linguistic contrast have not been successful; task-related differences using reading and narrative speech did not differ between low-cognitive and highcognitive groups with multiple sclerosis, using pausing and speech rate as measures [91,92]. It is arguable that reading and repetition also place cognitive demands on the speaker, for example, in requiring the pronunciation of relatively unfamiliar elements and structures and demands on short-term memory. In addition, it is difficult to see how lower cognitive demands (alleged by this theory to be inherent in reading and repetition) can be associated with improved voice measures, as has been consistently documented.
Instead, it is plausible that reading and repetition both provide an external model of the desired speech output while spontaneous speech requires the generation of an internal model of the motoric gesture. An external model reduces the burden on the motor speech neurological apparatus, which can proceed with less demand for initiation, sequential planning, and monitoring, thereby facilitating more efficient motor execution [88,93]. The observation that PD was associated with deficient recited speech (e.g. Humpty Dumpty), when compared to healthy speakers, further supports the hypothesis that highly routinized or procedural speech sequences with well-established internal models are impaired by basal ganglia dysfunction [94-98]. This perspective places task effects more solidly at the level of motor organization, initiation, planning, monitoring, and execution, leaving aside concerns about linguistic or cognitive characteristics. Support for this view can profitably be considered in the light of findings from gait and arm movement studies [99-101] whereby providing a model significantly improves execution of the movement [102-104]. Motor deficits in Parkinson’s disease are more severe in internally than in externally guided motor tasks [105,106] implying that, for speech, a deficient subcortical system can be expected to perform more poorly for spontaneous speech, when a newly generated internal model is required, than in repetition or reading, where an external model is provided.
Conclusion
This study examined Parkinsonian speech under stimulation on and off conditions to investigate subcortical influences on details of voice and articulation. Stimulation, allowing for enhanced subcortical function in basal ganglia disease, affected both of these components of motor speech, but in an orthogonal manner. Improvement in voice quality was accompanied by a reduction in fluency. Overall, intelligibility was reduced in the stimulated state. A stronger laryngeal component may be competing with a less competent articulatory system, leading to a compromised motor speech product and an impression of greater effort, in the PD speakers themselves, in the activity of speaking, as reported by one of our participants in the beginning of this report. From a speech motor control perspective, improvements in voice characteristics may have a disproportionate effect on speech, altering the parameters of the intended speech output most disruptively in spontaneous speech, when no external model is present. Analogously to gait and arm reach, the repetition mode provides an external model, aiding motor output efficiency in subcortical processing for speech.
Acknowledgement
We appreciate the contributions of Amy Alken, the Brain and Behavior Laboratory research assistants, and Michele Burgevin at various stages of this project. This work was supported by a grant from the National Institute of Deafness and Communicative Disorders (R01 DC 007658).
References
- Ponce FA, Lozano AM (2010) Deep brain stimulation state of the art and novel stimulation targets. Prog Brain Res 184: 311-324.
- Volkmann J (2007) Deep brain stimulation for Parkinson’s disease. Parkinsonism Relat Disord 13: S462-S465.
- De Bodt MS, Hernández-DÃaz HM, Van De Heyning PH (2002) Intelligibility as a linear combination of dimensions in dysarthric speech. J Commun Disord 35: 283-292.
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol 8: 67-81.
- McIntyre CC, Savasta M, Walter BL, Vitek JL (2004) How does deep brain stimulation work? Present understanding and future questions. J Clin Neurophysiol 21: 40-50.
- Montgomery EB (2007) Basal ganglia physiology and pathophysiology: A reappraisal. Parkinsonism Relat Disord 13: 455-465.
- Vitek JL (2008) Deep brain stimulation: How does it work? Cleve Clin J Med 75: S59-65.
- Perlmutter JS, Mink JW (2006) Deep brain stimulation. Annu Rev Neurosci 29: 229-257.
- Ciucci MR, Barkmeier-Kraemer JM, Sherman SJ (2008) Subthalamic nucleus deep brain stimulation improves deglutition in Parkinson’s disease. Mov Disord 23: 676-683.
- Kitashima A, Umemoto G, Tsuboi Y, Higuchi M, Baba Y, et al. (2012) Effects of subthalamic nucleus deep brain stimulation on the swallowing function of patients with Parkinson’s disease. Parkinsonism Relat Disord 19: 480-482.
- Troche MS, Brandimore AE, Foote KD, Okun MS (2013) Swallowing and deep brain stimulation in Parkinson's disease: A systematic review. Parkinsonism Relat Disord 19: 783-788.
- Fasano A, Romito LM, Daniele A, Piano C, Zinno M, et al. (2010) Motor and cognitive outcome in patients with Parkinson’s disease 8 years after subthalamic implants. Brain 133: 2664-2676.
- Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, et al. (2006) Subthalamic nucleus deep brain stimulation: Summary and meta-analysis of outcomes. Mov Disord 21: S290-304.
- Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, et al. (1998) Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology 51: 850-855.
- Astrm M, Tripoliti E, Hariz MI, Zrinzo LU, Martinez-Torres I, et al. (2010) Patient-specific model-based investigation of speech intelligibility and movement during deep brain stimulation. Stereotact Funct Neurosurg 88: 224-233.
- Kompoliti K, Wang QE, Goetz CG, Leurgans S, Raman R (2000) Effects of central dopaminergic stimulation by apomorphine on speech in Parkinson’s disease. Neurology 54: 458-462.
- Romito LM, Scerrati M, Contarino MF, Iacoangeli M, Bentivoglio AR, et al. (2003) Bilateral high frequency subthalamic stimulation in Parkinson’s disease: Long-term neurological follow-up. J Neurosurg Sci 47: 119-128.
- Skodda S, Rinsche H, Schlegel U (2009) Progression of dysprosody in Parkinson's disease over time--a longitudinal study. Mov Disord 24: 716-722.
- Gentil M, Pollak P (1998) Effects of levodopa on finger and articulatory movements in Parkinson’s disease. J Acoust Soc Am 103: 2892.
- Hammer MJ, Barlow SM, Lyons KE, Pahwa R (2010) Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson’s disease. J Neurol 257: 1692-1702.
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, et al. (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349: 1925-1934.
- Tripoliti E, Limousin P, Foltynie T, Candelario J, Aveles-Olmos I, et al. (2014) Predictive factors of speech Intelligibility following subthalamic nucleus stimulation in consecutive patients with Parkinson’s disease. Mov Disord 29: 532-538.
- Tripoliti E, Zrinzo L, Martinez-Torres I, Frost E, Pinto S, et al. (2011) Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology 76: 80-86.
- Tsuboi T, Watanabe H, Tanaka Y, Ohdake R, Yoneyama N, et al. (2015) Distinct phenotypes of speech and voice disorders in Parkinson’s disease after subthalamic nucleus deep brain stimulation. J Neurol Neurosurg Psychiatry 86: 856-864.
- Tornqvist AL, Schalén L, Rehncrona S (2005) Effects of different electrical parameter settings on the intelligibility of speech in patients with Parkinson’s disease treated with subthalamic deep brain stimulation. Mov Disord 20: 416-423.
- Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, et al. (2008) Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord 23: 2377-2383.
- Van Lancker Sidtis D, Cameron K, Bonura L, Sidtis JJ (2011) Speech intelligibility by listening in Parkinson speech with and without deep brain stimulation. J Neurolinguistics 25: 121-132.
- Aldridge D, Theodoros D, Angwin A, Vogel AP (2016) Speech outcomes in Parkinson's disease after subthalamic nucleus deep brain stimulation: A systematic review. Parkinsonism Relat Disord 33: 3-11.
- Iulianella I, Adams SG, Gow AK (2008) Effects of sub-thalamic deep brain stimulation on speech production in Parkinson’s disease: A critical review of the literature. Canadian Journal of Speech-Language Pathology and Audiology 32: 85-91.
- Farrell A, Theodoros D, Ward E, Hall B, Silburn P (2005) Effects of neurosurgical management of Parkinson's disease on speech characteristics and oromotor function. J Speech Lang Hear Res 48: 5-20.
- Gentil M, Garcia-Ruiz P, Pollak P, Benabid AL (1999) Effect of stimulation of the subthalamic nucleus on oral control of patients with parkinsonism. J Neurol Neurosurg Psychiatry 67: 329-333.
- Gentil M, Pinto S, Pollak P, Benabid AL (2003) Effect of bilateral stimulation of the subthalamic nucleus on Parkinsonian dysarthria. Brain Lang 85: 190-196.
- Arocho-Quinones EV, Hammer MJ, Bock JM, Pahapill PA (2017) Effects of deep brain stimulation on vocal fold immobility in Parkinson's disease. Surg Neurol Int 8: 22.
- Hoffman-Ruddy B, Schulz G, Vitek J, Evatt M (2001) A preliminary study of the effects on the subthalamic nucleus (STN) deep brain stimulation (DBS) on voice and speech characteristics in Parkinson’s disease. Clin Linguist Phon 15: 97-101.
- Wang E, Verhagen Metman L, Bakay R, Arzbaecher J, Bernard B (2003) The effect of unilateral electrostimulation of the subthalamic nucleus on respiratory/phonatory subsystems of speech production in Parkinson’s disease: A preliminary report. Clin Linguist Phon 17: 283-289.
- Hammer MJ, Barlow SM, Lyons KE, Pahwa R (2011) Subthalamic nucleus deep brain stimulation changes velopharyngeal control in Parkinson's disease. J Commun Disord 44: 37-48.
- Van Lancker Sidtis D, Rogers T, Godier V, Tagliati M, Sidtis JJ (2010) Voice and fluency changes as a function of speech task and deep brain stimulation. J Speech Lang Hear Res 53: 1167-1177.
- D’Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, et al. (2008) Effects of bilateral subthalamic nucleus stimulation and medication on Parkinsonian speech impairment. J Voice 22: 365-372.
- Gentil M, Chauvin P, Pinto S, Pollak P, Benabid AL (2001) Effect of bilateral stimulation of the subthalamic nucleus on parkinsonian voice. Brain Lang 78: 233-240.
- Sung JE, Kim H, Kim HS, Oh SH, and Hong JM, et al. (2004) Effects of subthalamic nucleus deep brain stimulation on the phonation and articulation of the patients with Parkinson’s disease. J Korean Neurol Assoc 22: 472-477.
- Dromey C, Kumar R, Lang AE, Lozano AM (2000) An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Mov Disord 15: 1132-1138.
- Karlsson F, Olofsson K, Blomstedt P, Linder P, van Doorn J (2013) Pitch variability in patients with Parkinson’s disease: Effects of deep brain stimulation of caudal zona incerta and subthalamic nucleus. J Speech Lang Hear Res 56: 150-158.
- Rousseax M, Krystkowiak P, Kozlowski O, Özsancak C, Blond S, et al. (2004) Effects of subthalamic nucleus stimulation on Parkinsonian dysarthria and speech intelligibility. J Neurol 251: 327-334.
- Lee VS, Zhou XP, Rahn DA, Wang EQ, Jiang JJ (2008) Perturbation and nonlinear dynamic analysis of acoustic phonatory signal in Parkinsonian patients receiving deep brain stimulation. J Commun Disord 41: 485-500.
- Santens P, de Letter M, van Borsel J, De Reuck J, Caemaert J (2003) Lateralized effects of subthalamic nucleus stimulation on different aspects of speech in Parkinson’s disease. Brain Lang 87: 253-258.
- Karlsson F, Blomstedt P, Olofsson K, Linder J, Nordh E, et al. (2012) Control of phonatory onset and offset in Parkinson patients following deep brain stimulation of the subthalamic nucleus and caudal zona incerta. Parkinsonism Relat Disord 18: 824-827.
- Tsuboi T, Watanabe H, Tanaka Y, Ohdake R, Yoneyama N, et al. (2015) Characteristic laryngoscopic findings in Parkinson’s disease patients after subthalamic nucleus deep brain stimulation and its correlation with voice disorder. J Neural Transm (Vienna) 122: 1663-1672.
- Moro E (2014) Parkinson disease: Let's listen to patients with PD after deep brain stimulation. Nat Rev Neurol 10: 550-552.
- Wertheimer J, Gottuso AY, Nuno M, Walton C, Duboille, et al. (2014) The impact of STN deep brain stimulation on speech in individuals with Parkinson’s disease: The patient's perspective. Parkinsonism Relat Disord 20: 1065-1070.
- Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, et al. (2009) Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 79: 522-529.
- Frost E, Tripoliti E, Hariz MI, Pring T, Limousin P (2010) Self-perception of speech changes in patients with Parkinson's disease following deep brain stimulation of the subthalamic nucleus. Int J Speech Lang Pathol 12: 399-404.
- Duffy JR (1995) Motor speech disorders: Substrates, differential diagnosis and management. (3rd edn) Mosby, St. Louis.
- Metter EJ, Hanson WR (1986) Clinical and acoustical variability in hypokinetic dysarthria. J Commun Disord 19: 347-366.
- Yorkston KM, Beukelman DR, Bell KR (1988) Clinical management of dysarthric speakers. College-Hill, Boston.
- Shames GH, Wiig EH (1990) Human communication disorders. Merrill, Columbus, OH: 470.
- Enderby PM (1983) Frenchay dysarthria assessment. College-Hill, San Diego.
- Yorkston KM, Beukelman DR (1981) Assessment of intelligibility of dysarthric speech. C.C. Publications, Tigard, OR.
- Snidecor J (1943) A comparative study of the pitch and duration characteristics of impromptu speaking and oral reading. Speech Monographs 10: 50-56.
- Lieberman P, Katz W, Jongman A, Zimmerman R, Miller M (1985) Measures of the sentence intonation of read and spontaneous speech in American English. J Acoust Soc Am 77: 649-657.
- Walker VG (1988) Durational characteristics of young adults during speaking and reading tasks. Folia Phoniatr Logop 40: 12-20.
- Brown A, Docherty GJ (1995) Phonetic variation in dysarthric speech as a function of sampling task. Eur J Disord Commun 30: 17-35.
- Kent RD, Kent JF (2000) Task-based profiles of the dysarthrias. Folia Phoniatr Logop 52: 48-53.
- Kent RD, Kent JF, Rosenbek JC, Vorperian HK, Weismer G (1997) A speaking task analysis of the dysarthria in cerebellar disease. Folia Phoniatr Logop 49: 63-82.
- Kempler D, Van Lancker D (2002) Effect of speech task on intelligibility in dysarthria: A case study of Parkinson's disease. Brain Lang 80: 449-464.
- Van Lancker Sidtis D, Cameron K, Sidtis JJ (2010) Dramatic effects of speech task on motor and linguistic planning in severely dysfluent Parkinsonian speech. Clin Linguist Phon 26: 695-711.
- Feenaughty L, Tjaden K, Sussman J (2014) Relationship between acoustic measures and judgments of intelligibility in Parkinson’s disease: A within-speaker approach. Clin Linguist Phon 28: 857-878.
- Sussman JE, Tjaden K (2012) Perceptual measures of speech from individuals with Parkinson's disease and multiple sclerosis: Intelligibility and beyond. J Speech Lang Hear Res 55: 1208-1219.
- Tjaden K, Sussman J, Liu G, Wilding G (2010) Long-term average spectral measures of dysarthria and their relationship to perceived severity. J Med Speech Lang Pathol 18: 125-132.
- Tjaden K, Wilding G (2011) Effects of speaking task on intelligibility in Parkinson's disease. Clin Linguist Phon 25: 155-168.
- Darley FL, Aronson AE, Brown JR (1975) Motor speech disorders. W.B. Saunders, Philadelphia.
- Ludlow CL, Bassich CJ (1984) Relationships between perceptual ratings and acoustic measures of hypokinetic speech. In McNeil MR, Rosenbek JC, Aronson AE (Eds.), The dysarthrias: Physiology, acoustics, perception, management. College Hill Press, San Diego: 163-195.
- Baken RJ (1987) Clinical measurement of speech and voice. Allyn and Bacon, Boston, pp: 383-384.
- Boersma P, Weenink D (2009) PRAAT: Doing phonetics by computer [Computer program].
- Canter GJ (1963) Speech characteristics of patients with Parkinson’s disease: I. intensity, pitch, and duration. J Speech Hear Disord 28: 221-229.
- Gamboa J, Jiménez-Jiménez FJ, Nieto A, Montojo J, OrtÃ-Pareja M, et al. (1997) Acoustic voice analysis in patients with Parkinson's disease treated with dopaminergic drugs. J Voice 11: 314-320.
- Sanabria J, Ruiz PG, Gutierrez R, Marquez F, Escobar P, et al. (2001) The effect of levodopa on vocal function in Parkinson's disease. Clin Neuropharmacol 24: 99-102.
- Yumoto E, Sasaki Y, Okamura H (1984) Harmonics-to-noise ratio and psychophysical measurement of the degree of hoarseness. J Speech Hear Res 27: 2-6.
- Sauvageau VM, Macoir J, Langlois M, Prud’Homme M, Cantin L, et al. (2014) Changes in vowel articulation with subthalamic nucleus deep brain stimulation in dysarthric speakers with Parkinson’s disease. Parkinsons Dis 2014: 487035.
- Van Lancker Sidtis D, Katsnelson D, Rogers T, Sidtis J (2008) Task effects on fluency and voice with ON and OFF DBS in Parkinson’s subjects: Evidence from acoustic measures and expert listeners. Paper presented at the Motor Speech Conference, Monterey, CA.
- Toft M, Dietrichs E (2011) Aggravated stuttering following subthalamic deep brain stimulation in Parkinson's disease-two cases. BMC Neurol 11: 44.
- Ahn JS, Van Lancker Sidtis D, Sidtis JJ (2014) Effects of deep brain stimulation on pausing during spontaneous speech in Parkinson’s disease. J Med Speech Lang Pathol 21: 179-186.
- Sidtis J, Alken A, Tagliati M, Alterman R, Van Lancker Sidtis D (2016) Subthalamic stimulation reduces vowel space at the initiation of sustained production: Implications for articulatory motor control in Parkinson’s disease. J Parkinsons Dis 6: 361-370.
- Canter GJ (1965) Speech characteristics of patients with Parkinson’s disease: III. Articulation, diadochokinesis and overall speech adequacy. J Speech Hear Disord 30: 217-224.
- Van Lancker Sidtis D, Hanson W, Jackson C, Lanto A, Kempler D, et al. (2004) Fundamental frequency (F0) measures comparing speech tasks in aphasia and Parkinson’s disease. J Med Speech Lang Pathol 12: 207-212.
- Frearson B (1985) A comparison of the AIDS sentence list and spontaneous speech intelligibility scores for dysarthric speech. Australian Journal of Human Communication Disorders 13: 5-21.
- Zraick RI, Marshall W, Smith-Olinde L, Montague JC (2004) The effect of task on determination of habitual loudness. J Voice 18: 176-182.
- Zraick RI, Gentry MA, Smith-Olinde L, Gregg BA (2006) The effect of speaking context on elicitation of habitual pitch. J Voice 20: 545-554.
- Huber JE, Darling M (2011) Effect of Parkinson's disease on the production of structured and unstructured speaking tasks: Respiratory physiologic and linguistic considerations. J Speech Lang Hear Res 54: 33-46.
- Rosen KM, Kent RD, Delaney AL, Duffy JR (2006) Parametric quantitative acoustic analysis of conversation produced by speakers with dysarthria and healthy speakers. J Speech Lang Hear Res 49: 395-411.
- Rusz J, Cmejla R, Tykalova T, Ruzickova H, Klempir J, et al. (2013) Imprecise vowel articulation as a potential early marker of Parkinson's disease: effect of speaking task. J Acoust Soc Am 134: 2171-2181.
- Feenaughty L, Tjaden K, Benedict RH, Weinstock-Guttman B (2013) Speech and pause characteristics in multiple sclerosis: A preliminary study of speakers with high and low neuropsychological performance. Clin Linguist Phon 27: 134-151.
- Rodgers JD, Tjaden K, Feenaughty L, Weinstock-Guttman B, Benedict RH (2013) Influence of cognitive function on speech and articulation rate in multiple sclerosis. J Int Neuropsychol Soc 19: 173-180.
- Hustad KC, Lee J (2008) Changes in speech production associated with alphabet supplementation. J Speech Lang Hear Res 51: 1438-1450.
- Bridges KA, Van Lancker Sidtis D, Sidtis JJ (2013) The role of subcortical structures in recited speech: Studies in Parkinson's disease. J Neurolinguistics 26: 594-601.
- Ullman MT (2004) Contributions of memory circuits to language: the declarative/procedural model. Cognition 92: 231-270.
- Graybiel AM (1995) Building action repertoires: Memory and learning functions of the basal ganglia. Curr Opin Neurobiol 5: 733-741.
- Baev KV (1995) Disturbances of learning processes in the basal ganglia in the pathogenesis of Parkinson's disease: A novel theory. Neurol Res 17: 38-48.
- Baev KV (1997) Highest level automatisms in the nervous system: A theory of functional principles underlying the highest forms of brain function. Prog Neurobiol 51: 129-166.
- Atchison PR, Thompson PD, Frackowiak RS, Marsden CD (1993) The syndrome of gait ignition failure: a report of six cases. Mov Disord 8: 285-292.
- Burleigh JA, Horak FB, Nutt JG, Obeso JA (1997) Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Mov Disord 12: 206-215.
- Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, et al. (1993) An evaluation of the role of internal cues in the pathogenesis of Parkinsonian hypokinesia. Brain 116: 1575-1587.
- Fimm B, Bartl G, Zimmermann P, Wallesch CW (1994) Different mechanisms underlie shifting set on external and internal cues in Parkinson’s disease. Brain Cogn 25: 287-304.
- Morris ME (2000) Movement disorders in people with Parkinson disease: A model for physical therapy. Phys Ther 80: 578-597.
- Georgiou N, Bradshaw JL, Iansek R, Phillips JG, Mattingley JB, et al. (1994) Reduction in external cues and movement sequencing in Parkinson's disease. J Neurol Neurosurg Psychiatry 57: 368-370.
- Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, et al. (2007) Task specific influences of Parkinson's disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience 147: 224-235.
- Schenk T, Baur B, Steude U, Bötzel K (2003) Effects of deep brain stimulation on prehensile movements in PD patients is less pronounced when external timing cues are provided. Neuropsychologia 41: 783-794.
Citation: Sidtis D, Sidtis JJ (2017) Subcortical Effects on Voice and Fluency in Dysarthria: Observations from Subthalamic Nucleus Stimulation. J Alzheimers Dis Parkinsonism 7: 392. DOI: 10.4172/2161-0460.1000392
Copyright: © 2017 Sidtis D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5907
- [From(publication date): 0-2017 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 5012
- PDF downloads: 895