Research Article Open Access
Sub-Acute Toxicity Studies of Alchornea cordifolia Leaf Extract in Swiss Albino Rats
Ezeokeke EE, Ene AC* and Igwe CUDepartment of Biochemistry, Federal University of Technology, Owerri, Nigeria
- *Corresponding Author:
- Ene AC
Department of Biochemistry
Federal University of Technology
Owerri, Nigeria
Tel: 23408038544994
E-mail: chineduene@gmail.com
Received date: January 27, 2017; Accepted date: March 04, 2017; Published date: March 10, 2017
Citation: Ezeokeke EE, Ene AC, Igwe CU (2017) Sub-Acute Toxicity Studies of Alchornea cordifolia Leaf Extract in Swiss Albino Rats. J Anal Bioanal Tech 8: 353. doi: 10.4172/2155-9872.1000353
Copyright: © 2017 Ezeokeke EE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The ethanolic leaf extract of Alchornea cordifolia (Schum. and Thonn.) Müll. Arg (Euphorbiaceae), a widely used traditional medicinal plant was assessed for possible sub-acute toxicity in Swiss albino rats. The rats were randomly distributed into five groups of four animals each. The groups were respectively administered 125, 250, 500 and 750 mg/kg body weight ethanolic leaf extract of Alchornea cordifolia intra peritoneally daily for (two weeks) 14 days. Normal saline was administered to the control group according to their body weights. The experimental animals were observed for another 14 days before the termination of the experiment. The weight of the animals was recorded daily throughout the duration of the study. The number of deaths in any group was recorded. All the surviving animals were sacrificed after 28 days. Blood samples were collected for biochemical and haematological analysis. Selected organs of the animals i.e., liver and kidney of both the dead and sacrificed animals were removed and stored in 10% formal saline ready for histopathological analysis. Administration of Alchornea cordifolia (0.125- 0.75 g/kg, po daily) for two weeks (14 days) did not affect significantly the relative organ weights, blood chemistry and renal function. Histology of liver and kidney at dose levels up to 0.5 g/kg was normal and similarto vehicletreated controls. However, liver sections of mice treated with 0.75 g/kg Alchornea cordifolia ethanolic leaf extract showed cloudy swelling of hepatocytes with vascular degeneration. These results suggest that Alchornea cordifolia is relatively non-toxic but has the propensity to induce hepatic injury at high doses.
Keywords
Sub-acute toxicity study; Ethanolic extract; Alchornea cordifolia
Introduction
Alchornea cordifolia (Schum and Thonn.), is a plant of the family Euphorbiaceae. It is also known as Agyama in Ghana, Susubolonta in Sierra Leone, Casamancebugong in Senegal, Tschiya in Togo, Bondji in Cameroon, Ewe ipa, Ubobo and Bambami in the northern part of Nigeria. Alchornea is pantropical and comprises about 60 species of which 6 occur in tropical Africa.
Alchornea cordifolia known as Christmas Bush is a shrub or small tree found abundantly along the coastal area of the West African Sub-region. This plant is used for treatment of a variety of diseases by traditional medical practitioners in Nigeria. Its different parts had been used to treat diarrhoea, wounds, sores, and cuts [1]. A. cordifolia is also reported to possess a multiplicity of biological effects. It is antibacterial [2], spasmolytic [3], anti-inflammatory [4], anti-diarrhoeal [5], antioxidant [6] and antimicrobial [7] agent. These diverse pharmacological actions have been linked to several active principles isolated from the leaves, root, and stem of A. cordifolia. In spite of the wide traditional use of A. cordifolia, very little is known about its anti-malarial effect. This study is therefore aimed at investigating the phytochemical composition and in vivo anti-malarial effect of ethanol and aqueous extracts of the leaves, stem bark and roots of the plant.
Materials and Methods
Plant identification
The Plant Alchornea cordifolia used for this research was collected from the Botanical Garden of School of Agricultural Technology, Federal University of Technology Owerri (FUTO) Nigeria. The plant was identified by Mr. Francis Iwunze of the Department of Forestry and Wild life, School of Agricultural Technology, FUTO. The plant was authenticated by a plant taxonomist, Dr FN Mbagwu of Imo State University Owerri, Nigeria. The plant was prepared and kept at the University herbarium with voucher number IMSU H524.
Plant extraction processes
The apparently healthy parts of the plant (leaves, root and stem bark) were harvested in large quantities and air-dried for about 3 weeks to a constant weight under shade in the laboratory. The dried samples were ground into powdered form using an electric grinder (Saisho 200 W) and stored separately. Using maceration method, 100 g of each powdered sample was soaked separately in 600 ml each of distilled water and ethanol of analytical grade respectively, for 72 hour. Each sample solution was filtered using Whatman No.1 filter paper. The aqueous and ethanol filtrates were separately concentrated using water-bath at 45°C. All the extracts were weighed and then stored in well stoppered containers and kept in a refrigerator at 4°C until used.
Experimental animals
Male albino rats of about 8 weeks old, weighing (84-100 g) were used for the experiment. The animals were housed in gauzed cages in the animal house behind the Biochemistry Department, Federal University of Technology, Owerri. Water and feed were made freely available for them and they were allowed to acclimatize for two weeks prior to commencement of experiment.
The male albino rats were divided into five groups, each consisting of four animals and fasted overnight prior to treatment. Group A, the control group received 2 ml/kg of normal saline daily intraperitoneally.
Groups B, C, D and E were treated intraperitoneally with daily doses of ethanolic leaf extract, (i.e., 0.125 g/kg, 0.25 g/kg, 0.5 g/kg and 0.75 g/kg respectively), for 14 consecutive days. The ethanolic leaf extract chosen for this study showed the highest antimalarial activity in a previous study [8]. All the animals were monitored for another 14 days. The animals were monitored closely for clinical signs of toxicity; i.e., mortality, changes in behavioural patterns, convulsion, loss of sight, loss of appetite, etc. Body weight changes were also recorded daily through-out the 28 days of the study.
Sample collection and processing
At the end of the 28 days of the experiment, the animals were sacrificed by mild ether anesthesia. Blood samples were collected from the animals by cardiac puncture and dispersed into ethylene diaminetetraacetic acid (EDTA) tubes for haematological studies and plain tubes for serum used for evaluation of biochemical parameters. The animal organs (i.e., liver, kidneys and heart) were collected, weighed and stored in 10% formal saline for histopathological analysis.
Assessment of haematological parameters
The haematological parameters were analysed with the aid of an automated analyser (Sysmex XE-2100, Canada).
Biochemical Analysis
Assay of serum aspartate aminotransferase (AST) activity
Determination of AST activity was based on the method of Reitman and Frankel [9] using commercially available test kit (Randox Laboratories Ltd, UK). Exactly 0.1 ml each of serum and distilled H2O were delivered into test tubes labeled Sample and Blank respectively. To both tubes, 0.5 ml of AST reagent I was added, appropriately mixed and incubated at 37°C for 30 min. Then, 0.5 ml of AST reagent II was added to all test tubes, mixed and incubated at 25°C for 20 min. Finally, 5.0 ml NaOH was added to the test tubes and mixed. Absorbance was taken at 510 nm against reagent blank after 5 min.
Assay of serum alanine aminotransferase (ALT) activity
This was based on the method of Reitman and Franke [9], using commercially available test kit (Randox Laboratories Ltd, UK). Briefly, 0.1 ml of serum and distilled water (dH2O) was delivered into test tubes labeled Sample and Blank respectively. Then, 0.5 ml of ALT reagent I was added into both tubes, mixed and incubated at 37°C for 30 min. Afterwards 0.5 ml of ALT reagent II was added to all test tubes, mixed and allowed to stand for 20 min at 25°C. Finally, 5.0 ml NaOH were added to the tubes, mixed and absorbance read against the reagent blank at 510 nm after 5 min and the activity calculated.
Assay of serum alkaline phosphatase (ALP) activity
ALP activity was assayed with the aid of a commercial test kit (Randox Laboratories Ltd, UK) according to the method described by Englehardt [10]. Briefly, 0.01 ml sample was added into labelled test tube. Then, 0.50 ml ALP reagent (containing diethanolamine buffer, magnesium chloride and p-nitrophenylphosphate) was added to the tube and mixed thoroughly. Absorbance was read against water blank at 405 nm and activity (U/l) determined.
Determination of serum total bilirubin concentration
The method that exploits the use of diazotized sulphanilic acid as described by Pearlman and Lee [11] and Zoppi et al. [12] was used with the aid of commercially available standard kit (Human Laboratories, Germany).
Determination of total protein concentration
The Biuret method as described by Gornall et al. [13] was employed for the determination of protein concentration. Test tubes were prepared and labelled as blank, standard and sample. To these 0.02 ml of distilled H2O, standard protein and serum were added respectively. Then, to all test tubes 1.0 ml of total protein Reagent 1 was added, mixed and incubated at 25°C for 30 min. Absorbance of sample and standard were read against reagent blank at 500 nm and protein concentration calculated.
Determination of serum albumin concentration
This was determined as described by Doumas et al. [14]. Briefly, test tubes were prepared and labelled blank, standard and sample. To these, 10 μl of distilled H2O, standard albumin and serum were respectively added. Then, 1.0 ml Reagent A (containing Bromocresol reagent) was added to all tubes, mixed and incubated at 25°C for 10 min. Absorbance of sample and standard were read against blank at 630 nm and albumin concentration calculated.
Determination of urea and creatinine concentrations
Serum urea and creatinine concentrations were determined by Jaffe’s reaction and urease enzymatic method respectively using standard kits (Human Laboratories, Germany) [15].
Histopathological analysis of the liver and kidney
A histological study was carried out with the liver and kidney samples from different groups of the experimental animals. This was carried out to crosscheck the results that were obtained from biochemical assay. The method used was that described by Okoro [16].
Statistical Analysis
Data obtained were expressed as mean ± standard deviation. Statistical analysis was carried out using one way analysis of variance (ANOVA) and significance taken at P<0.05.
Results
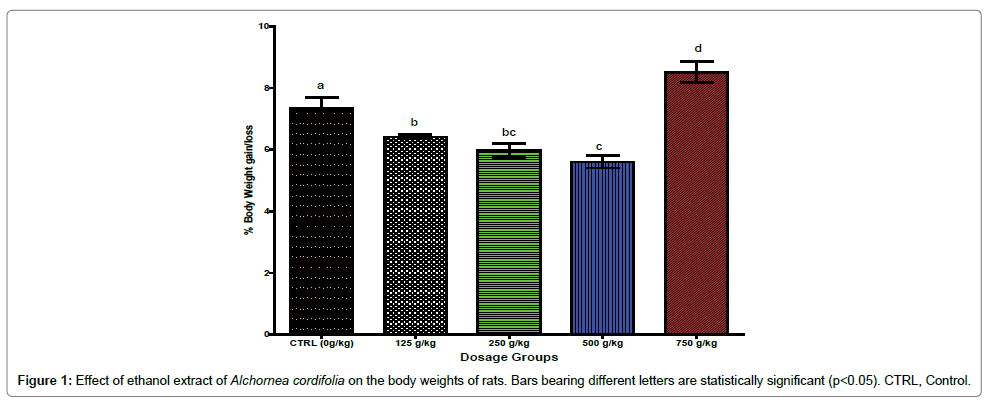
Figure 1 shows the effect of ethanol extract of A. cordifolia on the body weight of the albino rats. It shows that there were dosedependent significant (p< 0.05) decrease in percentage body weight of the animals administered 125, 250 and 500 g/kg body weight of the ethanol extract. However, the animals administered the highest extract dose of 750 g/kg bwt showed significant (p < 0.05) increase in the body weight when compared with the control group that was administered the extract.
Generally, the biochemical parameters of the extract treated animals did not differ significantly (p>0.05) from the control (Table 1), except for ALT activity which was observed to be generally low in the treated groups but significantly (p< 0.05) decreased only in the 250 g/kg and 750 g/kg extract groups, when compared with control.
| Parameters | Control | 125 | 250 | 500 | 750 | |
|---|---|---|---|---|---|---|
| AST (µ/L) | 46.40±2.87a | 47.75±2.14a | 42.80±2.67a | 45.00±4.24a | 42.35±2.25a | |
| ALT (µ/L) | 48.50±2.83a | 44.25±2.21a | 41.40±2.50b | 44.25±1.11a | 41.00±2.48b | |
| ALP (µ/L) | 115.40±3.69a | 115.40±3.69a | 109.56±2.75a | 114.00±4.25a | 108.01±5.82a | |
| Total bilirubin (µmol/L) | 27.04±6.48a | 26.51±5.94a | 31.47±5.46a | 32.49±10.59a | 29.23±3.59a | |
| Conjugated bilirubin (µmol/L) | 10.29±2.65a | 12.68±4.58a | 11.97±2.29a | 14.54±4.76a | 13.25±4.15a | |
| Total protein (g/L) | 81.60±3.17ab | 76.50±5.49ab | 74.20±4.91b | 84.25±2.10a | 84.25±2.06a | |
| Albumin (g/L) | 47.00±4.63a | 48.50±4.53a | 45.30±3.39a | 45.00±2.83a | 49.97±3.31a | |
| Creatinine (µmol/L) | 99.02±6.49a | 99.45±9.10a | 99.50±6.85a | 97.20±5.40a | 98.62±6.26a | |
| Urea (mmol/L) | 3.46±0.60a | 4.73±1.15a | 3.56±0.40a | 4.30±0.28a | 4.60±0.39a | |
Table 1: Effect of ethanolic extract of Alchorneacordifoliaon the serum biochemical parameters of rats treated with different doses of the extract.
Results of the study indicate that the administration of A. cordifolia leaf extract generally caused no variation in the haematological parameters of the rats. However, there was a significant (p< 0.05) dosedependent increase in the total white blood cell count of animals treated with the extract, when compared to the control (Table 2). On the other hand, there was a significant (p< 0.05) dose-dependent decrease in the monocyte count of the treated animals.
| Doses (Mg/kg) | |||||
|---|---|---|---|---|---|
| Parameters | Control | 125 | 250 | 500 | 750 |
| WBC (K/µl) | 5.10±0.63a | 6.83±0.70b | 6.80±1.07b | 7.15±0.16b | 7.31±0.21b |
| LYM (%) | 79.10±2.80a | 78.35±3.06a | 79.60±0.87a | 80.68±1.15a | 80.52±1.69a |
| NEU (%) | 5.68±1.40a | 5.98±2.09a | 5.98±1.03a | 6.20±0.66a | 7.86±2.59a |
| RBC (M/µL) | 5.98±0.21a | 5.55±0.51a | 5.77±0.14a | 5.68±1.40a | 5.97±0.31a |
| HGB (g/dl) | 13.48±0.51ab | 13.83±0.47ab | 13.02±0.51ab | 13.20±0.40ab | 12.62±0.26b |
| Monocyte(%) | 6.76±0.03a | 6.50±0.15bc | 6.56±0.08ab | 6.41±0.11bc | 6.30±0.10c |
| Eosinophil(%) | 4.10±1.47a | 4.35±0.96a | 4.60±0.99a | 5.68±0.80a | 5.54±0.66a |
| Basophil (%) | 1.20±1.42a | 1.60±1.01a | 1.63±0.88a | 1.67±0.73a | 1.72±0.86a |
| PLT (K/µL) | 276.40±95.49a | 302.20±60.01a | 301.70±65.66a | 348.50±69.70a | 386.20±99.82a |
Table 2: Effect of ethanolic extract of Alchorneacordifoliaon haematological indices in rats treated for two weeks.
The relative organ weights of the animals showed no significant (p>0.05) differences between those of the treated groups and the control group indicating that A. cordifolia at the doses used did not produce any damage in the form of organ swelling, atrophy or hypertrophy in the treated animals (Table 3).
| Organ | Doses (mg/kg) | ||||
|---|---|---|---|---|---|
| Control | 125 | 250 | 500 | 750 | |
| Heart | 0.47±0.04 | 0.49±0.06 | 0.47±0.02 | 0.40±0.04 | 0.38±0.03 |
| Liver | 4.46±0.59 | 5.08±0.67 | 5.71±0.63 | 4.67±0.82 | 4.20±0.48 |
| Kidney | 1.20±0.01 | 1.21±0.09 | 1.23±0.09 | 1.09±0.13 | 1.07±0.06 |
| Spleen | 0.64±0.13 | 0.74±0.11 | 0.97±0.26 | 0.73±0.21 | 0.74±0.09 |
| Stomach | 1.12±0.07 | 1.17±0.10 | 0.92±0.31 | 1.25±0.12 | 1.44±0.03 |
Table 3: Effect of different doses of leaf ethanolic extract of A. cordifoliaon relative organ weights of rats.
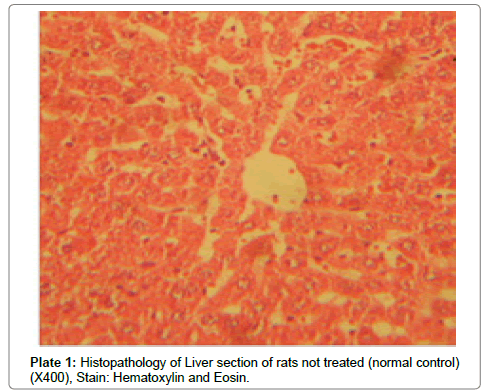
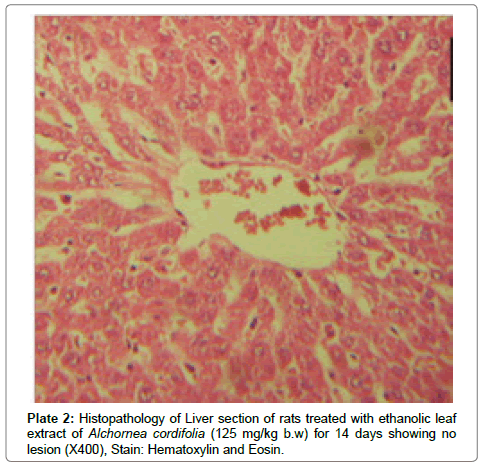
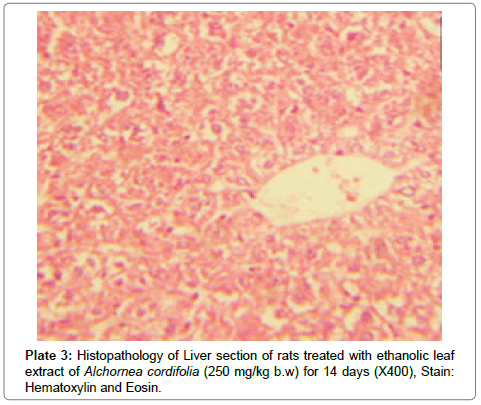
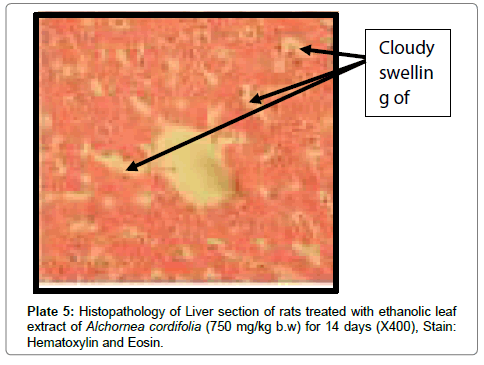
There were no lesions observed in the liver tissues of rats treated with the ethanol leaf extract of A. cordifolia at 125 mg/kg, 250 mg/ kg and 500 mg/kg body weight compared to the control (Plates 1-4). However, in the group treated with 750 mg/kg extract, a slight cloudy swelling of hepatocytes with vascular degeneration was observed (Plate 5). On the other hand, no lesions were observed in the kidney tissue sections of both the treated and control animals (Plates 6-10).
Discussion
The slight increase in body weight after the 14-day treatment can be ascribed to normal growth of the animals over the period. Food and water intake were normal and no deaths occurred. The relative weights of vital organs of animals did not show significant differences compared to the vehicle-treated control with no evidence of organ swelling, atrophy or hypertrophy in the animals. These observations indicate that the treatment had no effect on the general condition and functional behaviour of the animals. The haematopoetic system is an important index of physiological and pathological status in man and animals [17] and a sensitive target for toxic compounds [18] since it is initially exposed to a high concentration of toxic agents. This has a predictive value for toxicity in humans and animals and therefore analysis of blood is relevant to risk evaluation [19].
Results of the hematological analysis showed that Alchornea cordifolia has little or no effect on white blood cell (WBC), lymphocytes (LYM), neutrophils (NEU), red blood cells (RBC), haemoglobin concentration (HB), haematocrit (HCT), eosinophils, basophils and platelets (PLT). However, a dose dependent increase in neutrophils (NEU) was observed. Neutrophils interact with foreign compounds and microorganisms and destroy them by the process of phagocytosis. They are also known to be involved in the pathology of various inflammatory conditions. It is possible that the increase in neutrophils in the present study is related to the slight liver cell injury observed in the histopathological studies. The markers of renal function, urea and creatinine, were assessed based on reported kidney toxicity associated with the use of medicinal plants. These parameters were not affected in treated animals. Alchornea cordifolia has been shown to contain flavonoids [3]. Some of these flavonoids have been demonstrated to inhibit nephrotoxicity because of its strong antioxidant activity [20]. Alchornea cordifolia has also been reported to contain tannins and tannins are known to offer protection against nephrotoxicity [21].
It is possible that these constituents offered protection to the treated animals in the present study.
Aspartate transaminase (AST) and Alanine transaminase (ALT) are considered as indicators of liver function. High levels of AST,ALT and ALP in serum are usually indicative of disease and necrosis in the liver of animals. Alkaline phosphatase (ALP), Gamma glutamyl transpeptidase (GGT), albumin, globulin, bilirubin and total protein were the other biochemical parameters analyzed. Analysis of these parameters is important since there are several reports of liver toxicity related to the use of medicinal plant products [22]. These parameters did not differ in the treated and the vehicle-treated groups, indicating that Alchornea cordifolia caused no adverse effect on the hepatic and renal systems.
Histopathological observations in the liver however did not correlate with the biochemical findings especially at a high dose (750 mg/kg) of the extract. At a higher dose of the extract, it was observed that there were degenerations in the liver suggesting early signs of liver cell injury. This type of liver injury is however known to be reversible upon withdrawal of the toxicant allowing the liver cells to regenerate. This early stage of liver injury may not necessarily reflect in elevation of liver enzymes. In contrast to the present findings, Alchornea cordifolia at doses of 125-500 mg/kg was reported to protect rats against paracetamol induced liver damage [23]. Alchornea cordifolia evoked mild liver damage at rather high dose (700 mg/kg) in the present work. This may be intriguing, but the extract probably contains several components with different pharmacological actions. The proportion of specific toxicants may be increased at high doses. Though the slight liver damage detected in the histopathology studies was not associated with corresponding elevation of liver transaminases, the potential toxicity of the extract especially at high doses cannot be ignored.
Estimates based on recommended dosage of dried Alchornea cordifolia leaves (maximum 50 g per litre of water; 4 cups daily) in traditional medicine [23] suggest that humans could take a maximum of 200 mg/kg of the extract daily. Though this appears to be much lower than the dosages used in the present study, observation of potential liver damage in mice observed previously and the fact that dosages in herbal remedies are never precise due to non- standardization, call for caution in the routine use of the plant.
Conclusion
The results of the study indicate that though the ethanolic leaf extract of Alchornea cordifolia is relatively non-toxic to the haematological and renal systems, it is potentially hepatotoxic in rats if administered at high doses. Though these findings cannot be directly extrapolated to man in view of possible species differences and possible differences in metabolic activation, the present results suggest that caution should be taken in the use of the plant product especially at high doses.
Acknowledgements
We wish to express our gratitude to Rev. Chinekeokwu and Mr. Tony Nani, both of Biochemistry department, FUTO for their assistance during the laboratory work.
References
- Abbiw DK (1990) Useful plants of Ghana. Intermediate Technology Publications and Royal Botanic Gardens, Kew, London, UK, pp: 18-25.
- Ajali U (2000) Antibacterial activity of Alchornea cordifolia stem bark. Fitoterapia 71: 436-438.
- Ogungbamila FO, Samuelsson G (1990) Smooth muscle relaxing flavonoids from Alchornea cordifolia (leaves). Acta Pharmaceutica Nordica 2: 421-422.
- Marva-Manga H, Brkic D, Marie DEP, Quetin-Leclercq J (2004) In vivo antiinflammatory activity of Alchornea cordifolia (Schumach& Thonn.) Mull. Arg. (Euphorbiaceae). J Ethnopharmacol 92: 209-214.
- Agbor GA, Leopold T, Jeanne NY (2004) The antidiarrhoeal activityof Alchornea cordifolia leaf extract. Phytotherapy Research 18: 873-876.
- Olaley, MT, Rocha JB (2008) Acetaminophen-induced liver damage in mice: Effects of some medicinal plants on the oxidative defense system. Experimental Toxicology and Pathology 59: 319-327.
- Ebi GC (2001) Anti-microbial activities of Alchornea cordifolia. Fitotherapia 72: 69-73.
- Ezeokeke EE, Ene AC, Igwe CU (2015) In vivo anti-plasmodial effect of ethanol and aqueous extracts of Alchornea cordifolia. Biochemistry and Analytical Biochemistry 4: 1000221.
- Reitman S,Frankel S (1957) Glutamic – pyruvate transaminase assay by colorimetric method. Am J Clin Path 28: 56.
- Englehardt A (1970) Alkaline Phosphataseassay. Aerzti Labor 16: 42.
- Pearlman FC, Lee RTY (1974) Detection and measurement of total bilirubin in serum with use of surfactants as solubilizing agents. Clin Chem 20: 447-453.
- Zoppi F, Fenili D (1976) Enzymatic determination of total serum cholesterol with the Vickers D-300 analyzer. Clin Chem 22: 690.
- Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177: 751-766.
- Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta 31: 87-96.
- Tietz NW (1987) Fundamentals of Clinical Chemistry. Philadelphia, USA.
- Okoro I (2002) Manual of Practical Histology. Peace Publishers, Owerri, p: 171.
- Adeneye AA, Ajagbonna OP, Adeleke TI, Bello SO (2006) Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J Ethnopharmacol 105: 374-379.
- Harper HA (1973) Review of Physiological Chemistry. Lange MedicalPublications, California.
- Olson H, Betton G, Robinson D, Thomas K, Monro A, et al.(2000) Concordance of the Toxicity of Pharmaceuticals in Humans and in Animals. Regul Toxicol Pharmacol 32: 56-67.
- Satyanarayana PS, Singh D, Chopra K (2001)Quercetin, abioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Exp Clin Pharmacol23: 175-181.
- Yokozawa T, Fujioka K, Oura H, Nonaka G, Nishioka I (1991) Effects of rhubarb tannins on uremic toxins. Nephron 58: 155-160.
- Pittler MH, Ernst E (2003) Systematic review: hepatotoxic events associated with herbal medicinal products. Aliment Pharmacol Ther 18: 451-471.
- Adewunmi CO, Agbedahunsi JM, Adebajo AC, Aladesanmi AJ, Murphy N, et al.(2001)Ethnoveterinary medicine: screening of Nigerian medicinal plants for trypanocidal properties. Journal of Ethnopharmacology 77: 19-24.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 8389
- [From(publication date):
April-2017 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 7305
- PDF downloads : 1084