Study the Growth Indices, Physiological, and Productivity of Leafy Vegetables in the Hydroponic System
Received: 01-May-2024 / Manuscript No. rroa-24-138411 / Editor assigned: 04-May-2024 / PreQC No. rroa-24-138411(PQ) / Reviewed: 18-May-2024 / QC No. rroa-24-138411 / Revised: 22-May-2024 / Manuscript No. rroa-24-138411(R) / Published Date: 29-Aug-2024
Abstract
Due to rapid urbanization in developing countries, it is necessary for respective governments to seek new approaches to providing sustainable fresh food and chemical-free supplies. The implementation of a Hydroponic system (Nutrient Film Technique) is an innovative solution to shortages of organic and fresh food in urban areas. The study investigated the construction of the hydroponic system, the nutrient film technique was used for the production of leafy vegetables such as Lactuca sativa L and Amaranthus cruentus. This study was to assess the effects of commercial hydroponic fertilizer mix (CHFM) combined with clay ball, vermiculite, and coconuts coir bio-inoculated with N2 fixing and P-solubilizing bacteria Priestia aryabhattai (E3) and Enterobacter cloacae (P5), respectively to bring changes in N and P contents of the substrate to improve cultivation of leafy vegetables. Results reveal significant differences for fresh root weight (RW), shoot length (SL), fresh shoot weight (SW), total plant length (TL), total fresh weight (TW), root/shoot weight ratio (RSWR) and root/shoot length ratio (RSLR), in different treated conditions. While there was no significant difference in root length (RL). Bioinoculation of the substrate with nitrogen fixing and phosphate solubilizing bacteria enhanced the rate of germination of Amaranthus and lettuce seeds. This Hydroponic system can be used through a decentralized wastewater treatment and reuse system; being relatively cheap to construct and operate, having low maintenance requirements, and also a long lifespan.
Keywords
Hydroponic system, Nutrient Film Technique, Lactuca sativa L, Amaranthus cruentus. Priestia aryabhattai, Enterobacter cloacae
Introduction
Hydroponic: The hydroponic system is a modern scientific method, where plants grow in a soil-less medium, it is grown using nutrients, cocopeat, coco coir, gravels, sand, vermiculite, and clay balls, instead of soil. In this system mainly water is responsible for providing nutrients, hydration, and oxygen to the plant. So that the named hydroponic. Plants get food and water from this hydroponic system directly from their roots. The root stays smaller, so each plant can concentrate its energy on producing plant mass, rather than roots.
Using the technology to improve and address the real needs of consumers. The benefit of the hydroponic system is a faster growth cycle, reduced resource waste, and increased yield. It allows more plants to grow in a similar area and grow more quickly because they are not competing with other plants in the soil for the same nutrient. It is the gardening system for the most part recycling of water. The most important thing about the hydroponic system is that no wedding is involved and the vegetables taste better. It can deliver a greater harvest and is easy.
As per the world factbook, the total area of land with water is 196,939,900 sq miles, the water surface is 70.9% of the world (361, 132 million sq km), and the land is 29.1% (148,94 million sq km). The world’s Agricultural land has decreased due to population growth and new industries. The geographic expansion of urban settlements at the expense of rural localities through a transformation as per the FAO. In the year 2050, the world population growth will mainly occur in urban areas, it is expected to rise by 2.5 billion’s people for this growth of population, the land become short and polluted, where we can’t cultivate any kinds of plants and vegetables as for the scientist resource.
According to the United Nations for Food and Agricultural Organization (FAO) in the year 2050, the world population become a milestone in human history. The world population is expected to reach about 9-10 billion people. Over the years, the world has become increasingly urbanized, with 80% of the world’s rural people settling to live in urban areas and building many companies and factories to fulfill their needs. Due to this development, the free space becomes decreases.
According to Hickman (2011), the world’s hydroponic vegetable production is about 35,000 ha. As a result, the growing demands for food are in lockstep with population growth. Agriculture or FAO needs to make decisive and radical moves toward the efficiency and sustainability of biodiversity.
Today’s technology has advanced to monitor and automate many aspects of modern hydroponic gardening in greenhouses or growing rooms. Because of this technology, agriculture increases hydroponic benefits including a quicker growth cycle, higher quantity yield, and reduced resource waste. As technology continues to play a role in how we grow, the hydroponic future list is crystal clear as the water plant thrives.
- Types of hydroponic systems:
In the hydroponic system used to produce plants and leafy vegetables different types of systems defend the place according to the space, and area [1]. There are mainly seven types of hydroponic systems are can be used, such as:
- Nutrient Film Technique. (NFT),
- Deep Water Culture. (DWC),
- Wick Hydroponic,
- Aeroponics,
- Ebb and Flow,
- Drip system,
- Aquaponic
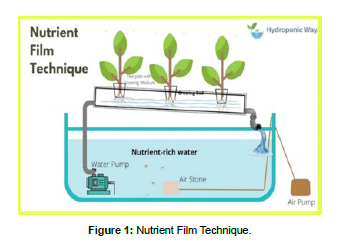
Nutrient film technique: In this system, the water and nutrient solution is recirculated, and the vegetables get nutrients by recycling the nutrient solution. This system gets higher quality crops using PVC pipe [2]. This system is easy to use because of the easy to wasted and draining of wastewater and nutrient solution. The cultivated plant’s root systems can be easily pulled from the cultivation hare pots (Figure 1).
Deepwater culture system: The deepwater system is a substrate-free method, there is no substrates are used for plant growth [3]. The plant roots are delighted in the nutrient solution. The rest except the root in this system used cork, wood bark, or polystyrene to support plant growth [4] (Figure 2).
Wick system: The wick system takes nutrients by the capillary action to the plant’s root zone. This is a passive form of hydroponic system because it works without the need for any motor, or pump on moving parts. The wick system is the simplest hydroponic system. In the wicky system, the suitable absorbance substance takes for the absorbed and riches water for substrate where the plant was sowing for the growth of the plant.
Aeroponic: In this system the plants are grown by hanging down without any substrates in the air with their roots, they get nutrients by the praying of a sprinkler system. The nutrients riches to the root of the plant by evaporation in the form of vapor air [4].
Ebb and flow: The Ebb and flow method is known as the flood and drain. The system is popularly used for homemade gardeners. The plants grow in the bed system and growth medium. The bed medium stays moist from flooding and the process is repeated.
Drip water system: In this system, the pump feeds nutrients and water regularly to the plant through the nutrient solution's dripped system. The nutrient solution is dripped or emitted directly onto plants and the solution is recirculated.
Aquaponic: This system used nutrient solution as the substrate for cevultivating the fish. The aquaponics system is a multi-hydroponic system of hydroponic and aquaponic. Different types of fish like Tinca tinca, Sander lucioperca, etc. [5,6].
The system is one type of closed system hydroponic system (CHS). CHSs have the benefit of using less water and fertilizer while also producing less wastewater that could potentially harm water sources. Contrarily, the persistent recirculation of drainage nutritional solution causes the concentration of salts, particularly NaCl, to gradually rise in the nutrient solution, rendering it eventually unusable and requiring replacement. However, CHSs are hardly ever used in greenhouses built in Mediterranean nations, where a lack of water and a decline in their quality are already significant issues. This is primarily because of the high investment costs, the high salinity of the groundwater used to prepare nutrient solutions, and other factors.
Hydroponic Substrates:
Properties of hydroponic substrates: The substrates that hold sufficient water and air to maintain optimum conditions for root and plant growth are the most important hydroponic growing medium requirement [1]. The substrates are the medium for plant growth and provide nutrients to the plant. The substrates are the key elements in the hydroponic system. These substrates should present the physical and chemical properties for providing a better environment for the plants.
Physical properties: The physical properties of substrates mostly present porosity, water retention, drainage, and capillary action. The porosity is related to the exchange of gas [7] and the aeration of the roots of plants. There are macro and micropores are present in the substrates.
Porosity: Porosity is the amount of space or tiny holes between sediments and depends on the sediments' size, shape, and sorting of sediments, which helps with gas exchanges and nutrient absorbency [7].
Water retention: The substrates are the consumption or the thorough evapotranspiration of water from the nutrient film technique of the floating raft which is the medium-filled grow bed [1].
Chemical properties: The chemical properties present in a substrate are pH, cation exchange, chemical stability, and nutrient retention. The characteristics of substrates provide nutrient-based hydroponic fertilizers and will increase the root zone’s stability. pH is also important for plant nutrient uptake through roots [8].
The hydroponic substrates are:
Clay ball: The clay ball mineral is rare in this world and a few places are found all over the world. The composition of clay balls is mica, quartz, and kaolinite. The properties of these balls are:
- It has a resistance to shrinkage.
- It also has water adsorption after firing, slow dry time, workability, and organic content.
Vermiculite: Polyurethane foam is used for expanded vermiculite powder. It has warp-knitted spacer fabric and this vermiculite's properties are cushioning and greater compression performance properties [9]. It has to produce higher porosity, specific surface area, and support materials for CO2 absorption [10].
Coconuts coir: It has a sound absorption coefficient index between 0.75 and 0.94 and it has the best result obtained at the low frequency of 500-2500Hz. It has the most investigated properties of porosity and tortuosity [11].
Nutrient solution: The nutrient is essential for plant growth in the hydroponic system, it provides essential micro and macronutrient sources for plants whatever need for growing [12]. There are present phytochemicals and nutrient supply for the hydroponic system plants, with accompanying improvements in harvesting yield and nutritional content for the plant growth and its uptakes the mineral present in the nutrient solution [13]. The nutrient may be different types used as a source for plant growth such as Hogland [14], wastewater, silica mineral water, and drainage water [15] (Table 1).
| Nutrients | Form of elements | Symbol | Nutrient solution |
|---|---|---|---|
| Phosphorus | PO43- | P | |
| Potassium | K+ | K | |
| Nitrogen | NO32-, NH4+ | N | CaCl2, MgSO4.7H2O, |
| Sulfur | SO42- | S | CaNO3, NH4NO3, |
| Calcium | Ca2+ | Ca | KH2PO4, K2SO4, |
| Iron | Fe2+, Fe3 | Fe | MnSO4, KNO3, |
| Magnesium | Mg2+ | Mg | ZnSO4, CuSO4, NaMoO4, |
| Boron | BO32-, B4O72- | B | K2SO4, KCL, KNO3 |
| Manganese | Mn2+ | Mn | |
| Copper | Cu2+ | Cu | |
| Zinc | Zn2+ | Zn | |
| Molybdenum Chlorine | MoO42- Cl | Mo Cl |
Table 1: The form of Elements for Plants is Present in the Nutrients and the Symbols of the Micro and Macro Nutrients.
Hydroponic Leafy vegetables
Lactuca sativa: The vegetables like Lettuce (Lactuca sativa) are a rich source of nitrogen for plant growth, it is essential to human bodies and one of the most important vegetables grown worldwide and consumed [16]. Mostly leafy vegetables have higher nitrogen sources, e.g.: lettuce (Lactuca sativa). The leafy vegetable has minerals, like nitrogen, and hydrolyzed amino acids, and the root of the lettuce has glycine betaine. Leafy vegetables are a composition of amino acids and minerals for growth in the hydroponic system [2]. It is one of the highest sources of antioxidants, flavonoids, vitamins, and carotenoids because this source of lettuce has healthy for human beings and presents phytochemicals [17].
The consumption of lettuce may contribute to lowering cholesterol, reducing the risks of coronary heart diseases, preventing some types of cancer, slowing down aging, and improving overall vitality. In addition, lettuce is preferably grown in a hydroponic system because of the short growth cycle. The hydroponic plant grows faster and yields higher compared to soil-grown plants under the same conditions. Also, the hydroponic system uses less water due to the constant reuse of nutrient solutions and also reduces the risk of soil-borne diseases The life cycle lettuce is applied to determine the water stress of environmental footprints in a Vertical farming system of electricity generation to migrate carbon footprints of food mils for the supplied chain in urban agriculture. This system applied different types of electric sources and field supply chains for transport [18]. The hydroponic system used a deep-water system, and the nutrient solution was in the tank for the floating of the vegetables. The nutrient solution is mixed up bio floc, and FLOCponics are used in the tank for the fish culture [3]. The plant uptake perfluoroalkyl acid (PFAAs) through the root and translocated in the form of perfluorooctanoic acid (PFOA) and perfluoro octane sulfonic acid (PFOS) [19].
The plant affects biochemical and phytochemical components of food security for benefits and applications. The nutrient components are determined as proteins hydrolyzed for plant growth and phenolic components [12]. The hydroponic system low a weight load substrate and short life cycle and the biomass have low phosphorus content in the struvite system, which is a benefit for urban Agriculture for controlling plant growth and nutritional value [20]. The greenhouse vegetable of lettuce has chemical and physical properties which help the harvester to variance analysis and response content moisture of the leaf, stem, and root of lettuce for the automatic harvesting machine [21]. Domestic sludge wastewater treatment contains numerous nutrients such as phosphorous, potassium, and nitrogen, which are used for the growth of lettuce and to affect the ozone-treated domestic sludge dilution contain nutrients such as chlorophyll, soluble sugar, and ascorbic acid [22].
Solanum lycopersicum: The tomato (Solanum lycopersicum) is the most valuable crop among the others, the economic growth generates social benefits by making efficient use of the resources. The tomato has a short life cycle assessment for the environmental impact and benefits from increasing renewable energy, which is the soilless horticulture with desalinated wastewater recycling. It is sustainable horticulture in the hydroponic system [23]. The hydroponic cultivation method used substrate perlite for tomato fertigation. The nutrient metabolites contain phosphorous, nitrogen, and calcium for growth and the industrial ecology need to balance the nutrient and the substance flow analysis of substrate [24].
The tomato contains higher levels of lycopene and β-carotene in the deepwater culture system. It grows more water use efficiently with DWC, capable of producing higher quality produce [25]. The wastewater treatment used effluent from the anaerobic baffled reactor (ABR) and commercial hydroponic fertilizer mix (EHFM) helps to increase shoot nitrogen potassium, calcium, and zinc for tomato growth in the hydroponic system. The Agricultural water management effect on nutrient absorbance and yield of crop biomass, production of horticulture for the Human excreta-derived materials of tomato [26].
The Dutch bucket system is used for the production of high-level crops such as tomatoes, peppers, and cucumber in control environment agriculture using substrates pine bark, peat, wood fiber, and coir for good fruit quality. The fruits contain phytochemicals and mineral nutrients such as nitrogen, phosphorous, potassium, calcium, sulfur, zinc, boron, copper, manganese, and total anthocyanin and phenol [7]. The tomato grows the drainage solution of a rotating advanced oxidation contactor with TiO2/zero lite composite sheet, which develops the inactivation plant pathogen bacterium Ralstonia solanacearum of a hydroponic system [15].
Triticum aestivum: Wheat contains bioavailability and toxicity of copper and cadmium absorbed properties for growth and also affects seedling growth, photosynthesis, and relative oxygen species (ROS) content [27]. Wheat (Triticum aestivum L.) is the present rich source of genetic variation for the genetically developing, plant growth, and high-yielding wheat genotypes under salinity conditions. Based on the PCA analyzed correlation showed total fresh weight (TW), the relative growth rate for the weight (RGR-Wt), shoot length (SL), new shoot weight (SW), and fresh root weight (RW) of parameters [28].
Zea mays: The maize (Zea mays) is highly dependent on environmental factors for plant growth and the tip of the primary root contains two main regions the root apical meristem (RAM), and the elongation zone (EZ) for the profiling of hormones. It studied the temperature of root tips and cell division of young maize seedlings in moist filter paper for a short period (24 hours) of moderate cooling (200C) condition. The improvement of nitrogen nutrition in crops used physiological and molecular characterization respond to provide different ratios of urea and ammonium. The real-time RT-PCR analyzed the gene expression of the mixture of nitrogen sources, nitrogen fertilizers, and nitrogen transporters of the root uptake [29] (Table 2).
| NAME OF THE PLANT | THE HYDROPONIC SYSTEM | pH | NUTRIENT MEDIUM | REFERENCES |
|---|---|---|---|---|
| Zea mays | Hydroponic (Plastic chambers) | 6.0 | Moist filter paper, | Ivan Friero et al. 2023 |
| Solanum lycopersicum | Hydroponic, (Dutch bucket system) | 5.8 | Water soluble fertilizer (calcium nitrate mixture) Pine bark, coir, wood fiber, | T. Yang et al.2023 |
| Lactuca sativa | Hydroponic, (NFT system) | 6.0 | Greenhouse, Nutrient solution | Kari Jokinen et al.2022 |
| Lactuca sativa | Vertical farming | Fossil fuel. | Leanne Casey et al. 2022 | |
| Triticum aestivum L. | Hydroponic (Iron tube) | 6.0 to 6.5 | Salinity condition | Muhammad Uzair et al. 2022 |
| Glycine max | Hydroponic (Tub) | Wastewater | Sarah A. Brecht et al.2022 | |
| Panax ginseng | Hydroponic(tub) | 6.5 to 7.5 | Silicate mineral water. | Tae Kyung Lee et al. 2022 |
| Lactuca sativa, Capsicum annum | Hydroponic, Pots. | 7.5 to 8 | The nutrient solution, and perlite | Veronica Arcas Pilz, 2022. |
| Fragaria ananassa (Fish-Tinca tinca) | Aquaponic (Multi-layer hydroponic channels NFT) | 4.5 | The nutrient solution, PVC biofilter | Victor M. Fernandez, (2022). |
| Solanum lycopersicum | Hydroponic | 6.2 | Drainage solution | Youhei Nomura et al. (2022). |
| Solanum lycopersicum | Hydroponic (Tank) | 8 to 8.5 | Desalinated seawater | B. Martin Gorriz et al. (2021). |
Table 2: Different Plants, the Scientific name of the Plant, Nutrients whenever used to Culture the Plant, and Reference of Research Paper of the Hydroponic System.
Aquaponic system: Aquaponic is the multi-cultural horticulture essential for human life as a pillar of the economic and multi-food cultural sector. It is a junction solution between hydroponic and aquaponic farming to the product of both plants (Lactuca sativa) and fish (Oreochromis niloticus, Carassius auratus) [30]. The nitrifier plays a vital role in the hydroponic system for the nitrogen cycle of various compartments (fish tank, biofilter, anaerobic digester). The aquaponics system analyzed bacteria, archaea, and tilapia fish (Oreochromis niloticus) community-based [31]. The combined aquaponic system accounted for the climate limitations of the energy consumption reduction during the summer and winter. It is a type of polyculture system and emerging technology in sustainable food production [32].
The strawberries (Fragaria ananassa) yield good quality fruits in aquaculture by using commercial multi-layered NFT channels of producing Tench (Tinca tinca) in a coupled aquaponic system. The system saves the water and substrate content as a circular economy for sustainable agriculture with fewer environmental issues [5]. The plant spinach (Spinachia oleracea) effect interacts with the essential macronutrient and micronutrients in the Nutrient film technique for the uptake and growth of Pangasius (Pangasianodon hypophthalmus) [33]. The traditional farming of the aquaponic systems mostly used goldfish. The two types of fishes such as goldfish (Carassius auratus var.) and crucian carps (Carassius auratus auratus) used for the testing of the intestinal microbiota of culturing different aquaponics and traditional farming. The fish intestinal microbiota plays a vital role in health maintenance such as metabolically promotion, energy utilization, immune function, and endocrine and enterocyte proliferation [34].
Materials and Methods
Experimental site
The Hydroponic system was constructed at MITS Institute of professional studies, Rayagada. The experiment was performed by covering of green polystyrene net (greenhouse). Treatment details of the experimental site are given below:
The material used for the construction of the Hydroponic system was purchased from the local market of Rayagada. The materials required are as follows:
Construction of hydroponic system: The material used for the construction of the Hydroponic system was purchased from the local market of Rayagada (Table 3) (Table 4).
| Sl. No | Material | Quantity | Measurement |
|---|---|---|---|
| 1 | Square pipe | 4 pcs | 20 feet |
| 2 | U PVC pipe | 9 pcs | 10 feet |
| 3 | Elbow | 18 pcs | 1 feet |
| 4 | PVC solvent | 1pcs | 250 ml |
| 5 | Hex a Blade | 2pcs | |
| 6 | Drill bit | 1pcs | 2 inch |
| 7 | Cooler motor | 2pcs | ½ inch |
| 8 | Flexible pipe | 5m | 3feet-1 feet |
| 9 | Reducer | 18pcs | 1-1/2 inch |
| 10 | Water tank | 2pcs | 80 lit |
| 11 | Air pump | 2pcs | |
| 12 | Pots | 1pcs |
Table 3: The Material Used for the Construction of the Hydroponic System.
| SI No. | Particulars | Measurement |
|---|---|---|
| 1 | Height | 6 feet |
| 2 | Length | 9 feet |
| 3 | Width | 5 feet |
| 4 | Hole diameter | 2 inch |
| 5 | Gap b/w hole | 10 cm |
| 6 | Standing | 40 |
| 7 | Gap b/w pipe | 1,5 feet |
Table 4: Construction Details.
Seeds used
Seeds of the lettuce (Lactuca sativa L.) and Amaranthus (Amaranthus cruentus), were purchased from the seed shop of Rayagada. Plants were maintained in the greenhouse at approximately 23–25 ºC, and 13/11 natural light/dark regime. Individual chive plants were separated from a clump and one plant was transplanted to each 10 cm diameter pot containing a substrate mixture of one-third of clay ball, one-third of vermiculite, and one-third of coco pit by volume. The substrate materials were sterilized using 1% sodium hypochlorite for 1h before rinsing with sterile distilled water. The plants were fed with water-soluble, formulated hydroponic fertilizer, Nutrified (Starke Ayres Pty. Ltd.). The fertilizer was dissolved in sterile distilled water at a concentration of 10 g/5 L and 100 mL of the mixture was added to each plant once a week. Each plant was watered with 100 mL reverse osmosis water once a week.
Different treatments: Treatment details of the experimental site are given below (Table 5).
| Condition | Coated seed |
|---|---|
| Control | Amaranthus seed |
| E3 | Amaranthus seed +Priestia aryabhattai |
| P5 | Amaranthus seed + Enterobacter cloacae |
| Control | Lettuce seed |
| E3 | Lettuce seed + Priestia aryabhattai |
| P5 | Lettuce seed +Enterobacter cloacae |
Table 5: Treatment Details of the Experimental site.
pH checking:
It was estimated in water suspension (1:2) using a glass electrode pH meter (Systronics 335) as described by Jackson (1973).
Plant growth measurement
Plant growth measurements such as plant height, root length and weight, shoot length, and weight were taken during the growth period based on the age of the plants. The height of the plant was taken by measuring the length of the plant from the base of the stem to the tip of the stem using a scale.
Spectrophotometric assay for chlorophyll
Estimation of total Chl was done according to Sartory and Grobbelaar (1984). To 1 mL of freshly beaded culture, 2 mL of 80 % methanol was added and agitated in a vortex for 2 min. The mixture was heated at 70°C in a water bath for 10 min. After cooling, the samples were centrifuged for 10 min (4 °C;6,000 × g). For quantification of Chl a and Chl b, absorbance was recorded in the supernatants at 666 nm and 653 nm and quantification was done using the equations of Wellburn (1994).
Result and Discussion
Construction of hydroponic system:
The experiment was performed by covering of green polystyrene net (greenhouse). In the hydroponic system, the Nutrient film technique used for the production of leafy vegetables was accommodated in NFT structures in a tank filled with the nutrient solution there are macro and micronutrients [26]. The experiment used four 80 ltr hydroponic tanks. The total area of the hydroponic system 4.181m2 (45 square feet) is considered for leafy vegetable production. The length, width, and height, of the systems, are 2.743m (9 feet), 1.52400m (5 feet), and 1.8288m (6 feet) respectively. The distance between two PVC pipes is 45 cm (1.5 feet). The distance between the two holes is 10cm and the diameter of the PVC pipe is 3 cm. At the end of the pipe, the U-shaped tubes are connected with an elbow-shaped that pass nutrient through the PVC pipe from the top of the NFT system downward to discharge the nutrient into the outlet bucket. The water is recycled from the bucket the through all parts of the hydroponic system and then inlet into the bucket by the motor [22].
The water is then recycled after travelin[ up through a pipe from the bucket to the system's top. Green crops like lettuce, Amaranthus, coriander, cabbage, and tomatoes are grown hydroponically in an NFT channel system with fertilizer solution and various substrate types under regulated environmental conditions. Green crops like lettuce, amaranth, coriander, cabbage, and tomatoes are grown hydroponically in an NFT channel system utilizing fertilizer solution and various substrate types in a controlled environment.
Evaluation of different conditions for maximum production of lettuce and Amaranthus:
Growth attributing characters
Germination: Seeds of the lettuce (Lactuca sativa L.) and Amaranthus (Amaranthus cruentus) were bio-inoculated with E3, and P5, and then left to dry in the open air. The seeds were then sowed after drying properly. The seeds were planted in three replicates. Within 2 days of sowing, the seeds of lettuce and Amaranthus bio-inoculated with Enterobacter cloacae (P5) showed germination, followed by seeds of lettuce and Amaranthus bio-inoculated with Priestia aryabhattai (E3) The untreated seeds showed complete germination after 4 days (Table 6).
| Lactuca sativa L | Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Control | - | - | + | + | ++ | +++ | +++ | |
| E3 | - | + | ++ | +++ | +++ | +++ | +++ | |
| P5 | - | +++ | +++ | +++ | +++ | +++ | +++ | |
| Amaranthus cruentus | Control | - | - | + | + | ++ | +++ | +++ |
| E3 | - | + | + | ++ | +++ | +++ | +++ | |
| P5 | - | ++ | +++ | +++ | +++ | +++ | +++ |
Table 6: Germination of the Plant.
To quantify the germination stages indicated by "+", we can assign numerical values to these symbols based on their relative levels of germination. Here is a possible interpretation:
- "-" indicates no germination (0%).
- "+" indicates initial germination (approximately 25% of seeds germinated).
- "++" indicates moderate germination (approximately 50% of seeds germinated).
- "+++" indicates full germination (approximately 75-100% of seeds germinated).
Shoot length and weight of Amaranthus cruentus
The data presented in Figure 3.1 clearly showed a distinct variation of the shoot length as observed in different treatments. The maximum shoot length was recorded with P5 (5.7 cm) which was significantly superior to all the treatments including the control (4.8 cm). However, the lowest shoot length was recorded in E3 (4.3 cm).
Maximum shoot weight was recorded in P5 (3.1 g) which also remained at par with E3 (2.7 cm). but differ significantly with control.
Root length and weight of Amaranthus cruentus
Root weight is the most commonly used parameter for studies of root growth in response to the environment. Generally, the washed roots are dried and then their weight is determined. The root length is measured after germination. The root length was measured every week and also measured the root weight.
Root length (a) and root weight (b) of Amaranthus cruentus
The data presented in Figure 3.2 clearly showed no distinct variation of the root length as observed in control and E3 bio-inoculated seeds. The maximum root length was recorded with P5 (6.4 cm) which was significantly high to control (5.4 cm). Maximum shoot weight was recorded in P5 (2.4 g) which also remained at par with control (2.7 cm).
Whole plant length and Weight of Amaranthus Cruentus
Data on the whole plant height of Amaranthus cruentus was recorded at 7, 14, and 28 days. Among all the treatments, plant heights of the crop ranged between 12.2 to 9.6 cm at harvest. The treatment P5 recorded the highest plant height 12.2 cm at 28 days, closely followed by the control which was 10.9 cm at 28 days at harvest. E3 treated maintained the lowest plant heights of 9.5.
Leaf chlorophyll content (mg/g)
From the data tabulated and presented in the Table recorded that the leaf chlorophyll was also significantly influenced by different treatments, but much difference is not noticed among the treatments. The maximum chlorophyll content in lettuce (Lactuca sativa L.) was recorded under the treatment P5 (2.81 mg/g) which was significantly superior to other treatments. However, the lowest value was recorded under E3 (0.78 mg/g). In Amaranthus (Amaranthus cruentus) the chlorophyll content was recorded in the range 2.2 – 1.5 mg/g in all treatments, including control.
pH of the growth medium
After 4 days of seedling, the pH of the system which is mostly required for plant growth was checked. The pH of the experimental system was in the range of 5.6 – 6.2 and it remained more or less the same throughout the crop growth period. pH gradually increased up to 6.5 till harvest in P5-treated seeds. pH value of around 6 is ideal for plant growth. After one week, the pH of the solution was 6.3. The purpose of this study was to evaluate the effects of different plant growth stages and pH (values 5-6). An important chemical property of nutrient solutions is their pH, which is calculated in a range of 1 to 14 and represents the acidity or alkalinity of the solution. Water has a pH of 7, which means that at room temperature, it is neither basic nor acidic. A solution is considered basic if its pH is greater than 7; otherwise, it is considered acidic. Since nutrients remain soluble in this pH range, the bulk of the literature agrees that the nutrition solution must be between 5 and 7. Fe and H2PO4 are less soluble at pH values over 7, which causes the development of Ca and Mg precipitates as well as other chemical reactions.
The development of crops and their yields:
The figure shows the outputs for the lettuce and Amaranthus cycles. Here, we can see the average yields for all three harvests and all treatments for their fresh and dry weights. In this experiment, two distinct crops, lettuce (Lactuca sativa L.) and amaranth (Amaranthus cruentus) were used to determine the dissolution and uptake of struvite.
Conclusion
The initial design, whether in the form of hydroponic barrels, was unable to produce an effluent that met accepted standards. However, the Nutrient film technique (NFT) proved effective. Bioinoculation of the substrate with nitrogen fixing and phosphate solubilizing bacteria enhanced the rate of germination of Amaranthus and lettuce. Enrichment of substrate with nitrogen fixing and phosphate solubilizing bacteria improved the reaction (pH) and plant whole length. The horizontal flow channels could be considered plug flow reactors. A hydroponic system using minimal substrate is suitable and/or feasible for green leafy vegetable production. Plant yield and growth were better in hydroponic horizontal channels. This Hydroponic system can be used through a decentralized wastewater treatment and reuse system; being relatively cheap to construct and operate, having low maintenance requirements, and also a long lifespan.
Acknowledgement
I would like to express my gratitude to my guide Dr. Shilalipi Samantharay and Asst. Prf. Miss Jayshree Jena, for their valuable and helpful recommendations during the planning stage and analysis of my research. Their Guidance, advice, patience, and granted carried me through all the stages of writing my project and thesis, and access to the technical equipment have helped me to use the latest technology innovations and complete this work. Especially thank Dr. Santunu Mahanty for his continuous support and understanding when undertaking my research and writing my project and providing the experimental setup through the college. I am forever thankful for the unconditional love and support throughout the entire thesis process.
Conflict of Interest
None
References
- Maucieri C, Nicoletto C, Junge R, Schmautz Z, Sambo P, et al. (2018) Hydroponic systems and water management in aquaponics: A review. Journal Italian of agronomy 13: 1012.
- Jokinen K, Salovaara AK, Wasonga DO, Edelmann M, Simpura I, et al. (2022) Root-applied glycine betaine decreases nitrate accumulation and improves quality in hydroponically grown lettuce. Journal ofFood Chemistry 366: 130558.
- Pinho SM, David LH, Garcia F, Portella MC, Keesman K J (2022) Sustainability assessment of FLOCponics compared to stand-alone hydroponic and biofloc systems using emergy synthesis. Ecological Indicators 141: 109092.
- Gillani SA, Abbasi R, Martinez P, Ahmad R (2023) Comparison of energy-use efficiency for lettuce plantation under nutrient film technique and deep-water culture hydroponic systems. Procedia Computer Science 217: 11-19.
- Fernández-Cabanás VM, Delgado A, Lobillo-Eguíbar JR., Pérez-Urrestarazu L (2022) Early production of strawberry in aquaponic systems using commercial hydroponic bands. Aquacultural Engineering 97: 102242.
- Delaide B, Teerlinck S, Decombel A, Bleyaert P (2019) Effect of wastewater from a pikeperch (Sander lucioperca L.) recirculated aquaculture system on hydroponic tomato production and quality. Agricultural Water Management 226: 105814.
- Yang T, Altland JE, Samarakoon UC (2023) Evaluation of substrates for cucumber production in the Dutch bucket hydroponic system. Scientia Horticulturae 308: 111578.
- Van Rooyen IL, Nicol W (2022) Nitrogen management in nitrification-hydroponic systems by utilizing their pH characteristics. Environmental Technology & Innovation 26: 102360.
- Li TT, Liu P, Wang H, Dai W, Wang J, et al. (2021) Preparation and characteristics of flexible polyurethane foam filled with expanded vermiculite powder and concave-convex structural panel. Journal of Materials Research and Technology 12: 1288-1302.
- Marcos C, Lahchich A, Álvarez-Lloret P (2023) Hydrothermally treated vermiculites: Ability to support products for CO2 adsorption and geological implications. Applied Clay Science 232: 106791.
- Khiari R., Ndiaye D (2022) The acoustic properties of coir coconut fiber 202. Coir Fiber and its Composites. Woodhead Publishing 359-372.
- Dass SM, Chai TT, Cao H, Ooi AL, Wong FC (2021) Application of enzyme-digested soy protein hydrolysate on hydroponic-planted lettuce: Effects on phytochemical contents, biochemical profiles and physical properties. Food Chemistry 12: 100132.
- Samreen T, Shah HU, Ullah S, Javid M (2017) Zinc effect on growth rate, chlorophyll, protein and mineral contents of hydroponically grown mungbeans plant (Vigna radiata). Arabian Journal of Chemistry 10: S1802-S1807.
- Aslani F, Juraimi AS, Ahmad-Hamdani MS, Hashemi FSG, Alam MA, et al. (2016) Effects of Tinospora tuberculata leaf methanol extract on seedling growth of rice and associated weed species in hydroponic culture. Journal of integrative agriculture 15: 1521-1531.
- Nomura Y, Koga K, Ohnishi K, Fukahori S, Fujiwara T (2022) Inactivation of plant pathogenic bacterium Ralstonia solanacearum in drainage solution from hydroponic system by a rotating advanced oxidation contactor equipped with TiO2/zeolite composite sheets. Journal of Water Process Engineering 48: 102936.
- Bo L, BIAN ZH, Yang QC, Jun W, CHENG RF, et al. (2018) The positive function of selenium supplementation on reducing nitrate accumulation in hydroponic lettuce (Lactuca sativa L.). Journal of integrative agriculture 17: 837-846.
- Mampholo BM, Maboko MM, Soundy P, Sivakumar D (2018) Variety-specific responses of lettuce grown in a gravel-film technique closed hydroponic system to N supply on yield, morphology, phytochemicals, mineral content and safety. Journal of integrative agriculture 17: 2447-2457.
- Casey L, Freeman B, Francis K, Brychkova G, McKeown P, Spillane C, Styles D (2022) Comparative environmental footprints of lettuce supplied by hydroponic controlled-environment agriculture and field-based supply chains. Journal of Cleaner Production 369: 133214.
- Felizeter S, Jürling H, Kotthoff M, De Voogt P, McLachlan MS (2020) Influence of soil on the uptake of perfluoroalkyl acids by lettuce: A comparison between a hydroponic study and a field study. Chemosphere 260: 127608.
- Arcas-Pilz V, Parada F, Rufi-Salis M, Stringari G, Gonzalez R, et al. (2022) Extended use and optimization of struvite in hydroponic cultivation systems. Resources Conservation and Recycling 179: 106130.
- Wang W, Ma Y, Fu L, Cui Y, Majeed Y (2021) Physical and mechanical properties of hydroponic lettuce for automatic harvesting. Information Processing in Agriculture 8: 550-559.
- Peng Y, Guo YZ, Ling Q (2018) Effects of ozone-treated domestic sludge on hydroponic lettuce growth and nutrition. Journal of integrative agriculture 17: 593-602.
- Martin-Gorriz B, Maestre-Valero JF, Gallego-Elvira B, Marín-Membrive P, Terrero P, et al. (2021) Recycling drainage effluents using reverse osmosis powered by photovoltaic solar energy in hydroponic tomato production: Environmental footprint analysis. Journal of Environmental Management 297: 113326.
- Sanjuan-Delmás D, Josa A, Muñoz P, Gassó S, Rieradevall J, et al. (2020) Applying nutrient dynamics to adjust the nutrient-water balance in hydroponic crops. A case study with open hydroponic tomato crops from Barcelona. Scientia Horticulturae 261: 108908.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1663
- [From(publication date): 0-2024 - Nov 16, 2025]
- Breakdown by view type
- HTML page views: 1371
- PDF downloads: 292