Subacute Oral Toxicity Studies of ZingiVir-H: A Herbomineral Antiviral Drug in Wistar Rats
Received: 01-Apr-2021 / Accepted Date: 15-Apr-2021 / Published Date: 22-Apr-2021 DOI: 10.4172/2476-2067.1000155
Abstract
Herbal and herbo-mineral preparations are being traditionally used in Indian system of medicines. These preparations have numerous health benefits that have been involved in their widespread use for the treatment of different diseases/disorders by traditional Ayurvedic medicinal practitioners. However, to the best of our knowledge, no systematic study regarding its toxicity profile has been reported for a herbomineral preparation with antiviral activity. This study aimed to investigate the subacute 28-days repeated dose oral toxicity of ZingiVir-H, herbomineral drug having antiviral activity in Wistar rats. According to OECD TG 407, rats were divided into 6 groups. The ZingiVir-H at the doses of 500, 1000 and 1500 mg/kg body weight daily was administered orally for 28 days for treatment and satellite groups, whereas an equal volume of vehicle was given to control groups. In order to access reversibility, satellite groups were kept for another 14 days post-treatment. Important parameters such as general behaviour, body and organ weight, haematological, serum biochemistry, organ macroscopy and microscopy were conducted to examine its safety. Treatment with ZingiVir-H daily for continuous 28 day did not show any signs of toxicity. Moreover, abnormalities on behaviour, food and water intakes, body weight, relative organ weights, haematological, and serum biochemical parameters were not observed. Histopathological examination of vital organs recorded normal architecture suggesting no morphological alterations after ZingiVir-H treatment. These results demonstrated that ZingiVir-H did not possess potential to induce any toxicity in the animals. Moreover, this is the first report on the toxicity study of herbo-mineral preparation with antiviral property.
Keywords: Herbomineral; Toxicity; Wistar rats; ZingiVir-H
Introduction
A toxicological investigation is considered very crucial for the development of novel drug formulations. The US Food and Drug Administration (FDA) have specified that it is very important to screen new molecules/formulations for toxicological and pharmacological activity in experimental animals before releasing into market [1]. Toxicology deals with the study of finding the adverse effect of chemicals/drugs on living organisms [2,3]. More over toxicology studies defines meticulous description of symptoms, bio-mechanisms, treatment and detection of poison; especially the poisoning effects during consumption of test chemicals [4]. It is also essential to know the concentration of dosage of any compound/drugs/herbal formulations/ by-products for humans through experimental animal models.
All traditional medicines used worldwide has a large range of formulations that can be classified as either phytochemicals (herbal formulations) or herbo-metallic/mineral formulations [5,6]. The use of various metals in medicinal preparations is widespread in Ayurveda, Traditional Chinese Medicine, Traditional Tibetan Medicine, and Nigerian Medicine etc. Some allopathic formulations also use metals like cisplatin, carboplatin, colloidal gold, silver sulphadiazene (silvadene), ferrous gluconate, etc., for the therapeutic purposes [7].
In Ayurveda, preparations involving heavy metals normally called bhasmas (ash) are very common [10]. These metals in combination with the herbal constituents are said to form an effective combination in disease management and treatment. The toxicity of the metallic constituent isbelieved to be removed bysubjectingtodetoxifying process which includes various heating and cooling cycles in oil, buttermilk, rice gruel, cow’s urine, herbal decoctions, etc. This detoxifying process will convert heavy metals to less toxic or non-toxic form [11,12]. In due course of time, herbo-mineral/metallic preparations came to occupy a significant seat in Ayurvedic Pharmacopoeia and have routinely been used in practicing different parts of India for several centuries. Yet, modern medical practitioners sturdily disagree with the therapeutic efficacy of such preparations due to the high content of heavy metals.
The past decade, however, we witnessed concerns raised by the western scientific community, regarding the safety profile of Ayurvedic Herbal, Herbo-mineral and metallic preparations, which is a major treat for the century-old Ayurvedic heritage [10]. These arguments have been further fuelled in recent years with reports of metal toxicity in patients who had consumed herbo-metallic preparations for a prolonged period of time [13,14]. Though this form of traditional medicine has been practiced for several centuries, the concepts are not fully admired by the chemists and western medical practitioners. Therefore, an in-depth assessment on the safety profile of herbomineral preparation becomes very necessary for convincing them.
We, Pankajakasthuri Herbals India Pvt. Ltd. recently formulated ZingiVir-H tablets (drug licence number: DL. NO: 50/25D/96), a novel herbo-mineral Ayurvedic formulation, which was proven to have antiviral, antipyretic and immunomodulatory properties. ZingiVir-H tablets were originally developed to combat against fever, various viral infection and viral fevers, Bronchitis and Acute Respiratory tract infections. This herbomineral formulation contains Eugenia caryophyllus, Zingiber officinalea, Cyperus rotundus, Hedyotis corymbosa, Trachyspermum ammi, processed and purified Mercuric sulphide and Arsenic trisulphide ingredients. A double-blind clinical trial of ZingiVir-H has revealed its significant antiviral effect for the treatment of deadly COVID-19 patients (data not published). However, safety issues are often raised on the use of mercury as well as arsenic containing Ayurvedic and other traditional drugs in disease condition due to the risk of mercury and similar other heavy metal toxicity to the patients [10,15-17]. So, the present study aimed to assess the toxicity profile of herbomineral antiviral drug ZingiVir-H in Wistar rats.
As per the OECD TG 407, subacute toxicity studies provide information on possible health hazards likely to arise from repeated exposure of drugs/chemical over a limited period of time and it is often considered as a preliminary study to find out the toxicity profile. In addition, subacute toxicology studies in rodents are considered to be a mandatory step to support the progression to various clinical trials and the eventual marketing of drug molecules for the use of general public [18]. No information is available in the literature till dated for the long-term toxicity study of herbomineral drug especially containing purified mercury and arsenic according to ancient Ayurvedic principles. So, the present work is regarded as the first study concerning the acute toxicity study of ZingiVir-H, herbomineral drug using the dose 500, 1000 and 15000 mg/kg body weight daily orally for 28 days to both genders of Wistar albino rats. In this study, hematological and biochemical parameters including liver enzymes activity, kidney function tests, and serum electrolytes were evaluated. Besides, histopathological examination of vital organs such as liver, heart, brain, lungs, spleen and kidney of both male and female rats.
Methodology
Chemicals and reagents
Commercial reagent kits for determination of serum biochemistry experiments were purchased from Agappe diagnostic (India). All other chemicals and reagents used were of the highest analytical quality obtained from commercial sources.
Ethics statement
All animal studies were performed according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and approved by the Institutional Animal Ethics Committee. The animals were examined and adapted to the new environmental conditions for a week before the formal initiation of the experiment.
Ethical approval
The experimental protocol of the present study was reviewed thoroughly and approved with reference no. PKHRF/IAEC/ NOC/01/2020 by the Institutional Animal Ethical Committee of Pankajakasthuri Herbal Research Foundation, Killy Kattakada, Thiruvananthapuram, Kerala, India (Reg. No. 2093/PO/ReRcBi/S/20/ CPCSEA) and animals were maintained as per the guidelines of the CPCSEA, India.
Animals
Healthy male and female Wistar rats (175–250 g) were purchased from the SCTMIST, Thiruvananthapuram, Kerala, India. The toxicity studies were performed strictly with the method of Compilation of Technical guidelines for Veterinary Drug Research and was also referred to Organization for Economic Cooperation and Development (OECD) Guideline No. 407. All rats were housed in plastic cages and kept separately according to sex (temperature 22 ± 2ºC, relative humidity 55 ± 10%, and 12-h light/dark cycle). All rats were allowed to access water and food freely throughout the study.
Subacute toxicity study of ZingiVir-H
Subacute oral toxicity study of ZingiVir-H was performed according to the OECD guideline 407 for testing of chemicals [19] and World Health Organization guideline [20]
According to OECD TG 407 [19], 52 rats (26 male and 26 female) were randomized into 6 groups (control/satellite control having 6 rats (three males and females) and treatment/satellite having 10 rats (five males and females). The experimental rats were marked to permit discrete identification of treatment groups. Male and female rats were kept in separate cages and cages were numbered. Rats groups used in the present study were as follows.
Group 1: Control group [6 rats (3 males and 3 female)] received a sodium chloride solution (0.9% w/v) for 28 days.
Group 2: Low dose group [10 rats (5 Males and 5 females)] received ZingiVir-H (500 mg/kg body weight orally/day) for 28 days.
Group 3: Mid dose group [10 rats (5 Males and 5 females)] received ZingiVir-H (1000 mg/kg body weight orally/day) for 28 days.
Group 4: High dose group [10 rats (5 Males and 5 females)] received ZingiVir-H (1500 mg/kg body weight orally/day) for 28 days.
Group 5: Satellite group control [10 rats (5 Males and 5 females)] received a sodium chloride solution (0.9% w/v) for 28 days and observed for another 14 days post treatment.
Group 6: Satellite group [10 rats (5 Males and 5 females)] received ZingiVir-H (1500 mg/kg body weight orally/day) for 28 days and observed for another 14 days post treatment.
General observations
During the study period (before and after dose administration), all the animals were observed twice daily for morbidity and mortality. Daily body temperature was recorded.
In-life clinical observations
Observations of clinical signs were recorded during handling and in open field on day 0, 7, 14, 21 and 28 for rats of the control and treatment groups. Animals of satellite group were observed on additional day 35 and 42. Clinical signs included changes in sensory organs (such as skin, eyes, nose), body secretion, autonomous activities (respiratory rate, irregular respiratory pattern, pupil size, piloerections), vocalization, lacrimation, salivation, convulsion, tremors, response while handling, walking pattern, overall body language, reflexes, changes in skin and fur texture, muscle tremor and behaviour, etc were observed daily [21,22].
Food and water consumption
Rats were allowed ad libitum access to food and water throughout the study. Food and water consumption for animals of treatment and control groups were measured daily. Rats were fasted overnight prior to termination of the study.
Body weight and body weight gain
Rats were weighed twice prior to grouping. Body weights were recorded on days 1 (prior to dosing), 7, 14, 21, 28 and 29 (fasting body weight prior to sacrifice for calculating relative body organ weight) in treatment and control groups. Body weight measurements of rats of satellite group were continued on a weekly interval on day 35, 42 and 43 (prior to sacrifice) [23,24]. At the end of the study period, mean body weight gains were calculated for each treatment group.
Necropsy and organ weight
All rats were fasted overnight prior to necropsy. The rats were euthanized using sodium pentobarbital (200 mg/kg body weight) followed by exsanguination. The macroscopic examination included a thorough study of the external surfaces, all orifices, which includes the thoracic, abdominal, cranial and pelvic cavity. During necropsy, vital organs such as liver, kidney, heart, lungs, spleen and brain were excised from both male and female rats respectively. These vital organs were carefully washed and placed in neutral buffered formalin (10%) solution. The macroscopic examination of the organs was also carried out for possible development of lesions or other abnormal signs. Then the organs were placed on absorbent papers for few minutes and after that organ weight (absolute organ weight) were recorded.
Haematological analysis
Blood samples were collected and placed into tubes containing EDTA-K2 for the hematological analyses. Haematological profile of the collected blood samples was analysed using automatic haematological analyser. The following haematological parameters were investigated: haemoglobin, total white blood cell (WBC) count, total red blood cell (RBC) count, total platelets, differential leucocyte count (neutrophils, lymphocytes, eosinophil, monocytes and basophil).
Serum biochemistry
The non-heparinized blood samples were collected for serum biochemical estimations. The coagulated blood samples were centrifuged at 2000 g to obtain serum. The serum was taken into new tubes and stored at -20˚C until analyzed. The serum biochemical parameters were estimated spectrophotometrically by using kits of analytical grade. The following parameters were recorded in the study: blood glucose, total cholesterol, triglycerides, HDL, LDL, urea, uric acid, creatinine, total protein, albumin, total bilirubin, direct bilirubin, aspartate transaminase, alanine transaminase, alkaline phosphatase, calcium, sodium, potassium, and chlorine, phosphate.
Histopathological studies
Histopathological studies were performed on organ samples of liver, kidney, spleen, heart, brain, and lungs from both male and female rats respectively. The major organs were surgically removed and were fixed in 10% buffered formalin (pH 7.4). After fixation, the tissue samples were dehydrated in graded series of ethanol (70–99.9%), washed in toluene, and then enclosed in paraffin. Using a rotary microtome, a thin tissue sections of 5 μm were obtained and this was stained with hematoxylin-eosin. The stained tissue sections were then analysed microscopically for pathological examinations and appropriate photos were recorded.
Statistical analysis
In the present study, data were expressed as mean value and standard deviations (mean ± SD). Statistical analyses containing body weight, organ weight, blood biochemical, and biochemistry data were performed by one-way analysis of variance (ANOVA) with SPSS Statistics 19.0 (IBM, Chicago, USA), and the graph was drawn by using origin software. A p <0.05 was considered statistically significant.
Ethical consideration
The study was conducted only after getting approval from the Institutional Animal Ethical committee of the Pankajakasthuri Herbal Research Foundation, and in line with the highest standard for the humane and compassionate use of animals in biomedical research. The rats used in this study were not subjected to any unnecessary painful and other terrifying situations. To keep the pain and suffering minimal during any surgical intervention, all animals were given sodium pentobarbital (200 mg/kg body weight) anaesthetic and the procedure was carried out by a well-trained person. The animals were protected from pathogens and placed in ideal environment. The numbers of animals were reduced to the minimum possible that allows investigators achieving the scientific objectives of the study [25].
Results
Subacute toxicity
In-life clinical observations: All rats were observed daily for mortality. Observations for any significant clinical signs during handling and in the open field were recorded on a weekly basis till the complesion of the study. In our study, no treatment related mortality was observed in animals of both sexes at the oral dose of ZingiVir-H at the doses of 500, 1000 and 1500 mg/kg body weight. The experimental rats were observed critically during handling and in open field; no prominent clinical signs in sensory organs (such as skin, eyes, nose), body secretion, autonomous activities (respiratory rate, irregular respiratory pattern, pupil size, piloerections), vocalization, lacrimation, salivation, convulsion, response while handling, walking pattern, overall body language, reflexes, muscle tremor and behaviour, etc. were observed in the rats of ZingiVir-H treatment groups and satellite group as compared to control groups. No gross or macroscopic abnormalities were observed in all rats of both sexes in the treatment groups.
General sign in the rat: All the rats fed with the ZingiVir-H were found to be healthy. There were no changes observed in their behaviour and locomotor activity. There was absence of any visual sign of intoxication during the 28-day treatment period. There were no changes observed in morphological characteristics of skin, eyes, nose, fur and mucous membrane. Animals also showed normal nutritional status. Any minor changes or activities in experimental rats observed during the study period can be considered common findings for Wistar rats.
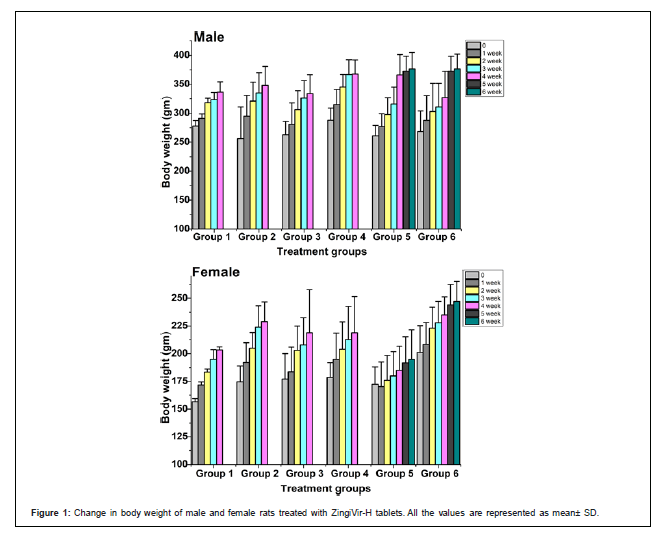
Body weight and body weight gain: The change in body weight of the control and treated rats were shown in Figure 1 In this study, all rats at each dosage group showed constant weight gain during the experimental periods which indicates that ZingiVir-H did not elicit any harmful effect on body weight. Moreover, no significant difference in the percentage increase of body weight was compared between the ZingiVir-H treated and control groups (Figure 1).
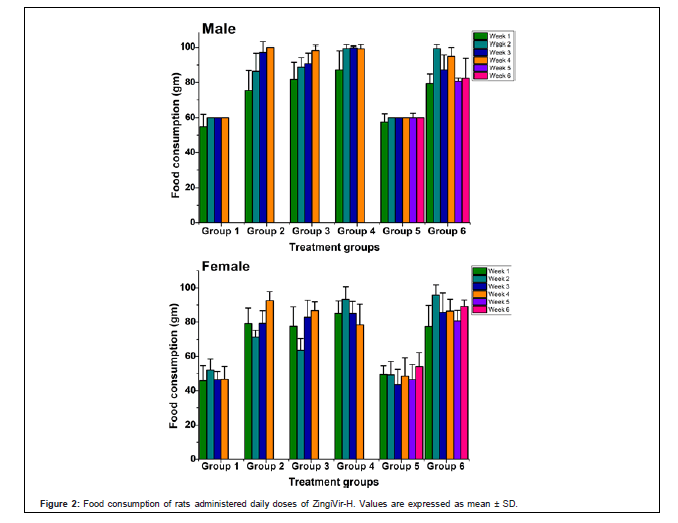
Food and water consumption: Food consumption for the treatment and satellite groups of male and female animals were compared with vehicle-treated control group. During the study, the constant increase in food consumption was observed in male and female animals of treatment and satellite groups. This exemplified that the ZingiVir-H has no obvious effect on food consumption in the experimental animals (Figure 2). Furthermore, oral administration of ZingiVir-H caused no major statistical alteration in water consumption in the treatment and satellite groups compared to control group.
Effect of ZingiVir-H on haematological parameters: Haematological parameters such as haemoglobin, red blood cells, white blood cells of ZingiVir-H treated groups were persist with that of the control groups (Table 1). All the parameters were within physiological range during the treatment period. More over the satellite groups also showed no significant change as compared to normal animals (Table 1). The treatment group retained normal increase in haemoglobin, red blood cell count and platelet count in male and female Wistar rats. In addition, white blood cells count of rats in treatment and satellite groups were observed to be in normal range and had no significant difference as compared to normal control groups. The eosinophil, monocyte and lymphocyte count in treated and satellite groups showed no significant change as compared to normal animals.
| Parameter | Control | Low dose | Mid dose | High dose | Control recovery | Recovery |
|---|---|---|---|---|---|---|
| Male | ||||||
| RBC | 4.05 ± 0.87 | 4.39 ± 1.47 | 3.48 ± 0.86 | 4.75 ± 1.35 | 3.23 ± 2.37 | 5.29 ± 0.91 |
| WBC | 5800.00 ± 2773.06 | 11020.00 ± 4963.06 | 6990.00 ± 2825.42 | 8500.00 ± 1541.10 | 9753.00 ± 1121.80 | 11974.00 ± 642.01 |
| Haemoglobin | 12.03±2.64 | 13.14±4.46 | 10.67±2.37 | 14.28±4.07 | 13.07 ± 2.88 | 15.86 ± 2.72 |
| Total Platelets | 6.27 ± 1.66 | 5.06 ± 1.07 | 4.46 ± 1.25 | 7.500 ± 0.81 | 6.17 ± 2.03 | 5.700 ± 2.68 |
| Neutrophil | 49.00 ± 4.36 | 61.80 ± 5.63 | 53.20 ± 3.96 | 47.60 ± 7.09 | 67.00 ± 1.41 | 62.00 ± 7.38 |
| Monocytes | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Lymphocytes | 50.33 ± 4.04 | 36.80 ± 6.30 | 45.40 ± 4.39 | 51.40 ± 6.84 | 29.50 ± 0.71 | 33.00 ± 7.55 |
| Eosinophil | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Basophil | 0.67 ± 0.56 | 1.40 ± 0.89 | 1.40 ± 0.55 | 1.00 ± 0.71 | 3.00 ± 1.41 | 4.60 ± 1.95 |
| Female | ||||||
| RBC | 4.20 ± 1.01 | 4.30 ± 1.76 | 4.88 ± 1.60 | 5.58 ± 0.91 | 6.13 ± 0.62 | 5.38 ± 0.28 |
| WBC | 12500.00±5047.77 | 6460.00 ± 2083.99 | 7860.00 ± 7670.27 | 6160.00 ± 808.08 | 10433.00 ± 930.98 | 11966.00 ± 20.35.89 |
| Haemoglobin | 12.60±3.02 | 15.06 ± 2.54 | 14.80 ± 4.64 | 16.71 ± 2.68 | 16.02 ± 0.54 | 16.14 ± 0.83 |
| Total Platelets | 3.93 ± 0.81 | 6.66 ± 1.82 | 7.04 ± 1.80 | 4.54 ± 1.29 | 2.97 ± 0.64 | 4.96 ± 1.90 |
| Neutrophil | 51.33 ± 5.03 | 46.80 ± 6.26 | 44.00 ± 2.83 | 51.60 ± 3.91 | 55.67 ± 7.10 | 66.20 ± 9.73 |
| Monocytes | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.000 ± 0.00 | 0.000 ± 0.00 |
| Lymphocytes | 47.33 ± 5.51 | 45.80 ± 15.14 | 55.00 ± 2.83 | 47.40 ± 3.51 | 38.33 ± 7.10 | 30.00 ± 8.80 |
| Eosinophil | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.000 ± 0.00 |
| Basophil | 1.33 ± 0.58 | 1.40 ± 2.61 | 1.00 ± 1.00 | 1.40 ± 0.55 | 6.00 ± 0.00 | 3.80 ± 2.17 |
Table 1: Haematological data of rats in the ZingiVir-H-treated group and the control group.
Serum biochemistry: The serum biochemical parameters in Table 2 are indicator of major toxic effects in the vital tissues especially kidney and liver. The various enzymes and proteins can be used to indicate hepatocellular effects (such as ALT, AST, ALP and bilirubin). Levels of cholesterol are an indirect indicator of liver function, whilst the levels of creatinine, uric acid and blood urea nitrogen act as biomarkers of nephron functional injury or kidney injury. All the biochemical parameters tested did not show any significant differences (p>0.05) between the treatment and control groups at all the tested doses for both sexes (Table 2).
| Parameter | Control | Low dose | Mid dose | High dose | Control recovery | Recovery |
|---|---|---|---|---|---|---|
| Male | ||||||
| Random blood sugar | 81.33 ± 3.80 | 84.19 ± 7.43 | 87.56 ± 17.90 | 97.23 ± 1.52 | 91.36 ± 16.41 | 95.64 ± 71.83 |
| Total cholesterol | 69.67± 11.93 | 58.93 ± 17.28 | 42.90 ± 6.09 | 72.57 ± 9.56 | 83.70 ± 14.37 | 70.50 ± 4.85 |
| Triglycerides | 70.47 ± 23.15 | 82.16 ± 53.32 | 60.13 ± 12.45 | 77.50 ±43.45 | 81.54 ± 19.06 | 69.77 ± 34.74 |
| HDL | 30.73 ± 10.76 | 26.47 ± 12.60 | 30.23 ± 13.16 | 20.79 ±11.06 | 22.05 ± 3.40 | 21.76 ± 4.30 |
| LDL | 86.37 ± 5.14 | 72.57 ± 18.85 | 84.99 ± 57.20 | 77.09 ±49.65 | 71.90 ± 9.79 | 72.51 ± 15.86 |
| Bilirubin total | 0.40 ± 0.07 | 0.28 ± 0.12 | 0.20 ± 0.08 | 0.33 ± 0.05 | 1.17 ± 1.01 | 0.18 ± 0.06 |
| Bilirubin direct | 0.10 ± 0.01 | 0.11 ± 0.05 | 0.29 ± 0.18 | 0.32 ± 0.07 | 1.04 ± 1.09 | 0.23 ± 0.05 |
| SGOT ALS | 17.87± 6.39 | 15.30 ± 9.19 | 15.52 ± 7.15 | 16.72 ± 3.56 | 7.63 ± 0.23 | 10.67 ± 1.58 |
| SGPT ALT | 11.80 ± 3.96 | 9.26 ± 1.01 | 9.64 ± 3.60 | 9.51 ± 2.39 | 16.43 ± 5.59 | 19.40 ± 0.10 |
| Alkaline Phosphatase | 99.25 ± 48.23 | 106.93 ± 13.22 | 124.60 ± 20.70 | 141.54 ± 10.88 | 66.50 ± 21.11 | 96.17 ± 27.48 |
| Total Protein | 6.63 ± 0.78 | 7.07 ± 0.70 | 5.87 ± 0.18 | 6.58 ± 0.53 | 7.30 ± 1.21 | 7.06 ± 2.43 |
| Serum albumin | 2.75 ± 0.06 | 2.58 ± 0.22 | 2.53 ± 0.28 | 2.91 ± 0.55 | 2.83 ± 0.09 | 2.77 ± 0.14 |
| Urea | 40.43 ± 2.38 | 32.60 ± 8.47 | 29.63 ± 9.24 | 29.47 ± 3.14 | 42.50 ± 2.01 | 38.98 ± 8.96 |
| Uric acid | 0.61 ± 0.10 | 0.63 ± 0.08 | 0.81 ± 0.46 | 0.39 ± 0.06 | 0.36 ± 0.17 | 0.24 ± 0.06 |
| Creatinine | 1.21 ± 0.10 | 1.17 ± 0.12 | 1.29 ± 0.28 | 1.22 ± 0.11 | 0.50 ± 0.02 | 0.57 ± 0.17 |
| Calcium | 12.82 ± 4.78 | 17.42 ± 1.09 | 18.48 ± 1.35 | 17.69 ± 2.00 | 18.87 ± 1.94 | 19.28 ± 1.59 |
| Chloride | 95.77 ± 5.26 | 98.25 ± 0.53 | 95.21 ± 5.90 | 85.27 ± 9.33 | 95.93 ± 6.03 | 100.93 ± 3.56 |
| Phosphorous | 21.49 ± 2.38 | 21.90 ± 1.48 | 22.59 ± 6.34 | 20.92 ± 0.56 | 20.93 ± 2.86 | 17.22 ± 2.88 |
| Potassium | 4.40 ± 0.95 | 5.60 ± 1.54 | 3.57 ± 0.57 | 5.33± 1.67 | 5.33 ± 2.49 | 7.466 ± 6.86 |
| Sodium | 204.68 ± 9.84 | 198.93 ± 9.94 | 192.40 ± 18.90 | 184.14 ± 25.06 | 245.26 ± 18.27 | 220.74 ± 17.43 |
| Female | ||||||
| Random blood sugar | 92.33±2.55 | 8032±10.53 | 116.48±17.90 | 110.56±8.14 | 75.77 ± 2.94 | 105.24 ± 14.43 |
| Total cholesterol | 60.03±8.26 | 53.77±9.35 | 74.60±2.13 | 50.90±5.11 | 85.10 ± 16.51 | 72.90 ± 7.64 |
| Triglycerides | 51.12±14.44 | 59.59±20.55 | 78.12±55 | 45.12±6.03 | 63.59 ± 5.91 | 73.69 ± 23.41 |
| HDL | 31.88±2.25 | 21.04±1.45 | 30.23±13.16 | 26.45±12.29 | 17.02 ± 3.30 | 22.34 ± 8.78 |
| LDL | 73.67±9.63 | 63.55±21.11 | 90.64±49.36 | 58.65±42.81 | 55.36 ± 13.98 | 74.64 ± 36.69 |
| Bilirubin total | 0.22±0.11 | 0.27±0.13 | 0.32±0.08 | 0.32±0.12 | 0.26 ± 0.13 | 0.35 ± 0.10 |
| Bilirubin direct | 0.18±0.06 | 0.12±0.02 | 0.25±0.16 | 0.15±0.07 | 0.26± 0.13 | 0.23 ± 0.13 |
| SGOT ALS | 18.43±3.35 | 13.88±7.20 | 19.78±10.58 | 17.96±2.65 | 21.77 ± 2.15 | 21.37 ± 0.64 |
| SGPT ALT | 7.63±3.47 | 12.03±6.67 | 15.71±9.58 | 10.19±1.73 | 10.87 ± 0.76 | 9.47 ± 0.06 |
| Alkaline Phosphatase | 93.76±27.53 | 92.17±6.43 | 130.46±21.96 | 98.99±44.42 | 66.17 ± 21.11 | 126.47±23.57 |
| Total Protein | 7.43±0.87 | 6.64±1.49 | 7.13±2.15 | 6.90±0.21 | 6.80 ± 0.36 | 6.80 ± 0.45 |
| Serum albumin | 2.47±0.26 | 2.46±0.50 | 3.02±0.27 | 3.19±0.25 | 3.00 ± 0.14 | 2.92 ± 0.45 |
| Urea | 38.80±5.56 | 33.68±11.26 | 27.69±9.87 | 37.46±9.20 | 45.45 ± 9.62 | 31.41 ± 4.95 |
| Uric acid | 0.70±0.20 | 0.61±0.05 | 0.40±0.26 | 0.43±0.20 | 0.26 ± 0.07 | 0.23 ± 0.02 |
| Creatinine | 1.22±0.21 | 1.01±0.27 | 1.06±0.17 | 1.15±0.09 | 0.58 ± 0.04 | 0.50 ± 0.04 |
| Calcium | 16.33±1.65 | 17.13±1.536 | 19.80±3.60 | 18.69±1.74 | 16.37 ± 7.94 | 21.75 ± 1.16 |
| Chloride | 79.02±23.32 | 95.34±7.15 | 92.50±7.11 | 92.69±4.42 | 96.50± 5.63 | 107.92 ± 8.20 |
| Phosphorous | 23.14±0.93 | 19.97±2.76 | 19.60±2.80 | 18.84±1.29 | 17.52 ± 0.59 | 15.80 ± 2.60 |
| Potassium | 4.30±0.95 | 4.20±1.65 | 4.90±0.61 | 5.27±1.00 | 7.79 ± 4.51 | 2.85 ± 0.78 |
| Sodium | 199.71±10.65 | 193.45±16.63 | 186.21±28.27 | 191.72±21.44 | 250.54 ± 7.03 | 191.28±17.65 |
Table 2: Serum biochemistry data of rats in the ZingiVir-H-treated group and the control group.
Additionally, no relevant changes were found in total protein, and albumin (Table 2). The effects of subacute administration of ZingiVir-H on serum electrolytes (Calcium, Chloride, Phosphorous, Potassium and Sodium) were also shown in Table 2. The results clearly indicated ZingiVir-H did not reveal any significant changes in the experimental animals.
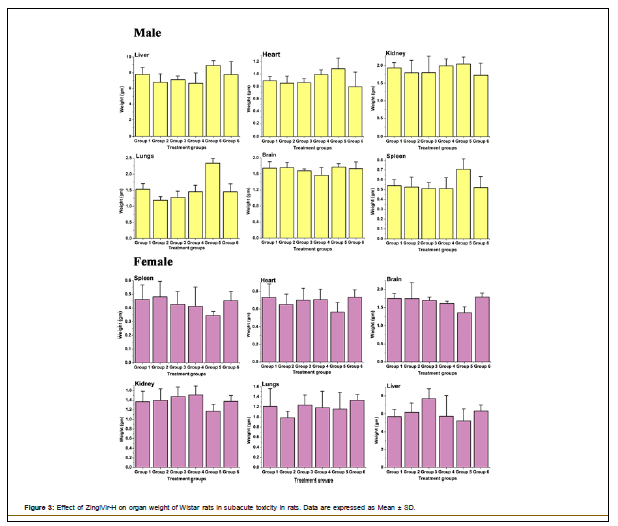
Histopathology: The organ weight of the liver, brain, kidney, heart, spleen, and lungs of treatment groups were shown in Figure 3. From the results it can be established that the administration of ZingiVir-H did not elicit any adverse effects to the vital organs.
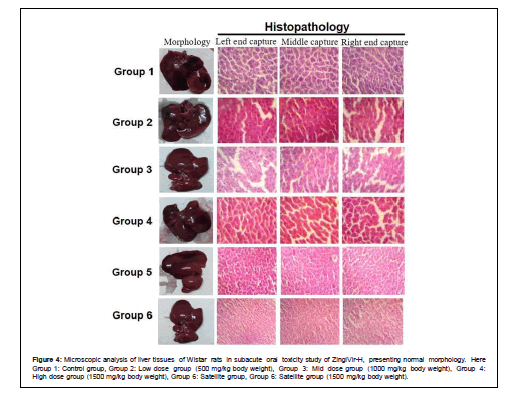
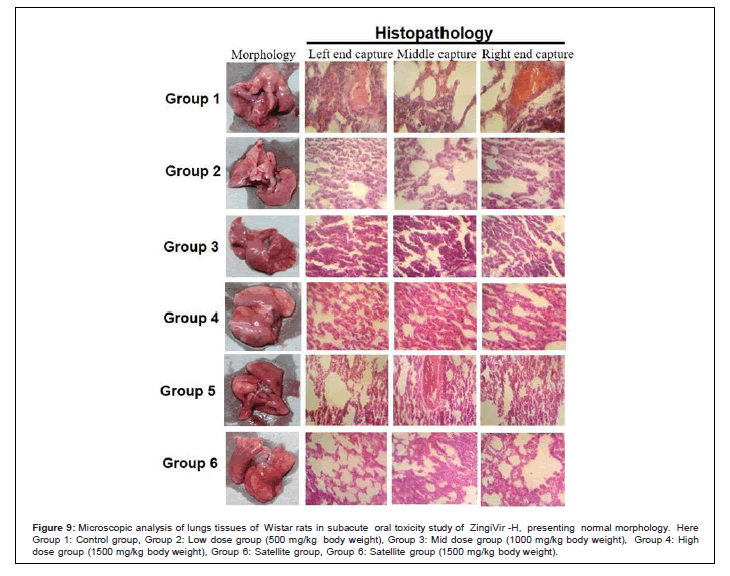
The histopathological results of liver, brain, kidney, heart, spleen, and lungs showed normal morphological aspects, and the tissues maintained their histopathological architecture, as represented in Figures 4-9.
Figure 4: Microscopic analysis of liver tissues of Wistar rats in subacute oral toxicity study of ZingiVir-H, presenting normal morphology. Here Group 1: Control group, Group 2: Low dose group (500 mg/kg body weight), Group 3: Mid dose group (1000 mg/kg body weight), Group 4: High dose group (1500 mg/kg body weight), Group 6: Satellite group, Group 6: Satellite group (1500 mg/kg body weight).
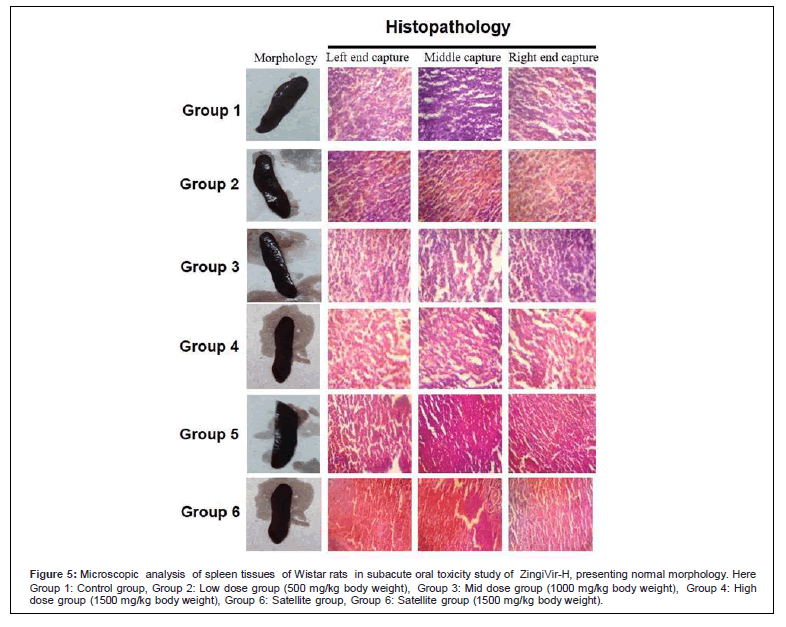
Figure 5: Microscopic analysis of spleen tissues of Wistar rats in subacute oral toxicity study of ZingiVir-H, presenting normal morphology. Here Group 1: Control group, Group 2: Low dose group (500 mg/kg body weight), Group 3: Mid dose group (1000 mg/kg body weight), Group 4: High dose group (1500 mg/kg body weight), Group 6: Satellite group, Group 6: Satellite group (1500 mg/kg body weight).
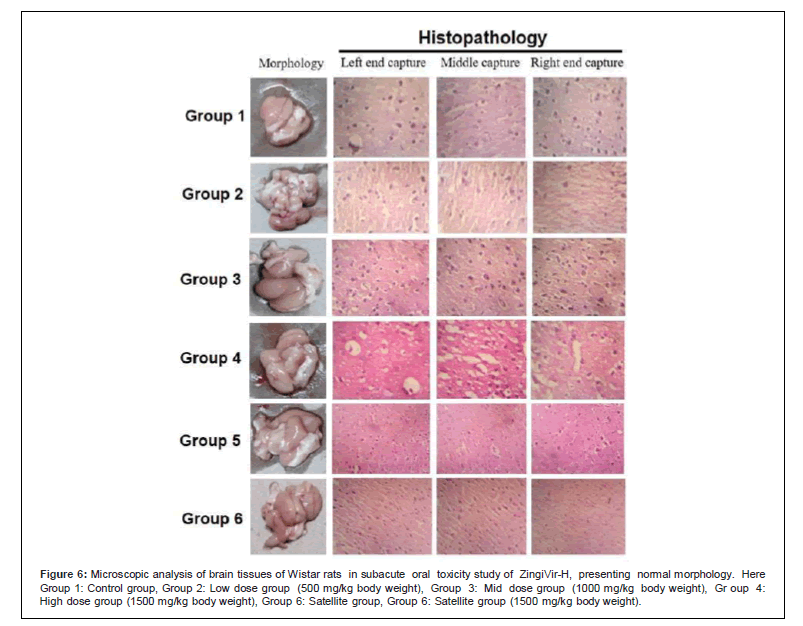
Figure 6: Microscopic analysis of brain tissues of Wistar rats in subacute oral toxicity study of ZingiVir-H, presenting normal morphology. Here Group 1: Control group, Group 2: Low dose group (500 mg/kg body weight), Group 3: Mid dose group (1000 mg/kg body weight), Gr oup 4: High dose group (1500 mg/kg body weight), Group 6: Satellite group, Group 6: Satellite group (1500 mg/kg body weight).
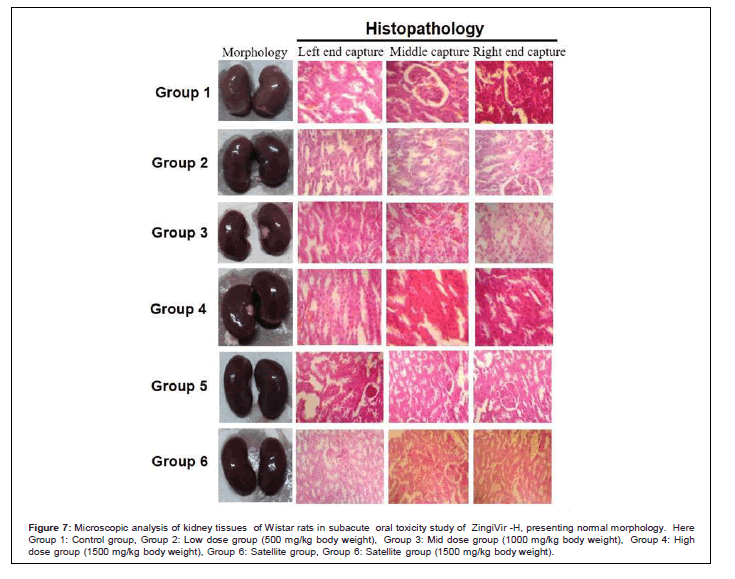
Figure 7: Microscopic analysis of kidney tissues of Wistar rats in subacute oral toxicity study of ZingiVir -H, presenting normal morphology. Here Group 1: Control group, Group 2: Low dose group (500 mg/kg body weight), Group 3: Mid dose group (1000 mg/kg body weight), Group 4: High dose group (1500 mg/kg body weight), Group 6: Satellite group, Group 6: Satellite group (1500 mg/kg body weight).
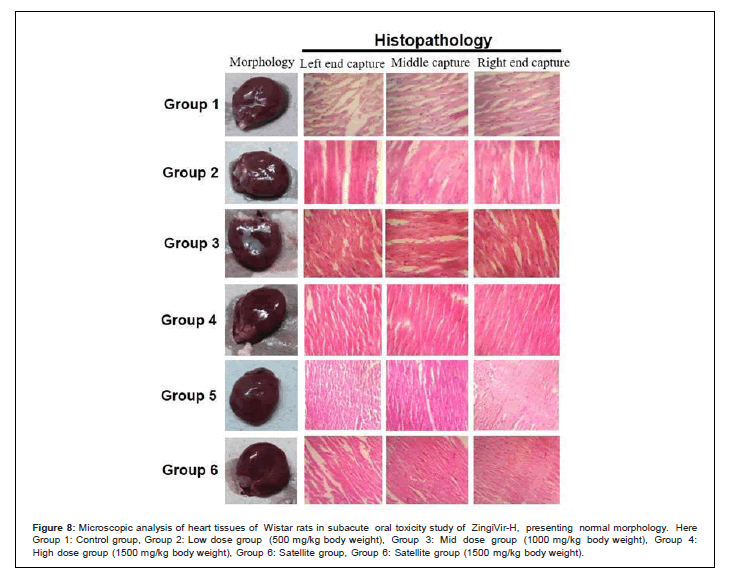
Figure 8: Microscopic analysis of heart tissues of Wistar rats in subacute oral toxicity study of ZingiVir-H, presenting normal morphology. Here Group 1: Control group, Group 2: Low dose group (500 mg/kg body weight), Group 3: Mid dose group (1000 mg/kg body weight), Group 4: High dose group (1500 mg/kg body weight), Group 6: Satellite group, Group 6: Satellite group (1500 mg/kg body weight).
Figure 9: Microscopic analysis of lungs tissues of Wistar rats in subacute oral toxicity study of ZingiVir -H, presenting normal morphology. Here Group 1: Control group, Group 2: Low dose group (500 mg/kg body weight), Group 3: Mid dose group (1000 mg/kg body weight), Group 4: High dose group (1500 mg/kg body weight), Group 6: Satellite group, Group 6: Satellite group (1500 mg/kg body weight).
The microscopic examination of the hepatic tissues recorded normal histological structure with cords of large polygonal hepatocyte with eosinophilic cytoplasm and prominent round nuclei. Normal sinusoidal space was recorded between the cords. Liver sections of the control and ZingiVir-H treated groups recorded normal hepatocytes and portal triad showing prominent central vein. Renal biopsy of the ZingiVir-H treated groups showed normal kidney structure with intact Bowman’s capsules, Proximate Convoluted Tubules and Distal Convoluted Tubules. The renal biopsy sections of the treated and control groups recorded normal structure. The brain sections of the treated and the control group shows neurons, glial cells, capillaries that appeared in normal structure. Spleen sections taken from control and ZingiVir-H treated groups recorded thin capsule, prominent red as well as white pulp. Histopathological examination of the sections of the heart tissue taken from both male and female rats recorded normal findings in control as well as ZingiVir-H treated rats. The heart sections from the control group displayed normal appearance and cardiac muscle fibres were well arranged with centrally nuclei. In lung tissue, normal pulmonary architecture was detected.
Discussion
For several decades, herbal medicines and their formulations have been considered to be highly safe and effective due to their negligible side effects. Even though, several herbs and their preparations have been identified for its toxic properties. This hypothesis may have prejudiced the indiscriminate use of these formulations to a great extent amongst the people especially residing in the rural area. The Food and Drug Administration has released warning reports regarding the possible toxic effects of several herbal remedies and/or herbal preparations commonly consumed among the common peoples [26]. These herbal formulations are generally consumed over a very long period of time without any proper dosage monitoring by the authorised medical practitioners [27]. Safety profiles especially toxicology tests are regularly needed to popularize the acceptance, standardization and or regulation of the market for herbal medicines which are currently under sale [28].
Prior to any pharmacological validation and the development of an herbal medicine, acute and subacute toxicity is mandatory according to standard published guidelines [29,30]. It is also a vital process for preclinical dose determination in drug discovery and development programme [31,32]. Thus, in recent times, scientists/researchers absolutely focused on the safety and efficacy of traditional medicine in order to provide data that meet all the required criteria to support its worldwide acceptance [32,33]. So, in the present study we aimed to study the subacute oral toxicity studies of ZingiVir-H tablets, a herbomineral antiviral drug against Wistar rats.
The absence of any specific toxicity after single dose of herbal drug is important; however, it does not assure the complete safety of the tested material. Moreover, use of herbal drug is often required for a prolonged period; so a long-term study may provide useful data regarding the toxicity profile of the test materials. In this study, ZingiVir-H was administered daily for 28 days orally at doses of 500, 1000, and 1500 mg/kg of body weight and no mortality was recorded during the study period. The observation of body weight in both male and female rats (Figure 1) may suggest that the rats were having normal growth pattern based on their healthy food intake. Reports from earlier studies showed that animals losing 10% of the initial body weight might not survive no longer and is a strong indication of any adverse side effects of the tested substance [34,35]. In the current study none of the rats recorded reduction in the body weight. So, from the result, it can be concluded that growth inhibition was not recorded during repeated doses of ZingiVir-H tablets for 28 days. Increase in the body weight of animals determines the positive health status [36]. Previous toxicological studies also reported that the decrease of body weight may be due to the reduction of dietary intake or any injury to the vital organ, which may be due to the toxicity associated with the test materials [37]. So, our results further supported that ZingiVir-H does not induce any toxicity effect on the test animals.
To successfully evaluate the safety of ZingiVir-H, it is very much necessary to demonstrate the toxicity by hematological analyses. The hematopoietic system can reflect toxic effects of any test materials, because it is one of the most sensitive targets for toxic chemicals, which are very important indexes that determine the physiological and pathological roles in humans and animals [38]. Blood is main transport system of nutrients and other foreign bodies, making its constituents like RBCs, WBCs, and platelets along with haemoglobin which are more vulnerable to toxic compounds. So, the evaluation of haematological parameters would be very helpful in determining the toxic effects of the extract/formulations/chemicals/drugs on animal’s blood and thus can be very useful to explain blood relevant function of extracts/chemicals/ drugs [39]. The haematological profile after treatment with ZingiVir-H showed values which were mainly non-significant when compared with the control and falls within the normal range for both male and female rats. Interestingly haematological parameters of ZingiVir-H treated rats is also comparable with that of the control rats. From this it was clear that, ZingiVir-H administration at all doses did not induce any major alterations in the haematological parameters of both sexes of rats, however few changes which were recorded does not carry any serious toxicological importance and therefore the ZingiVir-H at all the tested doses can be considered highly safe to the haematological profile.
Urea and creatinine are mainly considered as a suitable predictive indicator of renal dysfunction and kidney failure induced by any toxic compounds [40]. In this study, the absence of significant differences in these parameters after 28 days treatment with ZingiVir-H means that this particular drug has no harmful effect on the function of the kidney.
Alkaline phosphatase (ALP) is membrane bound and its alteration by any agent is likely to affect the membrane permeability and produce derangement in the transport of various metabolites throughout the body. An increase in serum ALP level is usually a main characteristic finding in cholestatic liver disease [41,42]. No significant effect in ALP was noticed after the 28th day. An increased serum ALP level is often associated with various disorders such as extrahepatic bile obstruction, intrahepatic cholestasis, infiltrative liver disease, as well as hepatitis and bone disease [43]. Therefore, from the enzyme study mentioned above, the ZingiVir-H caused any induction of some enzyme activities and suppression of some others when compared with the control. But from the results it is clear that the changes were all within their normal test ranges, hence the ZingiVir-H did not have adverse effect on the rat liver enzyme activities. This study further supports the research finding of Awotunde et al. [44].
Hepatic as well as renal function are crucial, with one being used for the metabolism of ingestion and where as the other for excretion of the waste product respectively [32,45]. To evaluate the toxicity of any new compound/drug/herbal extract, it is very much essential to know the state and response of these two vital organs, which can be confirmed through the biochemical estimation [32]. In our study, the renal and hepatic biomarkers showed that the ZingiVir-H is not toxic at the doses studied. In male and female rats, ZingiVir-H at all the doses administered did not alter the levels of total protein and albumin.
Similarly, serum lipid parameters have not changed in animals treated with ZingiVir-H.
The liver is one of the major organ of the adverse effects of toxic substances as it is mainly responsible for the biotransformation of xenobiotics. Due to this it is really important to evaluation the hepatic toxic effect of any compound or natural products. Elevation of serum enzymes like ALT, AST, and ALP indicate hepatocellular toxicity and liver damage [46]. In the present study, we observed that treatment with ZingiVir-H for 28-day does not produce any significant changes in ALT, AST, and ALP levels, indicating hepatoprotective action of ZingiVir-H. Bilirubin a yellow breakdown pigment product of normal heme catabolism, and its levels are elevated in certain diseases and it is a clear indication of liver damage [47].
The kidney function can be checked through the monitoring of secretory products especially the electrolyte concentration such as sodium, potassium, urea and creatinine. These electrolytes are typically required to assess the normal functioning capacity of different parts of the functioning unit of the kidney [48,49]. Moreover, balance in electrolyte is an indicator of osmotic regulation by blood, kidney and cardiac function, digestion and other intermediary metabolism [50,51]. Serum electrolytes especially Na, K, Ca and P levels were estimated in the present study and this was further supported by the studies of Unuofin et al. and Mei-Yin et al. [52,53]. No significant changes in the serum electrolytes were observed in treatment and satellite groups as compared to control group. This observation showed that the ZingiVir-H is not affecting the digestive system, metabolism process, cardiac and renal functioning which is further supported by the nonsignificant changes in the results of renal, lipid parameters (TG, HDL and TC) and normal histological finding of heart and liver. Hence it could be concluded that ZingiVir-H may not have any adverse effect on electrolyte balance in the experimental animals.
Histopathological investigation plays an important role in determining the morphology of tissues through microscopical evaluations. As we know histopathological examination of vital organs and tissues serves as a elementary test to measure the safety of any drug candidate drug molecules/herbal extracts [18]. The analysis of vital organs is considered as the major safety tool to determine any histological perturbations or distortions in the tissues [54,55]. Therefore, the histopathological assessment concretes the haematological and biochemical findings and which further substantiate the biological response factors of the body against the substance tested [56]. Histopathological examination (macroscopic and microscopic) of vital organs including liver, kidney, heart, lungs, brain, and spleen revealed a regular tissue architecture and no morphological alterations were observed in ZingiVir-H treatment groups compared to the control group. Our histopathogical studies demonstrated that ZingiVir-H does not possess any toxic effect on vital organ. These histopathological investigations are in agreement with the studies of Ping et al. [57,58], who reported no major morphological differences in tissues of control and treatment groups when treated candidate molecule. Therefore, from histopathological analysis, we conclude that ZingiVir-H did not produce any abnormal changes and structural alterations even at high dose (1500 mg) in experimental animals. The overall results of the present study permit to rank ZingiVir-H under the category 5 with low acute toxicity exposure, according to the Globally Harmonized System of Classification and Labelling of Chemicals (OECD guidelines 423) [59].
Conclusion
As we said earlier ZingiVir-H containing two purified heavy metal as per ayurvedic principles i.e. mercury and arsenic which according to modern toxicology principles are extremely poisonous even in small doses. But the two heavy metals we used for the preparation of ZingiVir-H are subjected to several ‘detoxifying’ processes according to the methods described in the century old classical Ayurveda literature. This detoxification process will convert the heavy metals into less toxic or non-toxic form But even also, modern medical practitioners strongly disagree with the therapeutic efficacy of such preparations due to high content of heavy metals in them. This may be due to the lack of scientific evidence especially through the toxicological and pharmacological studies. Therefore, an in-depth research on the safety profile of ZingiVir-H becomes very necessary for convincing them. So, we have conducted the safety study of ZingiVir-H in Wistar rats through oral feeding for continuous 28 days. Our current toxicological investigation on Wistar rats shows that ZingiVir-H does not have any significant toxicity even administered with higher doses to the candidates (1500 mg). The ZingiVir-H neither exhibited any alterations to the hematological and biochemical parameters nor showed any signs of physical or behavioural anomalies in the experimental animals. In addition to this, no mortalities were reported or no major histopathological changes were observed in ZingiVir-H treated rats when compared to the control groups. This establishes ZingiVir-H as a safe medicinal agent in appropriate or prescribed dosages. These results clearly depicted that ZingiVir-H does not possess any type of toxic effect on the experimental animals. However, for the advancement of the drug and its targeted therapeutic effects, well-designed future strategies should be developed based on pharmacological aspects.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors are grateful to the Managing Director, Pankajakasthuri Herbal Research Foundation, Kerala India, for providing the necessary financial support to conduct research.
References
- Parasuraman S (2011)  Toxicological screening. J Pharmacol Pharmacother 2: 74–79.
- Neuwinger HD (1996). African Ethnobotany: Poisons and Drugs: Chemistry, Pharmacology, Toxicology. CRC Press.
- Shan S, Sithara MS (2018). A study report of repeated dose 28-day oral toxicity study of Nirapara fresh wash in Wistar rats. Inter J Sci Res 7: 324-326.
- Ganesan AR, Subramani K, Balasubramanian B, Liu WC, Arasu MV, et al. (2020) Evaluation of in vivo sub-chronic and heavy metal toxicity of under-exploited seaweeds for food application. J. King Saud University – Sci. 32: 1088–1095.
- Bansal A, Sairam M, Prasad D, Sharma SK, Ilavazhagan G, Kumar D, et al. (2001) Cytoprotective and immunomodulatory properties of Geriforte, a herbomineral preparation in lymphocytes. Phytomedi 8: 438–444.
- Gupta VKL, Pallavi G, Patgiri BJ, Galib , Prajapati PK (2012) Critical review on the pharmaceutical vistas of Lauhakalpas (iron formulations). J. Ayurveda Integr. Med 3: 21–28.
- Nagarajana S, Sivajia K, Krishnaswamy S, Pemiah B, Rajana KS, et al (2014) Safety and toxicity issues associated with lead-based traditional herbo-metallic preparations. J. Ethnopharmacol 151: 1–11.
- Schoen A, Beck B, Sharma R, Dube E (2004) Arsenic toxicity at low doses: epidemiological and mode of action considerations. Toxicol Appl Pharmacol 198: 253–267.
- Michael JK, Richard PW, Stephen JR, Karen LH, Barbara LM, et al (2007) Recommendations for medical management of adult lead exposure. Environ. Health Perspect 115: 463–471.
- Saper RB, Kales SN, Paquin J, Burns MJ, Eisenberg DM, et al (2004). Heavy metal content of Ayurvedic herbal medicine products. JAMA 292: 2868-2873.
- Kumar A, Nair AG, Reddy AV, Garg AN (2006) Bhasmas: unique ayurvedic metallic-herbal preparations, chemical characterization. Biol Trace Elem Res 109:  231–254.
- Krishnamachary B,Purushothaman AK, Pemiah B , Krishnaswamy S,Krishnan UM, et al (2013). Bhanupaka: a green process in the preparation of an Indian ayurvedic medicine, Lauha bhasma. J Chem: 2013: 1-8.
- Kales SN, Christophi CA, Saper RB (2007) Hematopoietic toxicity from lead- containing ayurvedic medications. Med Sci Monit: 13:295–298.
- Karri SK, Saper RB, Kales NS (2008) Lead encephalopathy due to traditional medicines. Curr Drug Saf 3: 54–59.
- Kang-Yum E, Oransky SH (1992) Chinese patent medicine as a potential source of mercury poisoning. Vet Hum Toxicol 34: 235-238.
- Lynch E, Braithwaite R (2005) A review of the clinical and toxicological aspects of “traditional†(herbal) medicines adulterated with heavy metals. Expert Opin Drug Saf 4: 769-778.
- Cooper K, Noller B, Connell D, Yu J, Sadler R, et al (2007) Public health risks from heavy metals and metalloids present in traditional Chinese medicines. J Toxicol Environ Health Part A 70: 1694-1699.
- Greaves P (2012). Histopathology of preclinical toxicity studies: interpretation and relevance in drug safety evaluation (4th ed),USA.
- Organization for Economic Co-operation and Development (OECD) (2008). Guidance Document on Subacute Oral Toxicity Testing 407; Organization for Economic Co-operation and Development: Paris, France.
- World Health Organization (WHO) (2000) General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. WHO: Geneva, Switzerland.
-  Chandiran IS, Jayaveera KN, Karimulla S (2013) Preliminary phytochemical and preclinical toxicity studies of Grewia serrulata DC. Drug Invent. Today 5 : 267–274.
- Chandiran IS, Jayaveera KN, Karimulla S (2013) Preliminary phytochemical and preclinical toxicity studies of Grewia serrulata DC. Drug Invent. Today 5 : 267–274.
- Wu Z, Ma Y, Zhao L, Cai S, Cheng G (2018). Acute and subchronic toxicities of the ethanol and hot-water extracts from Chinese sumac (Rhuschinensis Mill.) fruits by oral administration in rats. Food Chem Toxicol 119: 14–23.
- Thanabhorn S, Jaijoy K, Thamaree S, Ingkaninan K, Panthong A (2006) Acute and subacute toxicity study of the ethanol extract from Lonicera japonica Thunb. J. Ethnopharmacol 107: 370–373.
- National Research Council (NRC) (2011) Guide for the Care and Use of Laboratory Animals.(8th edition) National Academy Press, Wash, DC, USA.
- Witthawaskul P, Panthong A, Kanjanapothi D, Taesothikul T, Lertprasertsuke N (2003) Acute and subacute toxicities of the saponin mixture isolated from Schefflera leucantha Viguier. J Ethnopharmacol 89: 115–121
- Porwal M, Khan N Ali, Maheshwari KK (2017) Evaluation of Acute and Subacute Oral Toxicity Induced by Ethanolic Extract of Marsdenia tenacissima Leaves in Experimental Rats. Sci Pharm 85: 29.
- Eran BA, Noah S, Lee HG, Kamer M, Suha O, et al (2016) Potential risks associated with traditional herbal medicine use in cancer care: A study of middle eastern oncology health care professionals. Cancer 122: 598–610.
- Sahoo N, Manchikanti P (2013) Herbal Drug Regulation and Commercialization: An Indian Industry Perspective. J Altern Complement Med 19: 957–963.
- Dramane P, Adama H, N’do J, Samson G, Ernest SN, et al.(2019)  Rotective effect of bioactive fractions of C. dalzielii against weight gain in mice feed with high fat-diet. Int J Recent Sci Res10: 34144–34153.
- Gazayerly ON, Makhlouf AI, Soelm AM, Mohmoud MA (2014) Antioxidant and hepatoprotective effects of silymarin phytosomes compared to milk thistle extract in CCl4 induced hepatotoxicity in rats. J Microencapsul 31: 23–30.
- Kumar N, Rai A, Reddy ND, Raj PV, Jain P, et al (2014) Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol Rep 66: 788–798.
- Kpemissi M, Metowogo K, Melila M, Veerapur VP, Negru M, et al.(2020) Acute and subchronic oral toxicity assessments of Combretum micranthum (Combretaceae) in Wistar rats. Toxicol Rep 7: 162–168.
- Kale OE, Awodele O, Akindele AJ (2019) Subacute and subchronic oral toxicity assessments of Acridocarpus smeathmannii (DC.) Guill. & Perr. root in Wistar rats. Toxicol Rep 6: 161–175.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA (2002) Effect of prolonged vigabatrin treatment on haematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharma 70: 135–145.
- Heywood (1983)“Long term toxicity,†in Animals and Alternatives in Toxicity Testing.Academic Press,London,UK.
- Rivas CAB, Castillo AA, MartÃnez HS, Zapata EP, Hernández JB, Tassé YM (2013) Acute oral toxicity of Azadirachta indica (Neem tree). Rev. cuba. plantas med 18: 502–507.
- Féres CAO, Madalosso RC, Rocha OA, Leite JPV, Guimarães TMDP, et al.(2006) Acute and chronic toxicological studies of Dimorphandra mollis in experimental animals. J. Ethnopharmacol 108: 450–456.
- Hu Z, Feng R, Xiang F,  Song X, Yin Z, et al.(2014) Acute and subchronic toxicity as well as evaluation of safety pharmacology of eucalyptus oil-water emulsions. Int J Clin Exp Med 7: 4835–4845.
- Yakubu M, Akanji M, Oladiji A (2007) Hematological evaluation in male albino rats following chronic administration of aqueous extract of Fadogia agrestis stem. Pharmacogn Mag 3: 34–8.
- Illodigwe EE, Akah PA, Nwosu CS (2010) Evaluation of the acute and subchronic toxicities of ethanol leaf extract of Spathodea campanulata P. Beauv. Int J Appl Res Nat Prod 3: 17-21.
- Gnanamani A, Sudha M, Deepa G, Sudha M, Deivanai K, et al (2008) Haematological and biochemical effects of polyphenolics in animal models. Chemosphere 72: 1321-1326.
- Angelico F, Del Ben M(2010) Towards predicting the therapeutic response in patients with hepatitis C: author’s reply. Aliment Pharmacol Ther 31: 339-340.
- Awotunde OS, Adewoye SO, Hawumba J, Joseph J, Alinaitwe L (2017) Histopathological and organ-body weight ratio alterations induced by oral administration of aqueous extract of Terminalia schimperiana root in some selected organs in male Wistar rats. Int J Adv Res Sci Eng Technol 4: 3416-3420.
- Jothy SL, Zakaria Z, Chen Y, Lau YL, Latha LY, et al. (2001) Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules 6: 5268-5282.
- Pare D, Hilou A, N’do J, Guenne S, Ernest SN, e al.(2019) Rotective effect of bioactive fractions of C. Dalzielii against weight gain in mice feed with high fat-diet. Int J Recent Sci Res 10: 34144–34153.
- Arika WM, Nyamai DW, Osano KO, Ngugi MP, Njagi ENM (2016) Biochemical Markers of In Vivo Hepatotoxicity. J Clin Toxicol 6:Â 297.
- Panda NC, Talwer GP, Stivastava LM, Moudgil KD (Eds.)(1989) Textbook of Biochemistry and Human Biology.(2nd edition). Prentice-Hall of India Private Ltd, India.
- Baranano DE, Rao M, Ferris CD, Snyder SH (2002) Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA 99: 16093–16098.
- Zilva JF, Panmall PR, Mayne PD (1991). Clinical Chemistry in Diagnosis and Treatment.(5th edition).England Clays Ltd. St Ives Plc.
- Rose BD, Post TW (2001) Clinical Physiology of Acid-Base and Electrolyte Disorders. (5th Edition) McGraw-Hill, New York.
- Simon A (2004) Fluid, electrolytes and nutrition. Clin Med 4: 573–578.
- Unuofin JO, Otunola GA, Afolayan AJ (2018) Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia Less. In Wistar rats. J Integr Med 16: 335–341.
- Mei-Yin C, Chih MY, Yi TL, Chao-Hsiang C (2018). Dihydromyricetin-rich herbal mixture extracts as a potential prescription for treatment of metabolic syndrome in rats fed a high-fat diet and subacute toxicity assessment in rats. J Tradit Complement Med 9: 221-226.
- Chitra B, Ramaswamy R, Suba V (2015) Toxicity evaluation of Purn a Cantirotaya Centuram, a Siddha medicine in Wistar rats. Int Sch Res Notices 2015: 1-10.
- Jaganathan R, Ravinayagam V, Panchanadham S, Palanivelu S (2012). Toxicological, biochemical and histopathological evaluation of Tridham, a siddha medicine in Wistar albino rats. J Biochem Toxicol 4: 541-548.
- Prabu P, Panchapakesan S, Raj CD (2013) Acute and sub-acute oral toxicity assessment of the hydroalcoholic extract of Withania somnifera roots in Wistar rats. Phytother Res 27: 1169-1178.
- Loha M, Mulu A, Abay SM, Ergete W, Geleta B (2019) Acute and subacute toxicity of methanol extract of Syzygium guineense leaves on the histology of the liver and kidney and biochemical compositions of blood in rats. Evid Based Complement Alternat Med 2019: 1–15.
- Yuet Ping K, Darah I, Chen Y, Sreeramanan S, Sasidharan S (2013) Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Res Inter 2013: 1–14.
Citation: Nair HJ, Sasidharan S (2021) Subacute Oral Toxicity Studies of ZingiVir-H: A Herbomineral Antiviral Drug in Wistar Rats. Toxicol Open Acess 7: 155. DOI: 10.4172/2476-2067.1000155
Copyright: © 2021 Nair HJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4104
- [From(publication date): 0-2021 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 3200
- PDF downloads: 904